Abstract
The effect of degumming pretreatment on whole cotton stalk alkaline peroxide mechanical pulp (APMP) was researched. Degumming pretreatment was used as the first stage of an APMP pulping process, replacing conventional hot water pretreatment. Two degumming agents of sodium hydroxide (NaOH) and sodium oxalate (Na2C2O4) were researched separately. The efficiency of hot water pretreatment, NaOH pretreatment, and Na2C2O4 pretreatment on pectin and metal ions removal was compared. After pretreatment of hot water, NaOH, and Na2C2O4, pectin content was reduced to 4.0%, 2.1%, 1.6%, respectively, compared to original material (4.3%), at removal rates of 7%, 51%, and 64%, respectively. For metal ions, especially transition metal ions, the removal rate was up to 20% after degumming pretreatment. The brightness of the handsheets was 64% ISO, 68% ISO, and 73% ISO, respectively. The dirt count was 2674 mm2·m-2, 533 mm2·m-2, and 132 mm2·m-2, respectively. After Na2C2O4 pretreatment, the tension index and tear index were increased to 40.5 N·m·g-1 and 4.5 mN·m2·g-1, respectively. Through degumming pretreatment, pectin, metal ions, and dirt count were reduced efficiently, and the brightness and physical strength were improved significantly.
Download PDF
Full Article
Degumming Pretreatment with Sodium Hydroxide and Sodium Oxalate in the Process of Whole Cotton Stalk APMP Pulping
Yu-Meng Zhao, Zhong Liu, Lan-Feng Hui,* Qun Li, Xin-Shou Li, and Ying-Jian Huang
The effect of degumming pretreatment on whole cotton stalk alkaline peroxide mechanical pulp (APMP) was researched. Degumming pretreatment was used as the first stage of an APMP pulping process, replacing conventional hot water pretreatment. Two degumming agents of sodium hydroxide (NaOH) and sodium oxalate (Na2C2O4) were researched separately. The efficiency of hot water pretreatment, NaOH pretreatment, and Na2C2O4 pretreatment on pectin and metal ions removal was compared. After pretreatment of hot water, NaOH, and Na2C2O4, pectin content was reduced to 4.0%, 2.1%, 1.6%, respectively, compared to original material (4.3%), at removal rates of 7%, 51%, and 64%, respectively. For metal ions, especially transition metal ions, the removal rate was up to 20% after degumming pretreatment. The brightness of the handsheets was 64% ISO, 68% ISO, and 73% ISO, respectively. The dirt count was 2674 mm2·m-2, 533 mm2·m-2, and 132 mm2·m-2, respectively. After Na2C2O4 pretreatment, the tension index and tear index were increased to 40.5 N·m·g-1 and 4.5 mN·m2·g-1, respectively. Through degumming pretreatment, pectin, metal ions, and dirt count were reduced efficiently, and the brightness and physical strength were improved significantly.
Keywords: Degumming; Whole cotton stalk; APMP
Contact information: Tianjin Key Lab of Pulp and Paper, Tianjin University of Science and Technology, Tianjin 300457, China; *Corresponding author: huipeak@163.com
INTRODUCTION
With the development of the papermaking industry in China, the deficiency of raw material has become a big problem (China Paper Association 2013). China lacks forest resources but is abundant with non-wood material, so research in recent years has focused on the exploitation and development of new technologies based on non-wood material.
Cotton stalk is a very good material for papermaking. It possesses bast fibers in the bark section and wood fibers in the core section. The bark fiber is long and strong, and the wood fiber is similar to hardwood. If whole cotton stalk is used as raw material for papermaking, it could produce high-quality pulp with excellent strength. Since the 1950’s, cotton stalk has been used for papermaking. Chemical pulping methods were mostly applied at that time. With more and more concern on environment protection and climate change, there are stricter emission limits. The research direction of cotton stalk has been transferred to re-mechanical pulping with a slight chemical treatment. Unfortunately, the high-content pectin in black bark (>8%) has a direct effect on brightness and dirt count of whole cotton stalk chemi-mechanical pulp. This is the main cause of the delayed development of whole cotton stalk pulping. Removing pectin in order to reduce the influence on brightness and dirt count is the key to development of whole cotton stalk chemi-mechanical pulping.
Degumming is widely used in the textile, food, and biofuel industry. In the textile industry it is a very important procedure to remove gel substance such as pectin, lignin, hemicelluloses, and water-soluble matter by chemical, physical, or biological methods. It aims to extract fibers from bast material (Quan 2013). The main methods include chemical degumming, biological degumming, and biochemical degumming. Among these methods, chemical degumming is widely used (Brühlmann et al. 2000; Hartzell and Hsieh 1998; Lenting and Zwier 2002). Sodium hydroxide (NaOH) is the most popular agent used for chemical degumming. During the process of alkali boiling for cotton stalk bark, the normal conditions are: temperature 80 C to 100 C, time 30 to 60 min, NaOH 8 to 14 g/L, and bath ratio 1:100 (Liu et al. 2009; Zhang et al. 2010). Sodium oxalate (Na2C2O4) is normally used as an aid to improve the dissolubility of degraded pectin substance (Brünhlmann et al. 2000; Pérez-Rigueiro et al. 2002; Wang et al. 2005; Fraeye et al. 2007). In the food and biofuel industries, degumming is mainly used in oil refinery. It aims to remove phospholipids. The main method is acid degumming.
In the paper industry pectin has not been given much attention except for its negative effect on pulp. With the development of cotton stalk pulping, the effect of pectin on the brightness of cotton stalk starts to be of concern. In 2007, degumming technology was induced in the pulping industry for the first time (Tian et al. 2007). With the condition of 90 C, 40 min, 1.5% usage of NaOH or Na2C2O4 (on the basis of oven dried material), and liquor-to-material 3:1, the brightness of whole cotton stalk bleached chemi-thermal mechanical pulp (BCTMP) was increased to 61% ISO (NaOH degumming) and 64.5% ISO (Na2C2O4 degumming). This opens a new thought for the development of whole cotton stalk chemi-mechanical pulping. On this basis, Yang et al. (2012) further compared the degumming reaction rate of different agents. It was shown that the reaction rate between Na2C2O4 and calcium pectinate was the fastest. Under the conditions of 90 C, 30 min, 3% Na2C2O4, and liquor-to-material 4:1, the brightness of whole cotton stalk chemi-mechanical pulp reached 76% ISO.
In this work, the effect of degumming pretreatment on whole cotton stalk alkaline peroxide mechanical pulp (APMP) was researched. The effect of degumming pretreatment on the change of metal ions, dirt count, and physical strength was further researched except on the change of pectin and brightness. At the same time, a detailed and deep analysis was made on every change. Referring to the previous work, NaOH and Na2C2O4 were used as degumming agents. The degumming efficiency of NaOH and Na2C2O4 were compared. The conditions of 90 C, 40 min, 3% NaOH or Na2C2O4 (on the basis of oven dried material), and liquor-to-material 4:1 were used.
EXPERIMENTAL
Materials
The dried cotton stalk was obtained from a paper mill in Tianjin, China. Before the experiment it was prepared by sorting, cutting, washing, and air drying. The prepared whole cotton stalk morphology can be seen from Fig. 1.
Methods
Whole cotton stalk composition
The chemical composition of whole cotton stalk was determined according to China national standard test methods (Shi and He 2009).
Fig. 1. (a) Prepared whole cotton stalk; (b) Bark section; (c) Core section
Removal of pectin
The experiment was performed in a 15 L rotary digester (Santasalo-Sohlberg Corp., Finland). For hot water pretreatment, only water was added and mixed with the material. The liquor-to-material ratio was 4:1. For degumming pretreatment, a 3% charge (on the basis of oven dried material) of alkali (NaOH) or sodium oxalate (Na2C2O4) was added. The liquid-to-material ratio was 4:1. The temperature was raised to 90 °C and held there for 40 min. After the treated whole cotton stalk was taken out, it was cleaned directly by distilled deionised water, air dried indoors, and finally ground into powder (P40/R60) for pectin testing. Pectin content was determined according to GB/T 10742 2008.
Desorption of metal ions
Three 10 g portions of ground whole cotton stalk powder (P40/R60) were weighed and placed separately into three small flasks. Water, 3% charge (on the basis of oven dried material) of NaOH, and 3% charge (on the basis of oven dried material) of Na2C2O4 were separately added into the three flasks. The liquor-to-material ratio was 4:1. After full mixture had been achieved, the flasks were immersed in a 90 °C water bath for 40 min. After that, the charged powder was taken out of the flask, cleaned with distilled deionised water, then air dried indoors in preparation for the metal ions test. The metal ions were tested by flame atomic absorption spectrometry (FAAS; Shimadzu Co., Ltd., Japan). Copper and iron contents were tested according to GB/T 8943.1-1988; manganese ions was according to the standard method of GB/T 8943.3-1988; and calcium and magnesium ions were tested according to GB/T 8943.4-1988.
APMP pulping process and technology conditions
A flowchart and technical conditions of whole cotton stalk APMP pulping are described in Fig. 2 and Table 1, respectively. At the beginning of APMP pulping, hot water, NaOH, and Na2C2O4 pretreatment were separately applied.
Hot water pretreatment, degumming pretreatment, and two stages of chemical treatment were all carried out in a rotary digester (Santasalo-Sohlberg Corp., Finland), while screw extruding was done in a single-screw extruder (Anqiuwenrui Machinery Manufacturing Co., Ltd., China). Grinding was carried out in a high consistency refiner (Kumagai Riki Kogyo Co., Ltd., Japan). Screening was performed with a flat vibrating screen (Lorentzen & Wettre, Sweden). Hydrogen peroxide supplementary bleaching was done in a thermostated water bath (Beijing Guangming Instrument Co., China). The detailed technology conditions are indicated in Table 1.
Degumming pretreatment
As Fig. 2 shows, at the beginning of whole cotton stalk APMP pulping, degumming pretreatment was applied, replacing hot water soaking. Alkaline and sodium oxalate as two kinds of degumming agents were used separately. The conditions of degumming pretreatment were: NaOH/Na2C2O4 3% (on the basis of oven dried material) at 90 °C, for 40 min.
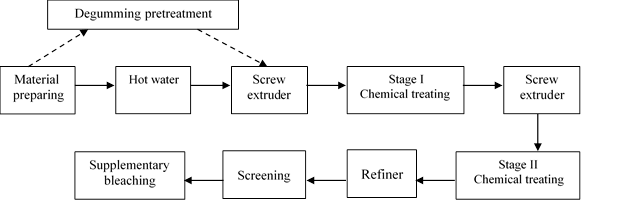
Fig. 2. Flow chart of whole cotton stalk APMP pulping
Table 1. Technical Conditions of Whole Cotton Stalk APMP Pulping
Brightness test
The whole cotton stalk APMP pulp with different pretreatment methods was made into handsheets according to TAPPI T205 sp-06 (2006) for brightness testing. Brightness was determined according to ISO 2470 (1999) using an ELREPHO device (Lorentzen & Wettre, Sweden).
Dirt count test
Such handsheets as mentioned above were also used for the test of dirt count. Dirt count was determined according to TAPPI T437 om-03 (2008) using Spec & Scan 2000 software (Apogee Systems, Inc., USA) and a Epson Perfection V500 Photo Scanner (Epson (China) Co., Ltd., China). The detailed parameters for dirt count test were as indicated in Table 2.
Table 2. Parameters Set for Dirt Count Test
Physical properties of whole cotton stalk pulp
Before physical properties testing, the whole cotton stalk APMP pulp was beaten to 40 ºSR using a PFI beater (Lorentzen & Wettre, Sweden), according to China national light industry standard QB/T 1463-2010. Then, it was made into handsheets according to TAPPI T205 sp-06 (2006). The bulk density, tear index, and tensile index all were tested according to TAPPI T220 sp-06 (2006). Brightness and opacity were determined according to ISO 2470 (1999) using an ELREPHO device (Lorentzen & Wettre, Sweden).
RESULTS AND DISCUSSION
Whole Cotton Stalk Composition
As shown in Table 3, the main chemical components of whole cotton stalk were holocellulose (76.2%), Klason lignin (26.6%), 1% NaOH extractives (25.9%), pectin (4.3%), and ash (3.6%).
Table 3. Chemical Composition of Whole Cotton Stalk (%)
Table 4 compares the composition of whole cotton stalk and other material including wood (aspen and Masson pine) and non-wood (wheat straw and reed). The composition data of wood and non-wood was from Li (2007). For whole cotton stalk, the pectin content (4.3%) was far higher than other materials. It was twice more than wood (1.8% of aspen) and ten times more than non-wood (0.3% of wheat straw) material. The ash content was lower than non-wood (6.0% of wheat straw) but far higher than wood material (0.3%), and almost ten times more than wood. The other composition such as 1% NaOH extractives, lignin, and holocellulose was more similar with wood materials.
From the comparison, it was concluded that whole cotton stalk was a good material for papermaking except for the high content of pectin and ash. So, in order to make good- quality cotton stalk pulp, it is mandatory to get rid of pectin and metal ions.
Table 4. Chemical Composition of Whole Cotton Stalk in Comparison with Other Materials
The pectin content in the different sections of cotton stalk was further analyzed, and results are shown in Table 5. The bark section had the highest pectin content (8.4%), which was almost double compared to the whole cotton stalk (4.3%) and threefold of the core section (2.5%). Thus, more than 80% of pectin was accumulated in the bark. Pectin is a key factor directly affecting brightness and dirt count of the pulp (Yang et al. 2012). According to this, usage of whole cotton stalk for papermaking requires pretreatment of the bark.
Table 6 shows the metal ions content analysis. In cotton stalk, the calcium ions content (13,100 mg/kg) was the highest, followed by magnesium ions (2300 mg/kg). The bark section contained the most metal ions, twice that of the core section. More than 70% of metal ions are contained in the bark.
The adverse effects of metal ions on pulp quality are mainly reflected in three aspects. First, some metal ions were colored themselves, which could affect the brightness of the pulp directly. Second, they could catalyze the unproductive decomposition of the bleaching agent during the process of hydrogen peroxide or oxygen bleaching to decrease bleaching efficiency (Liden and Ohman 1998; Ni et al. 2000). Third, they could form secondary chromophoric groups through binding with lignin to lower the brightness of pulp.
Table 5. Pectin Content from Different Parts of Cotton Stalk (%)
Table 6. Analysis of Metal Ions of Whole Cotton Stalk (mg/kg)
It follows that, if whole cotton stalk is to be employed, a way to remove both pectin and metal ions should be investigated to reduce the effect on the brightness and dirt counts of pulp.
Removal of Pectin
Degumming pretreatment was able to significantly decrease the pectin content of whole cotton stalk APMP pulp (Fig. 3); Na2C2O4 was more effective than NaOH. After hot water pretreatment, the pectin content changed from 4.3% to 4.0%, a decrease of only 7%. After NaOH and Na2C2O4 degumming treatment, the pectin content was reduced to 2.1% and 1.6%, respectively, at removal rates of 51% and 64%, respectively.
Pectins, which are more formally called methoxyl galacturonic acid or pectic acid, normally are present in nature plants in the form of pectic acid, pectic acid methyl ester, and pectin-metal complexes. Pectic acid is slightly soluble in water, but solubilization takes place very easily following a neutralization reaction with alkaline media. Pectic acid methyl ester is soluble in water, and a hydrolysis reaction proceeds very easily in an alkaline environment. Pectin-metal complexes are insoluble in water, but under the condition of strong alkalinity, the molecule chain, having an initial weight-average molecular weight (Mw) of more than 200,000g/mol, can be broken down and become small molecules (Mw of less than 20,000g/mol) to be dissolved out. So, after the effect of degumming pretreatment, the pectin content was decreased significantly.
The basic composite unit of pectin molecules backbone is D-pyran galacturonic acid. The carboxyl group (–COOH) on pectin unit C-6 ring is mostly in the form of methyl esterificated ( ), in which the free carboxyls connect with metal ions such as Ca2+, Mg2+ Mn2+ to form a structure of “
”. Under such effect of “bridge bonding” of metal ions, pectin molecules connect each other to form a gum substance having a web structure.
The degumming process could be considered to be a process of ion exchange of Na+ and other metal ions. Because calcium ions were the most prevalent ions in cotton stalk, it was taken as an example to indicate the exchanging process. Equation (1) and (2) show the reaction between calcium pectinate and degumming agents. Under alkaline environment, the stability of calcium pectinate decreased, and then the structure of “ ”started to loosen up. Finally, breakage of the Ca-O bond occurred, and at the same time, Na+replaced Ca2+. Therefore calcium pectinate gum macromolecule was degraded to small molecules. The freed calcium ions then can connect with free hydroxyl (-OH–) or oxalate (-C2O42-) to form precipitates of calcium hydroxide or calcium oxalate. Because calcium oxalate (CaC2O4) is more stable than calcium hydroxide (Ca(OH)2), the degumming reaction between sodium oxalate and calcium pectinate could proceed to the positive direction more easily than in the case of NaOH. That is, the degumming effects of Na2C2O4 were better than NaOH.
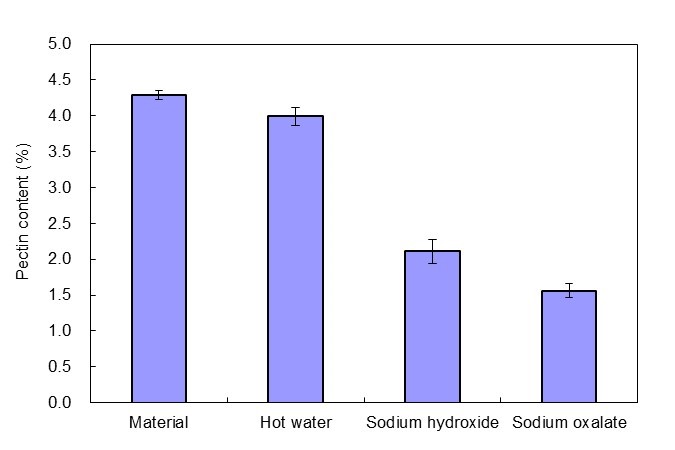
Fig. 3. Pectin content after the pretreatment of hot water, sodium hydroxide, and sodium oxalate
Desorption of Metal Ions
It was found that by using debarked cotton stalk and whole cotton stalk for papermaking, the brightness of the former was almost 10% ISO higher than the latter (Tang et al. 2005). The reason for this was thought to be related to the high content of metal ions in the bark. During the process of hydrogen peroxide bleaching, an excess of metal ions, especially transition metal ions, could catalyze the decomposition of hydrogen peroxide (H2O2); thereby, the bleaching efficiency of H2O2 was weakened, and the improvement of pulp brightness was hindered.
Based on this argument, the change in metal ion content after degumming treatment was tested. As Table 7 shows, all metal ion contents decreased after the degumming treatment. Sodium hydroxide and Na2C2O4 had different efficiencies for the removal of different metal ions. For the removal of Ca2+, Mg2+, and Mn2+, Na2C2O4 was more effective than NaOH, while for the removal of Fe2+/Fe3+ and Cu2+, NaOH was more effective than Na2C2O4. For transition metal ions, the removal rate was up to 20%.
As mentioned above, the metal ions normally exist in cotton stalk in the form of a gel substance of “pectin-metal-pectin” via connections with pectin. Under alkaline condition, metal ions could exchange with sodium ions and remove out from the gel of “pectin-metal-pectin”. The exchanging rate and removal rate are determined by the stabilization of the precipitate formed by metal ions and -OH– or – C2O42-. So, for different metal ions, the removal rate was different.
Table 7. Change in Metal Ions Content after Degumming Treatment (mg/kg)
Change in Brightness of Whole Cotton Stalk APMP Pulp
Applying alkaline or sodium oxalate degumming pretreatment caused the brightness of whole cotton stalk APMP pulp to increase significantly (Fig. 4). After hot water soaking, the brightness was 58.4% ISO, but while using alkaline and sodium oxalate degumming pretreatment, the brightness was 61.9% ISO and 66.7% ISO, respectively. After further bleaching with hydrogen peroxide, the brightness increased to 64.0% ISO (hot water), 67.9% ISO (NaOH), and 73.3% ISO (oxalate), respectively.
For brightness promotion, sodium oxalate’s effect was better than that of the alkaline. After H2O2 supplementary bleaching, the brightness of sodium oxalate degumming pretreatment increased almost 10% ISO compared to hot water pretreatment.
There were three main reasons of brightness promotion. First, pectin has a deep brown color, and it could therefore influence the pulp brightness directly. After degumming pretreatment, more than 50% of the pectin was removed (Fig. 3), and the direct effect of pectin on the brightness was alleviated. Second, after degumming pretreatment, transition metal ions were partly removed (Table 5). The unfavourable decomposition of hydrogen peroxide was reduced and hence bleaching efficiency was improved. Third, some metal ions themselves were colored and were removed by the degumming pretreatment, hence their impact on the brightness was lowered.
Fig. 4. The brightness change of whole cotton stalk APMP pulp after degumming pretreatment
Change of Dirt Count of Whole Cotton Stalk APMP Pulp
The analysis on the dirt count of whole cotton stalk APMP pulp was carried out at the same time. Dirt is any foreign matter embedded in or on the surface of a sheet, having a contrasting color to the rest of the sheet. As Fig. 5 shows, the dirt count of whole cotton stalk APMP pulp decreased significantly after NaOH and Na2C2O4 degumming pretreatments; Na2C2O4 was more effective than NaOH. With hot water pretreatment during the process of whole cotton stalk APMP pulping, the dirt count was 2674 mm2·m-2, while when NaOH and Na2C2O4 were applied, the dirt count was reduced to 533 mm2·m-2 and 132 mm2·m-2, respectively, the removal rate was 80% and 95%, respectively.
Fig. 5. Change in dirt count after sodium hydroxide and sodium oxalate degumming pretreatment
The dirt of whole cotton stalk pulp was mainly from two origins: pectin and black bark. Pectin is a deep brown gum substance, mainly existing in the middle lamella; its roles are to bond fibers and to transport nutrition to the plant (Luzio and Cameron 2008; Ngouémazong et al. 2011). During the process of APMP pulping of whole cotton stalk, pectin would normally be present in the pulp in the form of dirt. Through degumming pretreatment, more than 50% of pectin was removed, so the dirt counts induced by pectin were greatly reduced. At the same time, the removal of pectin made the bonding between fibers less tight; in the subsequent chemical treating process, the penetration of the various chemical agents became easier, so that NaOH and H2O2 could participate in the removal of lignin and pectin and bleaching reaction more efficiently. With the brightness improvement, the dirt count will be reduced. As a result, the dirt induced by black bark was also reduced.
Physical Property Analysis of Degummed Whole Cotton Stalk APMP Pulp
Table 8 compares the properties of whole cotton stalk APMP pulp with and without degumming pretreatment. After Na2C2O4 pretreatment, the physical strength of the pulp was improved significantly. The tensile index increased from 23.40 N·m·g-1 (hot water pretreatment) to 40.54 N·m·g-1 (degumming pretreatment), and the tear index improved from 3.95 mN·m2·g-1 to 4.52 mN·m2·g-1. In contrast, the bulk and opacity decreased to different degrees: bulk decreased from 2.96 cm3·g-1 (hot water pretreatment) to 2.10 cm3·g-1 (degumming pretreatment) and opacity from 93.4% to 84.3%.
As mentioned above, the removal of pectin made cell bonding less tight, and penetration into the inner cell wall became easier for NaOH and H2O2. On the one hand, NaOH could promote the effect of hydration and swelling of fibers, which could make fibers softer and the cohesion between fibers decrease; on the other hand, NaOH could dissolve partial hemicelluloses. This causes gaps to appear between the cell wall and middle lamella. Such a gap opens a passageway for water molecules to have access. Thereby, the hydration effect of fibers is further promoted. In this situation, the fibers could be separated more easily when ground. Also, the fiber length could be maintained to a high extent, ensuring physical strength.
The reduction of bulk and opacity occurred mainly because the hydration and swelling of fibers allowed for better defiberation during grinding. The fiber bonds were tighter; thus, the bulk density was reduced. There was a positive relationship between bulk and opacity (the higher bulk, the higher opacity). So, the opacity also was reduced.
Bulk is normally associated with the bonding properties of fibers, meaning that the more combined the compact fiber, the lower the bulk and opacity. As mentioned above, hydration and swelling of fibers were increased after degumming pretreatment. As fibers were ground, they could be de-fibered very well so that the bonding of fibers was strengthened. As a result, the bulk and opacity were reduced.
Table 8. Properties of Whole Cotton Stalk APMP Pulp after Degumming Pretreatment
CONCLUSIONS
- Degumming pretreatment removed pectin and metal ions efficiently. After pretreatment with NaOH or sodium oxalate, the removal extent of pectin reached 51% and 64%, respectively. Na2C2O4 was more efficient than NaOH. The transition metal ions were reduced more than 20%
- Degumming pretreatment greatly improved the brightness of whole cotton stalk pulp and reduced the dirt count efficiently. Na2C2O4 performed better than NaOH. After Na2C2O4degumming treatment, the brightness was increased to 73.3% ISO (after H2O2 supplementary bleaching), which was almost 10% ISO higher than that treated by hot water. The dirt count was decreased from 2674 mm2·m-2 (hot water) to 132 mm2·m-2 (removal extent 95%).
- The physical strength of degummed whole cotton stalk APMP pulp was greatly improved by pretreatment. Tensile index was increased from 23.40 N·m·g-1 (hot water pretreatment) to 40.54 N·m·g-1, and tear index from 3.95 mN·m2·g-1 to 4.52 mN·m2·g-1. The bulk and opacity were decreased.
- Based on the results of this study, degumming pretreatment is a very effective way to improve the whole quality of whole cotton stalk APMP pulp.
ACKNOWLEDGMENTS
The financial support for this project by the National Nature Science Foundation of China (NSFC, No. 31000283, 21076160 and 31270631) and Tianjin Municipal Science and Technology Commission (Grant No. 12ZCZDGX01100) are gratefully acknowledged.
REFERENCES CITED
Brühlmann, F., Leupin, M., Erismann, K. H., and Fiechter, A. (2000). “Enzymatic degumming of ramie bast fibers,” Journal of Biotechnology 76(1), 43-50. DOI: 10.1016/S0168-1656(99)00175-3
China Paper Association (2013). “2013 Annual Report of China Paper Industry”
Fraeye, I., De-Roeck, A., Duvetter, T., Verlent, I., Hendrickx, M., and Van-Loey, A. (2007). “Influence of pectin properties and processing conditions on thermal pectin degradation,” Food Chemistry 105(2), 555-563. DOI: 10.1016/j.foodchem.2007.04.009
Hartzell, M. M., and Hsieh, Y. L. (1998). “Enzymatic scouring to improve cotton fabric wettability,” Textile Research 68(4), 233-241. DOI: 10.1177/004051759806800401
Lenting, H. B. M., and Zwier, E. (2002). “Identifying important parameters for a continuous bioscouring process,” Textile Research 72(9), 825-831. DOI: 10.1177/004051750207200912
Li, Q. (2007). “The characteristic and chemimechanical pulping technology development of cotton stalk,” Tianjin Paper (2), 8-14.
Lidén, J., and Ohman, L. O. (1998). “On the prevention of Fe- and Mn-catalyzed H2O2 decomposition under bleaching conditions,” Journal of Pulp & Paper Science 24(9), 269-276.
Liu, L., Li, L. and Li, Y. (2009). “The research actuality of cotton-straw peel degumming process,” Textile Science and Technology Development (6), 1-2.
Luzio, G. A., and Cameron, R. G. (2008). “Demethylation of a model homogalacturonan with the salt-independent pectin methylesterase from citrus: Part II. Structure-function analysis,” Carbohydrate Polymers 71(2), 300-309. DOI: 10.1016/j.carbpol.2007.05.038
Ngouémazong, D. E., Tengweh, F. F., Duvetter, T., Fraeye, I., Loey, A. V., Moldenaers, P., and Hendrickx, M. (2011). “Quantifying structural characteristics of partially de-esterified pectins,” Food Hydrocolloids 25(3), 434-443. DOI: 10.1016/j.foodhyd.2010.07.015
Ni, Y., Ju, Y., and Ohi, H. (2000). “Further understanding of the manganese-induced decomposition of hydrogen peroxide,” Journal of Pulp and Paper Science 26(3), 90-94.
Pérez-Rigueiro, J., Elices, M., Llorca, J., and Viney, C. (2002). “Effect of degumming on the tensile properties of silkworm (Bombyx mori) silk fiber,” Journal of Applied Polymer Science 84(7), 1431-1437. DOI: 10.1002/app.10366
Quan, Q. Y. (2013).“Hemp degumming mechanism and optimization methods,” China Fiber Test (1), 87-88.
Shi, S. L., and He, F. W. (2009). “Analysis and testing of pulp and papermaking,” China Light Industry Press, Beijing.
Tang, Y. J., Liu, B. Y., Li, Y. M., and Wang, L. P. (2005). “APMP pulping performance of four kinds of non-wood resource,” Transactions of China Pulp and Paper 20(1), 28-32.
Wang, P., Wang, Q., and Lin, G. (2005). “Research on the pectin removal effect of different chelating agents on cotton fabrics,” Textile Auxiliaries 22(5), 33-35.
Yang, H. L., Li, Q., Niu, L. P., and Zhang, Y. J. (2012). “Improving the bleachability of whole cotton stalk chemimechanical pulp with depectinization agents,” BioResources 7(3), 4171-4178.
Zhang, S. J., Sun, J. L., and Li, L. (2010). “Comparative studies on degumming of cotton bast fiber,” Journal of Cellulose Science and Technology 18(3), 34-38.
Article submitted: December 8, 2014; Peer review completed: March 1, 2015; Revised version received and accepted: March 23, 2015; Published: March 30, 2015.
DOI: 10.15376/biores.10.2.2913-2925
