Abstract
Salix psammophila has been extensively used as a sand barrier material for various desertification control applications. Elucidating the long-term decomposition characteristics and nutrient cycling process of this sand barrier in desert environments is of great importance. In this study, which was conducted for 1 to 9 years, changes in the mass loss percentage and the residual percentage in the decomposition process were explored of S. psammophila sand barriers in arid Northwestern China. In addition, the S. psammophila analysis nutrient elements release rule and its influence on soil properties were evaluated. The results showed that the decomposition process of S. psammophila sand barriers exhibited a “slow-fast” trend. After decomposition time for 9 years, mass decreased remarkably, and the residual percentage was 33.6%. Further, the nutrient release characteristics differed. C, P, and K were in the release state, whereas N was in the enrichment state. The decomposition percentage of the sand barriers was significantly correlated with N, P, K, C/N, C/P, and N/P (p < 0.05). The soil nutrient contents of C, P, and K contents increased 3.43, 2.23, and 2.08 g/kg compared to the initial values, respectively. The soil nutrient contents of N contents decreased 0.19 g/kg.
Download PDF
Full Article
Decomposition Characteristics of Long-established Salix psammophila Sand Barriers in an Arid Area, Northwestern China
Ruidong Wang,a Xia Yang,a,* Yong Gao,a Xiaohong Dang,a,b Yumei Liang,a Shuai Qi,a Chen Zhao,a and Xiaoting Duan a
Salix psammophila has been extensively used as a sand barrier material for various desertification control applications. Elucidating the long-term decomposition characteristics and nutrient cycling process of this sand barrier in desert environments is of great importance. In this study, which was conducted for 1 to 9 years, changes in the mass loss percentage and the residual percentage in the decomposition process were explored of S. psammophila sand barriers in arid Northwestern China. In addition, the S. psammophila analysis nutrient elements release rule and its influence on soil properties were evaluated. The results showed that the decomposition process of S. psammophila sand barriers exhibited a “slow-fast” trend. After decomposition time for 9 years, mass decreased remarkably, and the residual percentage was 33.6%. Further, the nutrient release characteristics differed. C, P, and K were in the release state, whereas N was in the enrichment state. The decomposition percentage of the sand barriers was significantly correlated with N, P, K, C/N, C/P, and N/P (p < 0.05). The soil nutrient contents of C, P, and K contents increased 3.43, 2.23, and 2.08 g/kg compared to the initial values, respectively. The soil nutrient contents of N contents decreased 0.19 g/kg.
Keywords: Salix psammophila sand barriers; Desertification control; Degradation process; Nutrient release percentage; Soil nutrient; Mass loss percentage
Contact information: a: Key Laboratory of Eolian Physics and Desertification Control Engineering, College of Desert Control Science and Engineering, Inner Mongolia Agricultural University, 29 Erdos East Street, Hohhot 010018, China; b: Inner Mongolia Hangjin Desert Ecological Position Research Station, Ordos 017400, P.R. Inner Mongolia China; * Corresponding author: yangxiashamoyuan@imau.edu.cn
GRAPHICAL ABSTRACT
INTRODUCTION
Using plants to control the development of desertification has become the focus of desertification control technology research, and the comprehensive utilization and development of plant resources in sandy areas aims to make plants participate in the virtuous cycle of ecological environment (Zhang et al. 2019). Salix psammophila is a typical desert deciduous shrub or small tree that is drought-resistant and has other biological characteristics, such as strong adaptability, easy reproducibility, and rapid growth. It grows widely in harsh, windy, arid, sandy environments and is mainly distributed in mobile sand dunes, semifixed sand dunes, and lowlands between hills in northwest China (Gao and Gong 2013). To prevent wind erosion or sand burying and improve the growing environment of plants, sand barriers have been adopted to control desertification in northwest China (Wang et al. 2008). Various configurations of S. psammophila are set in the sand surface, and their stems control the direction, speed, and structure of the wind-sand flow. Research has shown that the use of fast-growing S. psammophila as a sand barrier remarkably reduces near-surface wind speed, increases surface roughness, and increases vegetation coverage (Zhao et al. 2008; Gong 2012). The sand barrier can effectively improve the microenvironment of the sand dune and accelerate the process of vegetation restoration (Gao and Yi 1996; Wang et al. 2019). Further, S. psammophila sand barriers are low-cost, durable, and have a stable protection time.
Salix psammophila sand barriers are set for long-term establishment in desert environments. As the setting duration increases, S. psammophila buried in sand must endure changes in environmental temperature and humidity, rainwater erosion, and microbial degradation. S. psammophila sand barriers degrade to different degrees due to decay (Gong 2012; Wang et al. 2020). The decomposition of natural biomaterials is the link between the growth of psammophytes and the nutrient cycle of soil, which can promote the recovery and growth of vegetation, improve soil fertility, and play an important role in the desert ecosystem (Yu et al. 2015; Parton et al. 2007). Information on the decomposition process and nutrient release characteristics of S. psammophila sand barriers is limited, and research on the decomposition of S. psammophila sand barriers in desert environments has yet to be published. Compared to other environments, the desert environment is more extreme and more sensitive to extreme climate change and disturbance by human activity (Allington and Valone 2010).
The decomposition of natural biomaterials and their nutrient release change the carbon and nutrient cycles of ecosystems and their responses to environmental changes, and they have important effects on the accumulation of soil organic matter and the dynamics and availability of major nutrients. Decomposition plays an important role in important ecological processes, such as material circulation, structure, and functional maintenance of ecosystems (Goebel et al. 2011; Xu et al. 2013; He et al. 2019). Thus, there is a need to better understand the general laws of nutrient release and its response to the environment during the degradation of S. psammophila sand barriers to predict the biogeochemical cycle of the desert ecosystem.
In this study, S. psammophila sand barriers set in arid northwestern China for 1 to 9 years were studied. The decomposition characteristics of S. psammophila sand barriers and changes in the degradable residue percentage and substrate quality during decomposition were analyzed. The release and enrichment characteristics of C, N, P, and K during S. psammophila sand barrier decomposition were studied. Combined with the variation characteristics of soil nutrients (C, N, P, and K) and elements in the area surrounding the barrier, the effects of S. psammophila sand barrier degradables on soil nutrients in the degradation process were considered to promote material circulation in the ecological environment of the desert and provide information on the biogeographic element circulation process in the desert ecosystem.
EXPERIMENTAL
Study Site Description
The study was conducted at the Hangjin Banner, Ordos, Inner Mongolia in arid northwestern China (108°41′21″E, 40°29′34″N), as illustrated in Fig. 1. The study area had a plateau continental climate. There was sufficient sunshine and a large temperature difference between day and night. In addition, it had an annual average temperature of 6.3 °C, a maximum temperature of 38.1 °C, a minimum temperature of -30.5 °C, and a frost-free period of 135 d. Sand dune and dune slack were distributed alternately in the vegetation restoration area, and the primary geomorphic types were active dune, semiactive dune, fixed dune, semifixed dune, and dune slack. The zonal soils in the region were chestnut soil and brown calcic soil, whereas the nonzonal soils were eolian sandy soil and meadow soil (Na 2010). Artificial vegetation was the primary vegetation type in the studied area, which included shrub species (e.g., Psammochloa villosa, Agriophyllum squarrosum, and Artemisia ordosica) (Na 2010).
Fig. 1. Location of the study area (Hangjin Banner, Ordos, Inner Mongolia, China)
Materials
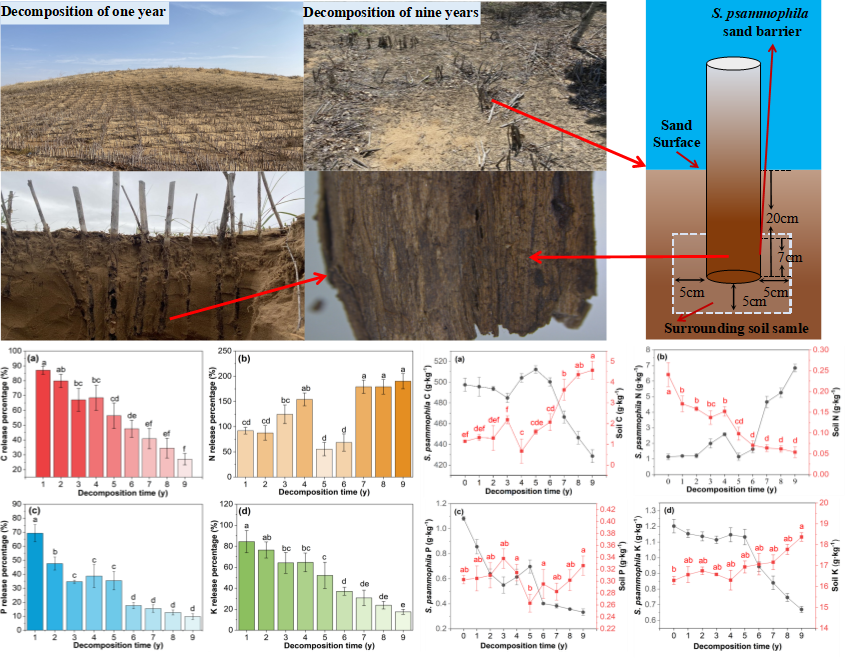
The experimental materials were collected from the research area in November 2019 via field investigation. The local S. psammophila sand barrier was divided accurately according to the setting year. After determining the overall situation of the sand barrier setting at the test site, the “space instead of time” method was used for sampling and investigation. From 2011 to 2019, the S. psammophila sand barrier and surrounding soil that had been setting for 1 y to 9 y were collected in gentle dunes from the sampling site and photographed, as illustrated in Fig. 2. At the same time, the sand barrier and soil just set up on the gentle dune for one month were taken as the control and recorded as 0 years.
S. psammophila sand barrier sampling
Sand barrier samples with a length of 7 cm was intercepted at a vertical depth of 20±0.5 cm from the dune surface. Three representative sample sites were selected in each year, and 6 samples were taken from each sample site to reach a total of 162 samples. After removal, photos were taken for recording.
The sand barriers collected from the field were pretreated, the soil and impurities attached to the surface were cleaned with a brush, and they were then stored in a sealed bag for sealing. After transportation to the laboratory, the samples were placed into the oven, dried at 80 °C to a constant weight, and then removed from the oven. After the samples were dried, they were ground into powder and passed through a 40-mesh to 60-mesh sieve to determine the nutrients of the S. psammophila sand barriers.
Fig. 2. Schematic diagram of S. psammophila sand barrier in desert environment
Surrounding soil sampling of S. psammophila sand barrier
The surrounding soil samples collection of surrounding soil corresponded to the collection of S. psammophila sand barrier plant material samples. The soil was collected within a horizontal radius of 5 cm and a vertical depth of the minimum and maximum distances from the soil surface are 13 and 25 cm respectively (including 7 cm of plant material and 5 cm below the plant material, a total of 12 cm). Three representative barriers on the same contour line of each year were selected, and three replicates were set in the area with an interval of 2 m. Fifteen samples were taken from each dune, and a total of 135 samples were taken and brought back to the laboratory.
Methods
The nutrient contents C, N, P, and K of the S. psammophila sand barrier were determined after the samples were crushed by a grinder and ground into powder through a 40-mesh to 60-mesh sieve. Soil samples collected from the field were naturally air-dried, and their nutrient content was determined. The total C contents of the S. psammophila sand barrier and the surrounding soil were determined via the potassium dichromate volumetric method. The N content was determined via the perchloric acid-sulfuric acid digestion method. The total N content of the soil was measured with the semimicro-kjeldahl method using a Kjeldahl apparatus (Kjeldahl 2200 Auto Distillation Unit, FOSS, Scandinavia, Sweden), whereas the total P and K concentrations were obtained via the HF-HClO4-HNO3 digestion method using a 6300 ICP-AES (Thermo Scientific, Waltham, MA, USA).
Data analyses
Because it is impossible to directly measure the quality of the sample before the decomposition of the S. psammophila sand barrier, it was simulated by using several calculations. The initial mass of the S. psammophila sand barrier was calculated with the density and volume method. Therefore, a large number of control samples were needed to simulate the density of S. psammophila sand barriers (Gong 2012). In the early stages, an experimental analysis was performed on the density of sand barriers, and 274 groups were sampled from the field test site. The density prior to decomposition the S. psammophila sand barrier was calculated as the average of the normal density, which was ρ0 = 0.5534 g/cm3. The mass loss percentage of the S. psammophila sand barrier was calculated with Eq. 1,
(1)
where Dmi (%) is the mass loss percentage, ρ0 is the average density (g/cm3) of the control sample, M is the mass of the S. psammophila sand barrier samples (g), and V is the volume of the S. psammophila sand barrier samples (g/cm3).
The nutrient accumulation index (NAI) was used to represent the accumulation or release of nutrient elements during the decomposition process of S. psammophila sand barrier (Eq. 2),
(2)
where NAI (%) is the nutrient accumulation percentage, Mt is the S. psammophila sand barrier residual mass (g), M0 is the initial contrastive mass (g), Ct is the remaining nutrient concentration (g/kg), and C0 is the contrastive nutrient concentration (g/kg).
All statistical analyses were conducted using the SPSS 19.0 statistical software package (SPSS Inc., Chicago, IL, USA) with a significance level of p <0.05, and figures were prepared with Origin 8.0 (OriginLab Corp., Northampton, MA, USA).
In addition, the correlations between mass loss percentage and the nutrient contents of the S. psammophila sand barriers were also examined using Pearson correlation coefficient analysis.
RESULTS
Changes in the Mass Residual Percentage and Mass Loss Percentage in the Decomposition Process of S. psammophila Sand Barriers
Figure 3 shows that the residual percentage of S. psammophila sand barrier decreased during the decomposition process from 1 y to 9 y. There were significant differences in the mass residual percentage during the decomposition process (p < 0.05). The decomposition process was divided into two stages. The initial stage (1 y to 5 y), in which the mass residual percentage changed a small amount, indicated that the decomposition of the S. psammophila sand barrier was slow. At the later stage (5 y to 9 y), the overall residual percentage reached the minimum of 33.62%.
Fig. 3. Changes in the mass residual percentage and mass loss percentage in the decomposition process of S. psammophila sand barriers. Note: Different lowercase letters in the figure indicate significant differences between different decomposition years of the sand barrier (p < 0.05).
The mass loss percentage of the S. psammophila sand barrier increased as decomposition time increased. The changes in the mass loss percentage were different in different stages. During the decomposition period of 1 to 3 years, the mass loss percentage was 14.6% after decomposition 3 y. With the change of environmental factors, the microbial activity was enhanced, and the decomposition speed was accelerated, which resulted in a rapid change in the mass loss percentage. The loss percentage was 60.3% after decomposition 5 y. Beyond after decomposition 5 y, the decomposition decreased and reached the maximum value of 66.4% after decomposition 9 y (later stage of decomposition).
Decomposition Nutrient Release Characteristics of the S. psammophila Sand Barriers
The release and enrichment of nutrients (C, N, P, and K) were different during the decomposition process of the S. psammophila sand barrier, and the release and enrichment percentage of nutrients accelerated as laying time increased. The period from 0 y to 3 y was a slow release and enrichment period, the period from 3 y to 5 y was a mixed period of enrichment and release, and the duration of 5 years later was the last instance of nutrient release or enrichment.
Fig. 4. The decomposition nutrient release characteristics of the S. psammophila sand barriers. Note: Different lowercase letters in the figure indicate significant differences between different decomposition years of the sand barrier (p < 0.05).
Figure 4a shows that in the desert environment, the degree of decomposition of the S. psammophila sand barrier varied with the time after decomposition time increased. The C content showed a fluctuating trend of decreasing, rising, and decreasing with the increase in decomposition time. Decomposition for 0 y to 5 y resulted in nutrient C content being basically maintained within a small range of fluctuations. After decomposition for 5 y to 9 y, the C content gradually decreased to the lowest figure at 9 y of setting, which represented a decrease of 68.7 g/kg from the initial value. Throughout the decomposition process, the change in the C accumulation index was the same as the change in C content, as shown in Fig. 5a. The change of the overall change rule is the release state, and the release percentage of C varies significantly with the increase of decomposition time (p < 0.05). Among them, 20.0% of the C content was released from 0 y to 3 y. The content of nutrient C fluctuated from 3 y to 5 y. After decomposition 5 y, the C content accumulation index gradually decreased to the lowest figure at 9 y of decomposition, 27.05%.
Figure 4b shows that the N content in the decomposition process of the S. psammophila sand barrier had a fluctuating trend of rising, decreasing, and rising as the decomposition time increased. There were significant differences in the decomposition process (p < 0.05), and the N content increased from 0 y to 4 y of decomposition time within a small range, and the maximum value was 2.58 g/kg. After decomposition time for 4 y, the N content gradually decreased. The N content showed an upward trend after 5 y with a large increase range, and it gradually decreased to the highest figure at 9 y of decomposition, 6.83 g/kg. The N accumulation index of the S. psammophila sand barrier showed a fluctuating increasing trend as the decomposition time increased. As shown in Fig. 5b, the S. psammophila sand barrier was in a state of enrichment overall. The sand barrier was in a state of slow enrichment from 0 y to 4 y of decomposition and showed a small release after 4 y. The sand barrier began to enrich after 5 y, and the C content accumulation index reached the maximum at 9 y of decomposition, 190%.
Fig. 5. The variation of the nutrient release percentages of the S. psammophila sand barriers during the decomposition process. Note: Different lowercase letters in the figure indicate significant differences between different decomposition years of the sand barrier (p < 0.05).
As shown in Fig. 4c, the P content of the S. psammophila sand barrier showed an decreasing, rising, and decreasing trend with the increase in decomposition time. After decomposition for 0 y to 3 y, the P content decreased rapidly, as it had a decrease of 0.53 g/kg compared to the initial value. Decomposition for 3 y to 5 y, showed an overall upward trend. After decomposition for 5 y, the P content showed a slow downward trend, the P content gradually decreased to the lowest figure at 9 y of decomposition, 0.33 g/kg. Throughout the decomposition process, the P content changed as the cumulative index law changed. Fig. 5c shows the overall performance was the state of first release followed by enrichment and then release, which included a C content of nutrient release of 34.68% from 0 y to 3 y, P enrichment from 3 y to 5 y, and a quick release 5 y later. And the accumulation index gradually decreased to the lowest figure at 9 y of decomposition, 9.73%.
As shown in Fig. 4d, the K content of S. psammophila sand barrier had a decreasing trend decomposition for 0 y to 3 y, and it exhibited a decrease of 0.08 g/kg compared to the initial value. Decomposition for 3 y to 4 y, the K content showed an upward trend. After decomposition for 5 y, the K content gradually decreased to the lowest figure at 9 y of decomposition, 0.53 g/kg. The K accumulation index of the S. psammophila sand barrier showed a fluctuating decreasing trend as decomposing time increased. As shown in Fig. 5d, the K content exhibited a release-enrichment-release trend. The K content was in a state of release from 0 y to 3 y of decomposition time and began to be slightly enriched from 3 y to 4 y. After decomposition for 5 y, it was released rapidly, and the C content accumulation index decreased to the lowest figure at 9 y of decomposition,17.52%.
Characterization of Stoichiometry During the Decomposition of the S. psammophila Sand Barriers
Figure 6a shows that the C/N content in the decomposition process of S. psammophila sand barrier had overall decreasing trend with the increase in decomposition time. In the decomposition process, C/N showed an decreasing trend from 0 y to 4 y, an increasing trend from 4 y to 5 y, and a decreasing trend after decomposition for 5 y. In the decomposition process, the C/P of the S. psammophila sand barrier showed an overall downward trend with the increase in setting years. As shown in Fig. 6b, C/P showed an increasing trend from 0 y to 3 y, and decomposition for 3 y showed an decreasing trend. After decomposition for 5 y, the C/P showed a upward trend, the C/P gradually increased to the highest figure at 9 y of decomposition. The change pattern of N/P was the same as that of C/P, as shown in Fig. 6c, in which N/P exhibited an increasing trend from 0 y to 3 y, a decreasing trend decomposition for 3 y, and increased decomposition for 5 y.
Fig. 6. Variation in the nutrient accumulation index of the S. psammophila sand barriers during the decomposition time. Note: Different lowercase letters in the figure indicate significant differences between decomposition years of the sand barrier (p < 0.05).
The Effect of the Decomposition of the S. psammophila Sand Barriers on Surrounding Soil Nutrients (C, N, P, and K)
As shown in Figure 7, the soil nutrient content surrounding the S. psammophila sand barrier were characterized by fluctuating increases and decreases with the increase in decomposition time. As shown in Fig. 7a, the surrounding soil C content showed increased when the C content of the S. psammophila sand barrier was released throughout the decomposition process, it gradually increased to the highest figure at 9 y of decomposition, which was an increase of 3.43 g/kg compared to the initial value. Figure 7b shows the soil N content had an overall downward trend, and it gradually decreased to the lowest figure at 9 y of decomposition, which was 0.19 g/kg lower than the initial value. Figure 7c shows that the soil P content featured a rising-declining-rising trend with decomposition time, showed an overall downward trend with the increase in setting years. This was due to the small amount of P contained in the S. psammophila sand barrier, which resulted in reduced accumulation of P in the soil during the decomposition process of S. psammophila sand barriers. Figure 7d shows that the soil K content was consistent with the change pattern of the C content of the soil, as it had a rising-declining-rising trend, and it it gradually increased to the highest figure at 9 y of decomposition, which was 2.08 g/kg higher than the initial value.
Fig. 7. The variation in the characteristics of surrounding soil C, N, P, and K during the decomposition process of the S. psammophila sand barriers. Note: Different lowercase letters in the figure indicate significant differences between different decomposition years of the sand barrier (p < 0.05).
DISCUSSION
The decomposition of S. psammophila sand barriers is a process of material exchange with the external environment that is governed by a variety of factors, in which soil biological metabolism interacts with the uptake and release of chemical elements under the action of soil leaching and decomposition (Burton et al. 2000). In the early stages of decomposition, soluble materials, such as carbohydrates, are rapidly leached due to environmental factors, such as soil temperature and soil water content (Parton et al. 2007; Tu et al. 2014) and decomposition liners. At a later stage, the decomposition process is mainly influenced by the biological action of depleting soluble compounds, whereas insoluble materials, such as lignin and cellulose, remain for microbial degradation. This study showed that the mass residual percentage of S. psammophila decreased as the time after decomposition time increased, and the mass residual percentage of the S. psammophila sand barrier was 73.8% after decomposition 3 y, but the mass residual percentage was only 39.7% after decomposition 7 y. In addition, the slope curve became slow after 7 y, and it reached the lowest value of 33.6% after decomposition 9 y. This result was related to the initial chemical properties of the S. psammophila sand barriers. However, the drought and low rainfall in the study area limited the decomposition of the S. psammophila sand barriers.
The decomposition of the S. psammophila sand barriers was accompanied by changes in the nutrient elements, and the release/enrichment of these elements plays an important role in the cycling process and is a remarkable determinant of the growth of vegetation in the desert environment (Aerts and de Caluwe 1997). In this study, the relationship between the decomposition of S. psammophila sand barriers and decomposition time was characterized by different degrees of release and enrichment of nutrients (C, N, P, and K). However, their contents tended to increase as decomposition time increased (Fig. 4), which was determined by the decomposition of the S. psammophila sand barriers and the characteristics of the elements themselves. From 0 y to 3 y of decomposition time, most of the C in the S. psammophila sand barriers existed as small molecules, such as soluble sugars and amino acids, P and K existed mainly as soluble inorganic ions, and C, N, and P were continuously exchanged with the soil through fine root leaching (Fahey et al. 1984). After 5 y of decomposition, C exists mainly as insoluble substances, such as lignin, cellulose, and tannin, and under the action of soil microorganisms, a series of irreversible changes occur in the structure and properties of S. psammophila sand barriers, and the chemical composition begins to disappear rapidly, which leads to a faster decomposition percentage (Wang et al. 2021). The decomposition percentage was mainly affected by the physical and chemical properties of the S. psammophila sand barrier, such as nutrient content, water-soluble carbon compounds, and structural carbon compounds, and it provides a sufficient environment for the growth and reproduction of microorganisms, so that the decomposition percentage is faster. In the later stage, decomposition was inhibited by lignin or cellulose (Yang et al. 2004).
The nutrient C content exhibits a variable release characteristic of S. psammophila sand barriers. The decomposition process of S. psammophila sand barriers is mainly dominated by C in the material cycling pattern. The leaching of soluble materials and the decomposition of easily decomposable carbohydrates both cause the release of C (Yang et al. 2006). The nutrient N content showed a small enrichment-release-enrichment pattern, and the enrichment of N content from 3 y to 5 y may have been due to the variation of N content related to the water and heat conditions, soil temperature and humidity, and the initial nutrients in the desert environment. Microbial reproduction and development require the uptake of large amounts of exogenous N from the external environment; therefore, N elements are enriched (Wu et al. 2007). From 3 y to 5 y of decomposition, there was a decreasing trend of the N content in the S. psammophila sand barriers, and microbial activities are frequent enough to satisfy the decomposition activities of soil microorganisms without absorbing N from the external environment for decomposition (Manzoni et al. 2008).
Table 1. Correlation Coefficients Between Mass Loss Percentage and Nutrient Contents of S. psammophila Sand Barriers
There was a significant positive correlation between the mass loss percentage and the N content variation of the S. psammophila sand barriers, and a there was highly significant negative correlation between the mass loss percentage and C/N (p < 0.05) (Table 1). Related studies have suggested that decomposition percentages are influenced by both internal (preserved within the vegetation) and external (acquired by external factors) N concentrations (Tu et al. 2015). In other studies, the decomposition percentage was mainly dependent on the amount of lignin and initial C content rather than the initial N concentration (Chen et al. 2001), which was inconsistent with the results of this study. This may be due to the inhibition of lignin decomposition by remarkable lower soil temperature and moisture, whereas nitrogen is enriched during the decomposition of S. psammophila sand barriers.
During the decomposition process of the S. psammophila sand barriers, the P content was generally in a state of release, as it had essentially the same characteristics as the release of C content. The mass loss percentage was highly remarkable and negatively correlated with the nutrient P content. As P elements are easily leached, they exist mainly in the form of phosphate or compounds. Therefore, the release of P elements from S. psammophila sand barriers is faster, and the microbial decomposition is supported by the continuous release of phosphorus from the sand barriers themselves (Li et al. 2008). This results in a faster conversion percentage for S. psammophila sand barriers and a faster percentage of decomposition between 5 y and 9 y of decomposition time when soil microbial activity is intense and enzymatic activity for decomposing organic matter is enhanced (Lin et al. 2011). In other studies, nutrients were reported to be more likely to be released into the environment at higher initial concentrations, whereas nutrients are enriched at lower initial concentrations (Vogt et al. 1986).
As decomposition occurs, different nutrient elements in the S. psammophila sand barriers were accumulated or released, which resulted in different trends in their stoichiometric characteristics (Reich and Oleksyn 2004). In this study, C/N showed an overall decreasing trend during the decomposition of the S. psammophila sand barriers, whereas C/P and N/P showed a fluctuating trend of first decreasing and then increasing. The C/N content did not change notably with the C content, and the N content was the key factor that regulated the change of C/N during the decomposition of the S. psammophila sand barriers. The change in P content played an important role in the change of C/P and N/P, and if the value of C/P and N/P was small, the S. psammophila sand barriers were easily decomposed. In addition, due to the different environmental factors in the study area, the threshold values of C/N also differed greatly, which further led to the differences in the correlation between the decomposition process of the S. psammophila sand barriers and the dynamic change process of nutrients C and N.
The decomposition and nutrient cycling of S. psammophila sand barriers in arid ecosystems are important for the study of regional carbon and nitrogen cycles, and they provide a theoretical basis for vegetation restoration. The degradable S. psammophila sand barrier on soil nutrients promote material circulation in the ecological environment of the desert. The content of C, N, P, and K released during the decomposition of the S. psammophila sand barriers participated in the material cycle and returned nutrients to the soil, which remarkably affected the soil physical and chemical properties (McFee and Stone 1966). As the decomposition speed of the S. psammophila sand barriers increased, the impact on the physicochemical properties of the soil increased, which obviously improved the soil. This study showed that the decomposition of S. psammophila sand barriers could improve the C content of the soil, which was consistent with other findings on desert ecosystems (Hu et al. 2016). Several studies have shown that the decomposition of natural biological materials is important for soil C input (Hobbie et al. 2010). Research has mentioned the different contributions of decomposers to soil organic carbon accumulation and the differences in their accumulation mechanisms (Peng and Liu 2002). Organic carbon stock has an important influence on the regional carbon cycle, and it influences the growth of vegetation in desert environments. The nutrients released during the decomposition of S. psammophila sand barriers (N and P) are important sources of soil N and P and determine the effectiveness of soil nutrients.
CONCLUSIONS
- During the decomposition process from the after one to nine years of decomposition (1 y to 9 y), the overall decomposition trend of S. psammophila sand barriers was slow at first and then fast.
- After decomposition for 9 years, the C, P, and K contents of the S. psammophila sand barriers were released in a fluctuating state. The N appeared to be enriched during the decomposition process.
- There was obvious migration of C, N, P, and K between the S. psammophila sand barriers and the soil, and the decomposition of the S. psammophila sand barriers affected the change of soil C, N, P, and K contents.
ACKNOWLEDGMENTS
This research was supported by the National Key Research and Development Program of China: (Grant No.2018YFC0507101), “Research and development, screening, application technology and effect of new materials fixed dune of different climates in an arid area.” and the National Natural Science Foundation of China (Grant No. 41861044), “The enhancement mechanism of the atmospheric-sand dynamic process on Salix sand barrier resistance.”
REFERENCES CITED
Aerts, R., and de Caluwe, H. (1997). “Nutritional and plant-mediated controls on leaf litter decomposition of Carex species,” Ecology 78(1), 244-260. DOI:
10.1890/0012-9658(1997)078[0244:NAPMCO]2.0.CO;2
Allington, G. R. H., and Valone, T. J. (2010). “Reversal of desertification: The role of physical and chemical soil properties,” Journal of Arid Environments 74(8), 973-977. DOI: 10.1016/j.jaridenv.2009.12.005
Burton, A. J., Pregitzer, K. S., and Hendrick, R. L. (2000). “Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests,” Oecologia 125(3), 389-399. DOI: 10.1007 /s004420000455
Chen, H., Harmon, M. E., and Griffiths, R. P. (2001). “Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: A chronosequence approach,” Canadian Journal of Forest Research 31(2), 246-260. DOI: 10.1139/x00-167
Gao, Y., and Gong, P. (2013). Salix Sand Barrier, Beijing Science Press, Beijing, China.
Gao, Y., and Yi, Y. (1996). “Studies on suitable vegetational cover rate for Salix psammophila forest,” Inner Mongolia Forestry Science & Technology 3(Z1), 38-42.
Goebel, M., Hobbie, S. E., Bulaj, B., Zadworny, M., Archibald, D. D., Oleksyn, J., Reich, P. B., and Eissenstat, D. M. (2011). “Decomposition of the finest root branching orders: Linking belowground dynamics to fine‐root function and structure,” Ecological Monographs 81(1), 89-102. DOI: 10.1890/09‐2390.1
Gong, P. (2012). The Corrosion Processes of Salix Sand-barrier and the Effect of Anticorrosion, Ph.D. Dissertation, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia.
He, L.-X., Jia, Z.-Q., Li, Q.-X., Feng, L.-L., and Yang, K.-Y. (2019). “Fine-root decomposition characteristics of four typical shrubs in sandy areas of an arid and semiarid alpine region in western China,” Ecology and Evolution 9(9), 5407-5419. DOI: 10.1002/ece3.5133
Hobbie, S. E., Oleksyn, J., Eissenstat, D. M., and Reich, P. B. (2010). “Fine root decomposition rates do not mirror those of leaf litter among temperate tree species,” Oecologia, 162(2), 505–513. DOI: 10.1007/s00442-009-1479-6
Hu, N., Ma, Z., Lan. J., Wu, Y., Fu, W., Yuan, H., and Lou, L. (2016). “Leaf litter decomposition characters and impact on soil organic carbon/nitrogen in different vegetation restorations of karst rocky desertification: An example of the Zhongliang mountain in Chongqing,” Carsologica Sinica 35(5), 539-549. DOI: 10.11932/karst20160510
Lin, C., Yang, Y., Guo, J., Chen, G., and Xie, J. (2011). “Fine root decomposition of evergreen broadleaved and coniferous tree species in mid‐subtropical China: Dynamics of dry mass, nutrient and organic fractions,” Plant and Soil 338(1), 311-327. DOI: 10.1007/s11104-010-0547-3
Li, Y., Wang, L. Q., and Zhang, R. L. (2008). “Nutrient release from decomposition of submerged macrophytes of Lake Dianshan,” Environmental Pollution & Control 30(2), 45-48, 52. DOI: 10.3969/j.issn.1001-3865.2008.02.013
Manzoni, S., Jackson, R. B., Trofymow, J. A., and Porporato, A. (2008). “The global stoichiometry of litter nitrogen mineralization,” Science 321(5889), 684-686. DOI: 10.1126/science.1159792
McFee, W. W., and Stone, E. L. (1966). “The persistence of decaying wood in the humus layers of northern forests,” Soil Science Society of America Journal 30(4), 513-516. DOI: 10.2136/sssaj1966.03615995003000040032x
Na, Q. (2010). Study on Salix Psammophila Checkerboard Corrosion Rule and Soil Corrosive Factors, Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia.
Parton, W., Silver, W. L., Burke, I. C., Grassens, L., Harmon, M. E., Currie, W. S., King, J. Y., Adair, E. C., Brandt, L. A., and Hart, S. C., et al. (2007). “Global‐scale similarities in nitrogen release patterns during long‐term decomposition,” Science 315(5810), 361-364. DOI: 10.1126/science.1134853\
Peng, S. L., and Liu, Q. (2002). “The dynamics of forest litter and its responses to global warming,” Acta Ecologica Sinica 22(9), 1534-1544. DOI: 10.3321/j.issn:1000-0933.2002.09.024
Reich, P. B., and Oleksyn, J. (2004). “Global patterns of plant leaf N and P in relation to temperature and latitude,” Proceedings of the National Academy of Sciences 101(30), 11001-11006. DOI: 10.1073/pnas.0403588101
Tu, D., Su, X., Zhang, T., Fan, W., and Zhou, Q. (2014). “Thermo‐mechanical densification of Populus tomentosa var. tomentosa with low moisture content,” BioResources 9(3), 3846-3856. DOI: 10.15376/biores.9.3.3846-3856
Tu, L.-H., Peng, Y., Chen, G., Hu, H.-L., Xiao, Y.-L., Hu, T.-X., and Tang, Y. (2015). “Direct and indirect effects of nitrogen additions on fine root decomposition in a subtropical bamboo forest,” Plant and Soil 389(1), 273-288. DOI: 10.1007/s11104-014-2353-9
Vogt, K. A., Grier, C. C., and Vogt, D. J. (1986). “Production, turnover, and nutrient dynamics of above‐and belowground detritus of world forests,” Advances in Ecological Research 15, 303-377. DOI: 10.1016/S0065-2504(08)60122-1
Wang, R. D., Dang, X. H., Gao, Y., Yang, X., Liang, Y. M., Qi, S., and Zhao, C. (2020). “Alternated desorption-absorption accelerated aging of Salix psammophila sand barrier,” BioResources 15(3), 6696-6713. DOI: 10.15376/biores.15.3.6696-6713
Wang, R. D., Gao, Y., Dang, X. H., Yang, X., Liang, Y. M., and Zhao, C. (2021). “Microstructure and biodegradation of long-established Salix psammophila sand barriers on sand dunes,” Environmental Technology & Innovation 21(2), 101366. DOI: 10.1016/j.eti.2021.101366
Wang, R.D, Dang, X., Gao, Y., Yang, X., Zhang, C., and Li, W. (2019). “Damage law and vegetation restoration of Salix psammophila sand barrier in Mu Us,” Journal of Southwest Forestry University 39(3), 71-77. DOI: 10.11929/j.swfu.201812066
Wang, X.-Y., Ding, G.-D., Gao, H., Zhang, J.-H., Hu, J.-R., and Zhang, Y.-R. (2008). “Effect of zonal willow Salix psammophila checkerboard on reducing wind and stabilizing sand,” Journal of Soil and Water Conservation 22(2), 42-46. DOI: 10.3321/j.issn:1009-2242.2008.02.010
Wu, H., Lv, X., Yang, Q., Jiang, M., and Tong, S. (2007). “Early-stage litter decom position and its influencing factors in the wetland of the Sanjiang Plain, China,” Acta Ecologica Sinica 27(10), 4027-4035. DOI: 10.1016/S1872-2032(07)60088-2
Xu, W., Liu, J., Liu, X., Li, K., Zhang, D., and Yan, J. (2013). “Fine root production, turnover, and decomposition in a fast‐growth Eucalyptus urophylla plantation in southern China,” Journal of Soils and Sediments 13(7), 1150-1160. DOI: 10.1007/s11368-013-0718-y
Yang, J. S., Liu, J. S., Yu, J. B., Wang, J. D., and Zhao, W. (2006). “Dynamics of major elements in Deyeuxia angustifolia litter during its decomposition in Sanjiang Plain,” Chinese Journal of Ecology 25(6), 597-602.
Yang, Y., Guo, J., and Zheng, Y.. (2004). “Comparative study on litter decomposition and nutrient dynamics between plantations of Fokienia hodginsii and Cunninghamia lanceolata,” Scientia Silvae Sinicae 40(3), 19-25.
Yu, Y., Jia, Z. Q., Zhu, Y. J., Liu, Y. S., Liu, H. T., and Li, Q. X. (2015). “Changes of carbon pools of alpine sandy Salix cheilophila shelterbelts with stand age,” Acta Ecologica Sinica 35(6), 1752-1760. DOI: 10.5846/stxb201305271202
Zhang, S., Ding, G.-D., Yu, M.-H., Gao, G.-L., Zhao, Y.-Y., Wang, L., and Wang, Y.-Z. (2019). “Application of boundary layer displacement thickness in wind erosion protection evaluation: Case study of a Salix psammophila sand barrier,” International Journal of Environmental Research and Public Health 16(4), 592. DOI: 10.3390/ijerph16040592
Zhao, G. P., Zuo, H. J., Xu, L. X., Hu, C. Y., and Fu, Y. R. (2008). “Effect of Salix deserts barrier on reducing wind and stabilizing sand,” Journal of Soil and Water Conservation 22(2), 38-41, 65. DOI: 10.13870/j.cnki.stbcxb.2008.02.002
Article submitted: March 26, 2021; Peer review completed: June 23, 2021; Revised version received and accepted: July 4, 2021; Published: July 13, 2021.
DOI: 10.15376/biores.16.3.5947-5963
