Abstract
Download PDF
Full Article
Designing the Epoxy Adhesive Formulations for Manufacturing Engineered Woods
Utai Meekum,* and Waree Wangkheeree
The design of an epoxy adhesive was investigated by a 2K design of experiment (DOE). All the assigned parameters showed no significant effect for both curing and mechanical properties, except for bisphenol A (BPA), which showed a significantly negative effect on the heat distortion temperature (HDT) of the cured samples. Adding dicyandiamine (DICY) into the hardener retarded cure time and also caused an incomplete curing at room temperature. Curing at 110 °C and 150 °C post curing were the optimal conditions and 20 g of DICY with 50 g of triethylenetetramine (TETA) was optimized. Adduct obtained from aliphatic epoxy (RD108, 14.63 g) and TETA (7.71 g) were selected and employed as hardener ingredients. The incomplete crosslinking reaction was the main reason for the inferior properties at high RD108 loadings. The toughening by blending with polycarbonate (PC) was explored, and 5 phr of PC was selected. Limitation of resin/fiber infusion due to high viscosity was observed. Dilution of the solvents using ethyl acetate (EA) and methyl ethyl ketone (MEK) to reduce viscosity was explored. The mechanical properties of the wood samples manufactured from the EA-diluted epoxy were superior to the MEK dilution. The lower boiling point and good solubility of EA were explained.
Keywords: Epoxy adhesives; Engineered wood; Mechanical and durability properties
Contact information: Institute of Engineering, Suranaree University of Technology, Maung, Nakorn Ratchasima, Thailand; *Corresponding author: umsut@g.sut.ac.th
INTRODUCTION
Typically, formaldehyde-based adhesives, namely phenol-formaldehyde (PF), urea-formaldehyde (UF), and melamine-formaldehyde (MF), have been widely used for manufacturing engineered woods (Kim 2009; Jang et al. 2011; Luo et al. 2016). The main benefits of these woods are that they have decent bonding properties and are inexpensive. The main drawbacks are the low moisture resistance and high content of toxic volatile organic compounds (VOC) and formaldehyde (Jang et al. 2011; Lei et al. 2014). Recently, strict standards and requirements were established to define acceptable formaldehyde emission levels, which has been an enormous concern. The California Air Resources Board (CARB) regulated formaldehyde emissions in 2008, and laws went into effect in 2009 (Lei et al. 2014). Many manufacturers have changed to methylene diphenyl diisocyanate (MDI) and epoxy resin to bio-based adhesives (Ormondroyd 2015). Epoxy resin is known to be a high performance adhesive in the advanced engineering composite industries. It can also be used as an adhesive for the manufacturing of high performance engineered woods.
Many research studies have investigated the influence of epoxy-based adhesives on wood products. Osmannezhad et al. (2014) used epoxy-based adhesives to manufacture specially reinforced glued laminated timbers (glulams) made from poplar (Populus deltoids) and beech (Fagus orientalis) wood to investigate the bending strength of the glulams with glass fiber reinforced plastic (GFRP). The incorporation of GFRP was done using four methods: 1 layer of GFRP under the glulam layers, 2 layers of GFRP under the glulam layers, 2 layers of GFRP between the glulam layers, and 4 layers of GFRP between the glulam layers. The results showed that the highest strength was obtained using epoxy glue, followed by solid wood. Also, the highest strength was obtained using 4 layers of GFRP. The beech wood had a higher strength than the poplar wood (Osmannezhad et al. 2014). Ahmad et al. (2010) investigated the fracture toughness of three room-temperature cured epoxy adhesives formulated for in situ timber bonding. Commercial TimbersetTM wood was used and cured with VESTAMIN® TMD, which is mainly composed of trimethyl hexamethylene diamine. It was found that, under a high impact rate, the toughness was ductile in the unnotched wood and brittle in the notched wood. Therefore, the toughness of the thixotropic adhesives could be enhanced by the inclusion of phase separated liquid rubber in the epoxy matrix (Ahmad et al. 2010). The FRP-wood composite bonded using the epoxy adhesive was manufactured by Raftery et al. (2009). The bonding of the commercially available FRPs to wood using three commercial epoxy adhesives was studied. The findings showed that for specific adhesives, thin bond lines had the capacity to resist severe hygrothermal stresses imposed at the FRP/wood interface. Also, the integrity of the bond depended not only on the epoxy adhesive in question, but also on the FRP type (Raftery et al. 2009). The study focused on composites made from pine wood dust and epoxy resin. An artificial neural network (ANN) was implemented to analyze the wear performance of a new class of epoxy-based composites filled with pine wood dust. The composites were prepared with three different compositions, 0 wt.%, 5 wt.%, and 10 wt.% of pine wood dust reinforced in epoxy resin. Based on a design of experiments (DOE), significant control factors predominantly influencing the wear rate were identified. An ANN concluded that pine wood dust possessed good filler characteristics to improve the sliding wear resistance of the polymeric resin. The factors, filler content, sliding velocity, and normal load, in that order, were the most significant factors that affected the specific wear rate (Kranthi and Satapathy 2010). Wood composites processed and filled with seven types of epoxy adhesives were also studied by Kumar et al. (2014). It was found that the mechanical properties increased up to a certain filler loading, and then they gradually decreased (Kumar et al. 2014).
With respect to the commercial application aspects, the properties of epoxy resin that have well-known drawbacks are the low impact toughness and low deformation at break. There have been research studies performed to improve the properties and curing parameters of epoxy formulations. For example, the use of polyethersulfone (PES) to improve both the heat resistance and toughness of cured biphenyl-type epoxy resins has been studied. The glass transition temperature (Tg) of resin containing 10 wt.% PES increased to 208 °C, and also, the value of the fracture toughness was approximately 60% higher than that of the unmodified resin (Mimura et al. 2000). A study on the enhancement of the interlaminar toughness of carbon/epoxy composites, produced by resin transfer molding (RTM), by modifying the resin with hyperbranched polymers (HBPs) was also published. An HBP content of 7.5% in the resin was selected as the best compromise between a slight loss in stiffness and a gain of 60% in fracture toughness. The fiber pullout tests indicated that there was poor fiber–matrix interface adhesion in the case of the modified epoxy (Verrey et al. 2005). Francis et al. (2006) reported on a diglycidyl ether of bisphenol A epoxy resin blended with hydroxyl terminated poly (ether ketone) based on tert-butyl hydroquinone (PEEKTOH) and cured with a diamine, 4,4’-diaminodiphenyl sulfone. The cured blends exhibited two-phase morphology at all of the curing temperatures. From the dynamic mechanical spectrum, it was evident that there were two Tg values that corresponded to the epoxy-rich and thermoplastic-rich phases (Francis et al. 2006). For the curing agent, two aliphatic amines, linear propanediamine (PDA), and branched N,N,N’,N’-tetra(3-aminopropyl)-1,3-propanediamine (TAPA), were used to cure the diglycidyl ether of bisphenol A (DGEBA). The dynamic mechanical analysis (DMA) showed that the cured DGEBA/TAPA exhibited higher glass- and β-relaxation temperatures and crosslink density than the DGEBA/PDA (Wan et al. 2012). Also, a phenyl glycidyl ether and solid metaphenylene diamine (MPDA) formulation system was studied. The higher Tg of the cured resin was determined to be the main advantage over the unmodified resin (Liu et al. 2013).
This publication is one of the series of the research on the manufacturing of the lightweight sandwich structure using wood foam core. Designing of the epoxy adhesive to serve with the special requirements throughout the production steps, with respect to both timing parameters and mechanical performance, was crucial. In this publication, five improvement aspects of designing epoxy formulations for use as adhesives in engineered wood manufacturing were studied. They were (i) the design of experiment (DOE), (ii) dicyandiamine (DICY) addition, (iii) amine adduct, (iv) PC blended epoxy resin, and (v) solvent dilution. The ultimate goal was to determine the best performance by means of the mechanical properties, especially fracture toughness and durability.
EXPERIMENTAL
Materials
There were three categories of the materials employed in the study: (a) commercially available as base resins, which included bisphenol A type (YD 127), diluted bisphenol A type (YD 515), Novolac type (YDPN 631), and aliphatic type (RD108) epoxy resins, supplied from Aditya Birla Chemicals Ltd. (Bangkok, Thailand); (b) amine chemicals for the hardener formulation including isophorone diamine (IPDA) supplied from BASF (Thai) Ltd. (Bangkok, Thailand) and diamio diphenyl sulphone (DDS) available from Vantico Co. Ltd. (Bangkok, Thailand); and (c) miscellaneous chemicals, such as bisphenol A (BPA) supplied from Bayer Thai Co. Ltd. (Bangkok, Thailand), polycarbonate (PC, Iupillon H-4000) available from Mitsubishi Engineering-Plastics Corporation (Bangkok, Thailand), epoxidized silane (Silquest®A187) purchased from Optimal Tech Co. Ltd. (Bangkok, Thailand), and ethyl acetate (EA), methyl ethyl ketone (MEK) available from Finechem Pty Ltd. (Bangkok, Thailand), and dichloromethane purchased from Optimal Tech. Co. Ltd. (Bangkok, Thailand), which were used for viscosity dilution of the epoxy resins. The fiberglass woven with an area density of 100 g/m2 was kindly supplied from Cobra International Co., Ltd. (Chonburi, Thailand), and the eucalyptus wood pulp fiber and medium-density fiberboard (MDF) sample for termite testing were supplied from Agro Fiber Ltd. (Krabinburee, Thailand). Teak veneer and also teak wood used for termite testing, and Para-rubber wood were locally available (Nakorn Ratchasima, Thailand). All of the materials and chemicals were used as received.
Epoxy Adhesives Formulation
Formulation of resin part
The mixed resin was designed by mixing the commercial base resins, YD 127, YD 515, and YDPN 631, at the given ratios. For the resin blended with PC, 10 phr of the PC corresponded to the amount of the designed base resin mixture that was prepared as a stock solution beforehand, as shown in Fig. 1. The assigned amounts of PC powder were dissolved in dichloromethane (CH2Cl2) at 60 °C for at least 30 min. Then, the mixed epoxy resin was added into the PC solution and a clear liquid mixture was obtained after continuous stirring for a few minutes. The CH2Cl2 was removed via evaporation. The PC blended epoxy resins with lower PC contents were obtained by dilution of the stock solution with the base resin mixture.
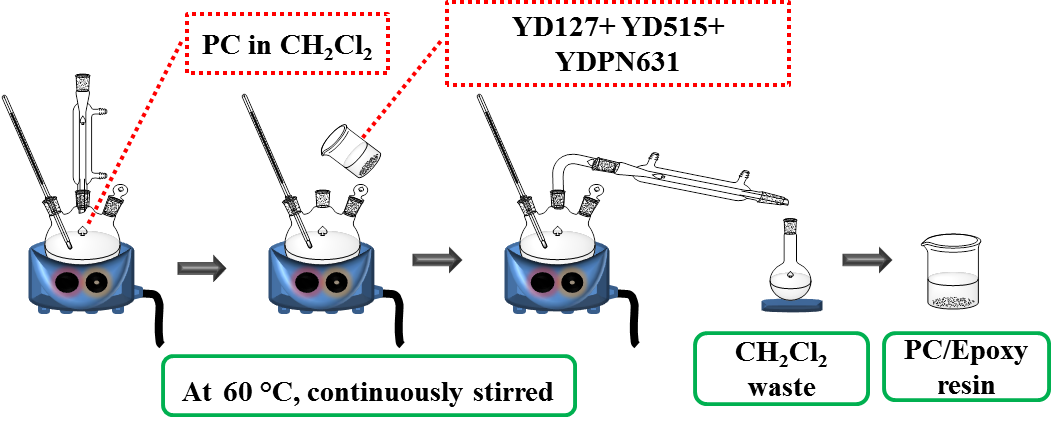
Fig. 1. PC/Epoxy resin preparation
Formulation of hardener
The main ingredients of the hardener were triethylenetetamine (TETA), isophorone diamine (IPDA), diamino diphenylsulfone (DDS), and bisphenol A (BPA). All of the amines were gently mixed at 60 °C until a clear yellowish solution was formed. In the case of the hardener comprised of DICY, the powder was slowly added into the amines mixture at 90 °C and stirred for at least 2 h. For the hardener with the aliphatic epoxy (RD108)/TETA adduct, the RD108 reacted with TETA at 60 °C for approximately 5 min. Next, different amounts of IPDA, BPA, DDS, and DICY were slowly added into the RD108/TETA adduct. The solution mixtures were continuously stirred for at least 4 h. Adduct hardeners that were syrupy and clear yellowish in color were obtained.
Test Specimen Preparation
Neat cured epoxy
Neat cured epoxy samples with a thickness of approximately 5.0 mm were prepared by mold casting. At the calculated stoichiometric ratio (phr), 150 g of resin and hardener were thoroughly mixed using a high speed mixer for at least 30 seconds. The mixed epoxy was cast onto the mold and degassed in a vacuum oven for 15 min. For the room temperature cure resin formulations, the resin was cured in an oven at room temperature for 12 h. The formulation that contained DICY, known as the high temperature cure system, was cured at 90 °C for 12 h. The solidified neat cured epoxy resin was then demolded. Standard size specimens were obtained by saw cutting, and were then sand-paper polished. Finally, the specimens underwent post curing at 90 °C for at least 8 h.
Fiber glass prepreg
The resin formula containing DICY showed the characteristics of prepreg-type resin. Therefore, prepreg on glass fiber was prepared, and 10 layers of 20 × 20 cm2 GFRP specimens were produced and tested. The glass woven fiber was wet with the prepreg epoxy formulation by a hand lay-up process. The resin and fiber weight ratio was constantly controlled at 70:100. The laminated prepreg was pre-consolidated under a vacuum bag at 150 °C for 30 min, and then it was allowed to completely cure at 150 °C for 2 h without applying the vacuum pressure. The standard sized GFRP test pieces were obtained by saw cutting, sand-paper polishing, and post curing at 150 °C for 12 h.
Engineered wood
Test samples of MDF-type engineered wood with a density of 1.10 g/cm3 were manufactured. On a 20 cm × 20 cm × 0.50 cm (W × L × H) compression mold, 220 g of fiber/epoxy resin mixture was evenly filled. The mold was vacuum dried at 105 °C for 4 h. Eucalyptus wood fiber was vigorously pulped in a high speed mixer for 2 min. Onto the 157 g of fiber, 63 g of mixed epoxy resin, adhesive formulation was added, which was approximately 40 phr corresponding to the fiber. High-speed mixing was then performed. The action of mixing/pause/mixing at 20 sec/5 sec/20 sec was applied for the high speed mixing process. The adhesive coated fiber was evenly transferred into the mold cavity lined with poly(tetrafluoroethylene) (PTFE) sheets. Cold compression was applied for the pre-forming. Finally, the MDF engineered wood was manufactured by compression at 180 °C and 120 kgf/cm2 for 8 min. The wood was demolded and annealed at room temperature overnight. Standard sized test specimens were saw cut, edge polished, and post cured at 120 °C for 12 h before testing.
Methods
The viscosity of the epoxy resins and hardener were measured by using a BrookfieldTM viscometer (Model LVDV-II+P, Brookfield Engineering Laboratories, Inc., Middleboro, Massachusetts, USA) at 25 °C at the specified spindle. The curing parameters of the room temperature cure epoxy formulation, cure time (`tcure) and time to reach 50 °C (t50), were obtained from the time/temperature profile plotted. A multi-meter equipped with a type-J thermocouple (BryMenTM BM 817, Brymen Technology Corp., Taipei, Taiwan) was used to collect the temperature rise within a one second interval of reaction time on 50 g of mixed epoxy formulation. The tcure was recorded as the time to reach the maximum temperature (Tmax), and t50 was the time that it took the mixed epoxy to reach 50 °C. For the high temperature cure epoxy system, which was used for the formulation cured with the hardener that contained the adducts, the cure parameter was measured by using a Differential Scanning Calorimeter (DSC) (Phoenix®204F1, NETZSCH-Gerätebau GmbH, Selb, Germany).
The mechanical properties, flexural and Izod impact strengths, were measured in accordance with ASTM D790-10 (2010) and ASTM D256-10 (2010), respectively. The heat distortion temperature (HDT) was evaluated by ASTM D648-07 (2007). Standard loads of 455 kPa, 1820 kPa, and 7280 kPa were assigned for the neat cured epoxy, wood, and prepreg samples, respectively. The durability of the woods were measured by water absorption (WAi, %), thickness swelling (TSi, %), and dimension stability after removing the moisture, or thickness swelling after drying (TSdried,I, %), of the samples after immersion in water for 1 d and 7 d. The testing methods followed ASTM D570-98 (2010).
2K Design of Experiment (DOE)
The properties of the cured epoxy were determined by either type of resin or hardener parts. In this study, for the given base resin mixture, the hardener ingredients were chosen for exploration of the improvement of the mechanical and curing properties. A 2K DOE method was employed. The IPDA, DDS, and BPA were referred to as A, B, and C design parameters, respectively, as shown in Table 1. Accordingly, 10.0 g of IPDA(A), 5.0 g of DDS(B), and 7.5 g of BPA(C) were given the label “High Level (+).” In contrast, 5.0 g of IPDA(A), 2.5 g of DDS(B), and 2.5 g of BPA(C) were labeled “Low Level (-).” With a K of 3, eight experimental design matrices were constructed, as seen in Table 1. Table 2 summarizes the design responses obtained for the curing parameters (t50 and tcure) of the 50 g mixed samples, and properties tested of the neat cured specimens.
Table 1. 2K Design Matrix of IPDA(A), DDS(B), and BPA(C)
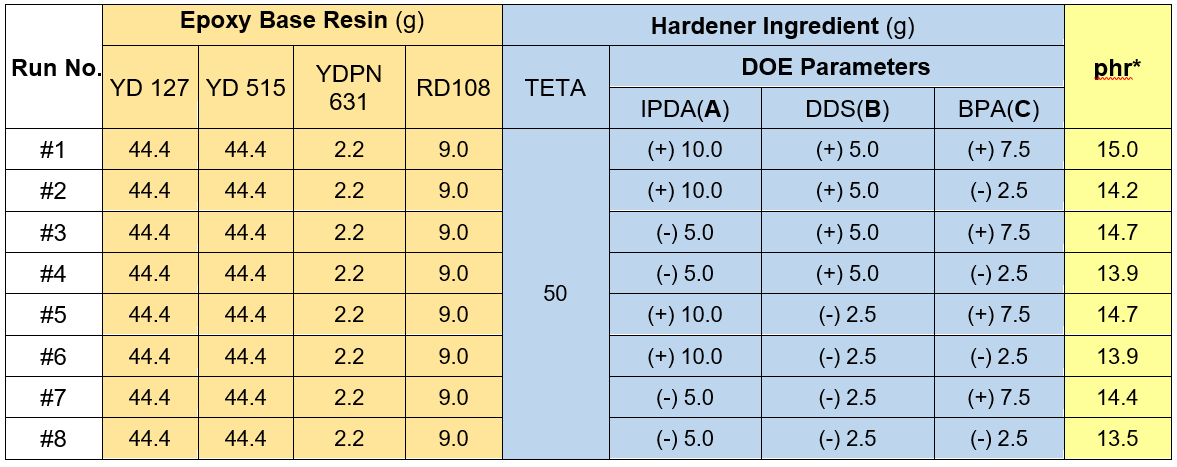
Note: *phr = part per hundred of resin
RESULTS AND DISCUSSION
An analysis of variance (ANOVA) with an a = 0.05 was conducted via the Design ExpertTM software (Stat-Ease, Inc., Minneapolis, Minnesota, USA). It was concluded that there were no significant effects from the design parameters on the curing parameters and mechanical properties of the epoxy formulations, except for BPA, which had a significantly negative effect on the HDT of the cured epoxy.
The resultant regression model for the HDT and BPA(C) of the solid cured epoxy was 80.85 to 0.24(C). A more detailed analysis of this DOE can be found in the study by Wangkheeree and Meekum (2015). With regards to the DOE conclusion, the Run #4 formulation showed a very interesting toughness, and therefore, it was chosen as the primary hardener formulation for further improvement for using as the adhesive in engineered wood manufacturing.
Table 2. Curing Parameters, Mechanical Properties, and HDT as the Design Responses of the 2K DOE
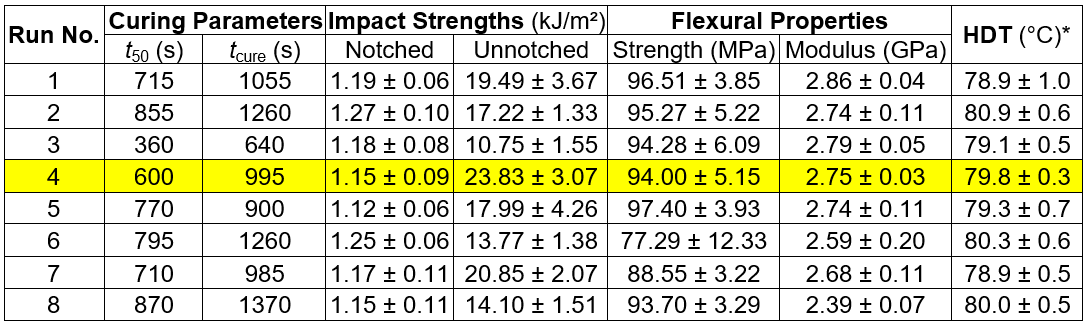
*Standard load of 450 kPa
DICY Added Hardener
The DICY, a catalytic amino amine, was selected for the properties improvement study. To the Run#4 hardener recipe, 5 g to 30 g of DICY was added, as shown in Table 3. With the assigned base resin, it was found that, at the high DICY contents, the prepreg characteristic was observed for the epoxy formulations with the B-stage cure. The performance measurements of the DICY added epoxy resin were tested on both the neat and GFRP cured samples. For the GFRP manufacturing, the epoxidized silane, Silquest®A187, at 1 phr that corresponded to the mixed resin, was added to enhance the fiber/matrix interfacial adhesion. The prepreg mixtures were cured in vacuum bagging at 150 °C.
Table 3 summarizes the properties of the DICY added epoxy formulations, and it can be seen that the viscosity of the obtained hardeners exponentially increased with added DICY. It was also seen that the hardeners with the 20.0 g and 30.0 g DICY contents had milky solutions after storage for a few days, which was due to the recrystallization of DICY. The calculated mixed resin viscosity is also reported in Table 3 at the given phr and based on a measured epoxy resin viscosity of 2351.0 ± 0.0 cP. The viscosity gradually increased with the DICY content. However, the magnitude of the viscosities was acceptable for the hand lay-up process. For the cure parameters, t50, tcure, and Tmax, it was seen that those indicators decreased with increased DICY content, except at 20.0 g and 30.0 g, where the time parameters increased.
Table 3. Effect of DICY Content on the Hardener Ingredients
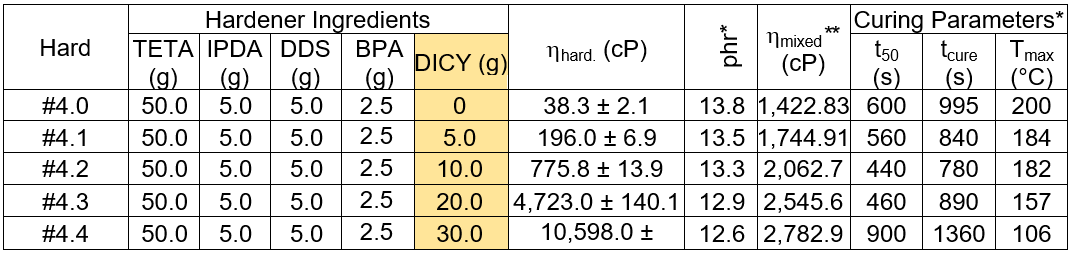
*Formulated with the base resin; **Calculated from ln(hmixed) = wresin × ln(hresin) + whard. × ln(hhard.)
Lower t50 and tcure values meant longer curing rates. The systems were fully catalyzed by DICY, but at the saturated DICY loadings, 20.0 g and 30.0 g, the reaction rates and heat accumulation due to the curing reaction, Tmax, were reversibly lower. In the completely soluble DICY hardeners, the reaction rate on the liquid/liquid phase was fast. In contrast, a slower rate in the liquid/solid reaction was found for the DICY saturated hardeners and incomplete chain crosslinking could have resulted, as shown in Table 4.
Table 4 summarizes the test outcomes of the neat cured and GFRP samples using the epoxy formulation cured with the DICY added hardener, respectively. The results showed that the impact strengths, for both the notched and unnotched neat cured samples after post curing at 110 °C, decreased with increased DICY content. The flexural properties also likely decreased with increased DICY loading. The measured HDT marginally increased with higher DICY contents. The decreasing of the mechanical properties was due to the incomplete cure of the epoxy, especially for the high loadings of DICY. This conclusion is supported by the weight loss measurement under the CH2Cl2 etching. It was seen that there was more than 80 wt.% loss when more than 20.0 g of DICY was added into the hardener. This piece of evidence indicated that curing and post curing the epoxy formulation using the DICY added hardener at 90 °C and 110 °C, respectively, was not sufficient for the complete crosslinking reaction. Hence, the inferior mechanical properties resulted from the partially crosslinked epoxy. The properties of GFRP are shown in Table 4. The samples were cured at 150 °C for 2 h, and were then post cured for 12 h. According to the measured DW, it was confirmed that all of the GFRP samples were fully crosslinked at the given curing conditions. It was also seen that the toughness decreased with increased DICY loading, which was determined via impact and flexural tests. The HDT of the GFRPs increased with higher loadings of DICY.
Table 4. Effect of DICY on the Properties of (a) Neat Cured Epoxy and (b) Prepreg GFRP
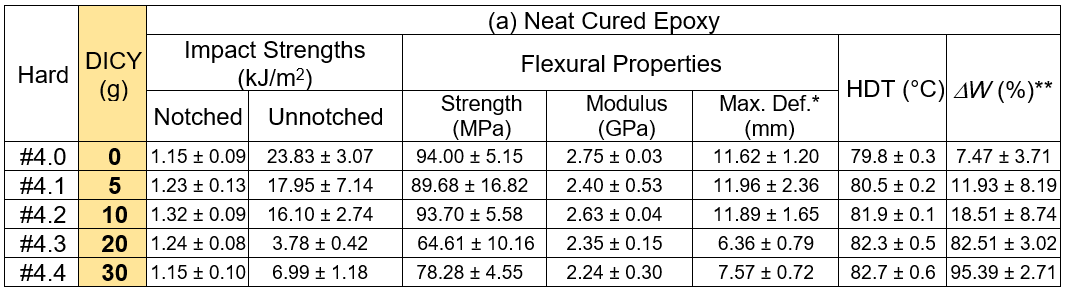
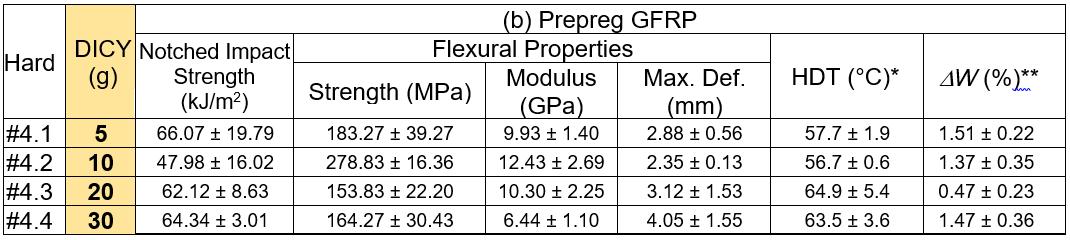
*Maximum deformation at break, **Sample post cured at 110 °C
*Standard load at 7280 kPa and **Sample post cured at 150 °C
The effects on the properties may have been due to the chemical bonding categories. Amino ester bonding was formed by the DICY and epoxide reaction, which commonly has higher service temperatures than the amide derived from the active amine hydrogen and epoxide group.
Amine Adduct Hardener
For the manufacturing of the engineered woods, the outstanding mechanical properties of the epoxy adhesive were not the only prime concerns, but the curing time was also critical. The wood production line involved several time-consuming steps, such as fiber/adhesive mixing, pre-shape forming, and consolidation. Premature curing of the adhesive during the production process would cause inferior properties. The agent TETA is typically known as a fast curing agent. The adducted hardener synthesized by the pre-reaction between the TETA and flexible aliphatic epoxy, RD108, was one of the approaches used to extend the hardening time. In this work, the hardener with 20.0 g of DICY, Hard#4.3, manifested the desired properties in the prepreg sample results. Therefore, it was chosen for further improvement by adding the TETA and RD108 ingredients into the adduct product beforehand. Hereby, the adducted hardeners were designated as Hard#4.3.X. Table 5 shows an example of the epoxy formulation having 7.81 g of TETA and 9.76 g of RD108 pre-reacted as the adduct product, and it was called Hard#4.3.1. When the amount of TETA was at 7.81 g, the amount of RD108 for the adduct reaction varied from 9.76 g to 29.27 g. The series of Hard#4.3.X samples were constructed as shown in Table 5. The viscosity of the resin and adduct hardeners were measured and reported in Table 5. The curing times, B-stage cure time (tB) and tcure, of the resin cured with the adduct hardeners were measured by the isothermal DSC method at 60 °C for 1 h. These results are also summarized in Table 6. It was seen that the Brookfield viscosity of the adduct hardeners decreased with increasing amounts of RD108. By using the adduct hardeners derived from the pre-reaction between TETA and the linear aliphatic epoxy, RD108, the prepreg epoxy formulations were obtained. The measured curing parameters showed that the tB of the adduct hardener epoxy formulations was almost constant, at around 33 s, which was shorter than the tB of the non-adduct hardener, Hard#4.3. Meanwhile, the tcure of the adduct formulations decreased with increasing amounts of RD108, which indicated faster curing times, especially for Hard#4.3.5. According to the cure parameters examined, it was concluded that the tB of the adduct hardener epoxy formulations did not depend on the amount of RD108. However, the tcure decreased with increasing amounts of RD108.
Table 5. Formulation of TETA/RD108 Adduct Hardener
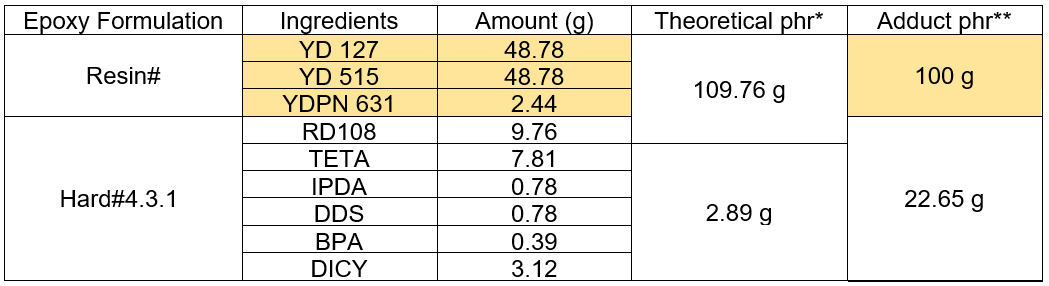
*RD108 as part of the resin; **RD108 pre-reacted with TETA and counted as hardener part
Table 6. Viscosity and Cure Parameters of Adduct Epoxy Formulation
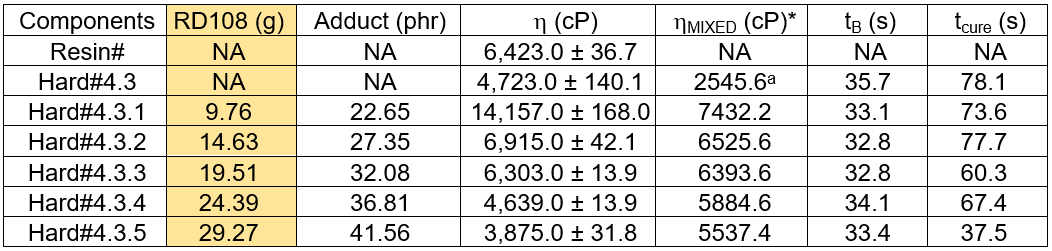
*Calculated based on adduct (phr)
The performance properties of the epoxy formulations using the adduct hardener were evaluated on both the GFRP and 1.10 g/cm3 panel board types of engineered wood samples, and the measured results are shown in Table 7.
Table 7. Tested Properties of (a) GFRP and (b) Engineered Wood Manufactured Using the Adduct Epoxy Formula
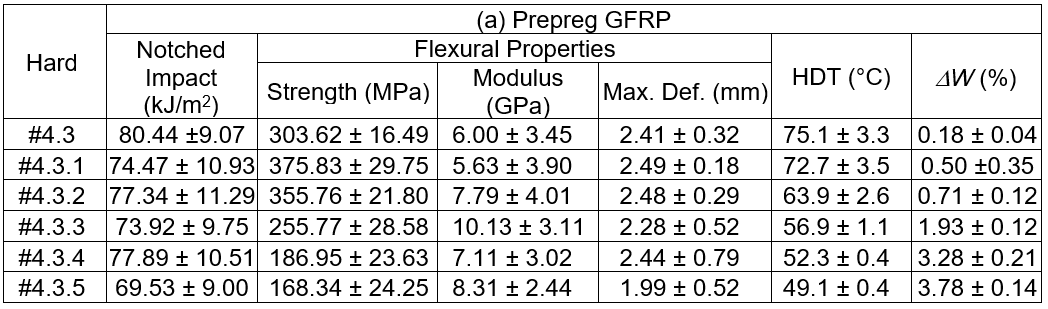
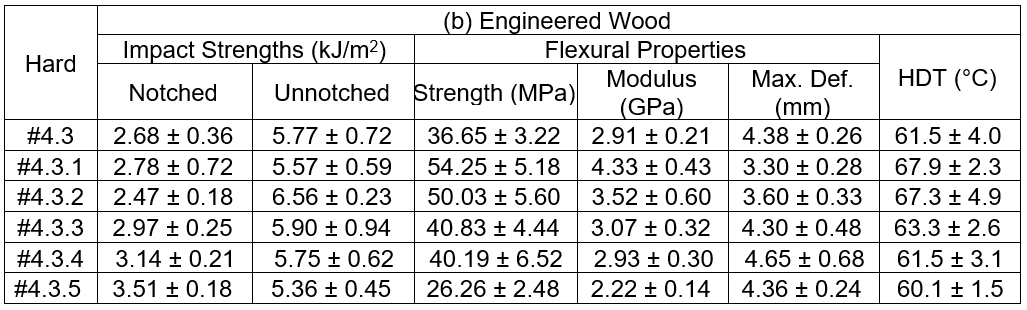
The test with no adduct formulation, Hard#4.3, was shown for comparison. The notched impact strength of the GFRP tended to marginally decrease as the RD108 ratio increased, and it was lower than for the Hard#4.3 formulation. However, the notched impact strength of the woods using the adduct epoxy resins as adhesive increased with higher RD108 additions, and they were superior to the Hard#4.3 formulation. Meanwhile, the strength of the unnotched samples seemed to have been unchanged. After the flexural testing, lower strength, higher modulus, and lower deformation at break were observed for the GFRP samples with increasing amounts of RD108. It was interpreted that the flexural toughness was weaker at higher RD108 loadings. Similarly, lower strength, lower modulus, and a marginal increase in the deformation at break after the flexural testing were seen for the wood when the RD108 was increased. This meant that the material was brittle. A trend similar for the mechanical properties was seen for the HDT. The service temperature for the GFRP and wood were lower at higher RD108 contents. The weight loss by CH2Cl2 etching of the GFRP was found to increase with increased RD108. This was evidence there was incomplete crosslinking of the epoxy formulation. Based on the test results obtained, especially for the high RD108 ratio, it was initially suggested that the GFRP and engineered woods, manufactured from the adduct epoxy adhesive, showed inferior properties with increased RD108 weight ratios in the adduct hardeners. One of the main reasons for this inferiority was explained by the incomplete crosslinking reaction, which was confirmed by the weight loss measurement. The short chains, or unreacted constituents, due to the incomplete cure reaction would act as the contaminant in the matrix phase. Accordingly, the properties of the matrix would be inferior.
The durability properties of the woods using the adduct epoxy adhesive, water absorption (WAi, %), thickness swelling (TSi, %), and thickness swelling after drying (TSdried, I, %), after 1 d and 7 d of immersion were measured and reported in Table 8. For all of the tested parameters for 1 d and 7 d, there was a tendency to increase with increased RD108 weight fraction, especially at the high loading ratios. The most likely explanation for the decreased water resistance with an increasing RD108 fraction was the incomplete crosslinking reaction, as previously mentioned in the weight loss examination.
Table 8. Durability Test Results of the 1.10 g/cm3 Panel Type Engineered Woods
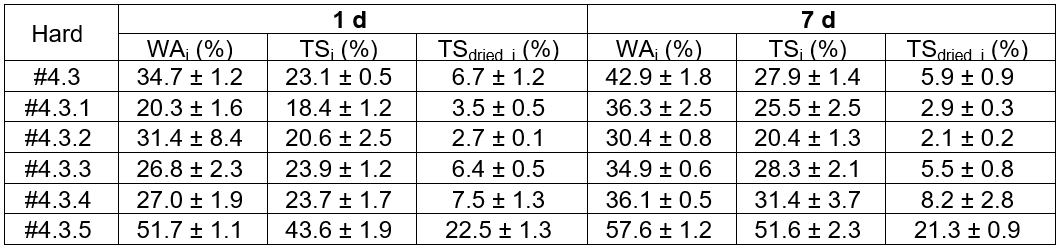
With respect to the above results, the inferiority of the properties of the adduct hardener epoxy formula was caused by the incomplete crosslinking reaction, especially for the high RD108 ratios. To further improve the epoxy, mainly in terms of the toughness properties especially for applying as the engineered wood adhesive, the epoxy adhesive formulation included thermoplastic blending in the resin part and polycarbonate (PC) was the candidate. The Hard#4.3.2 formulation, with a RD108 to TETA ratio of 14.63 g to 7.71 g, was selected as the curing agent.
PC Blended Epoxy Resin
The base Resin# was modified by solution blending with polycarbonate (PC) and named the Resin#2.X series. The PC contents varied from 1 phr to 10 phr with respect to the base resin mixture. Hard#4.3.2 was chosen as curing agent and the adduct content was 27.35 phr. Table 9 summarizes the measured viscosity of the PC blended epoxy resins. The calculated viscosity of the epoxy formulation for Hard#4.3.2 is also reported. As was expected, the Brookfield viscosity of the PC blended resins increased remarkably with increased PC loading. Also, the calculated viscosities of the mixed resins ranged from 4700 cP to 20000 cP. Normally, a viscosity less than 10000 cP for a mixed resin is acceptable for the hand lay-up process, especially for the manufacturing of the prepreg. In this case, for a PC loading exceeding 7 phr, the viscosities were above 10,000 cP. This limits the hand lay-up process. Consequently, the impregnation and wettability of the resin onto the fiber filament would be difficult, and hence, there would be weak mechanical properties.
Table 9. Viscosity of the PC Blended Epoxy Resins
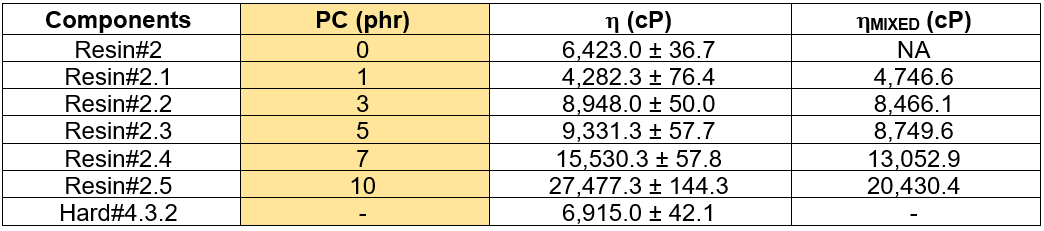
The performance evaluation of the PC blended epoxy adhesives was conducted on the prepreg GFRP and engineered woods manufacturing. The test results are summarized in Table 10. In the prepreg specimens, epoxy matrices that contained PC contents from 1 phr to 7 phr were employed. Meanwhile, the PC contents from 1 phr to 10 phr of epoxy adhesive were employed for making the engineered wood samples.
Table 10. Effect of the PC Blended Epoxy Resins on the Properties of (a) GFRP and (b) Engineered Woods
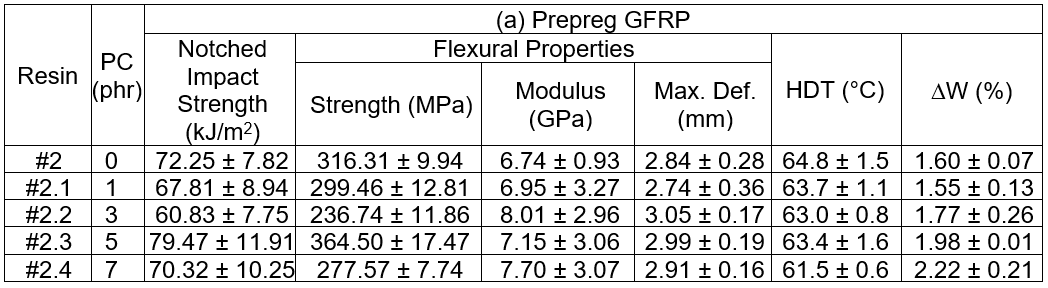
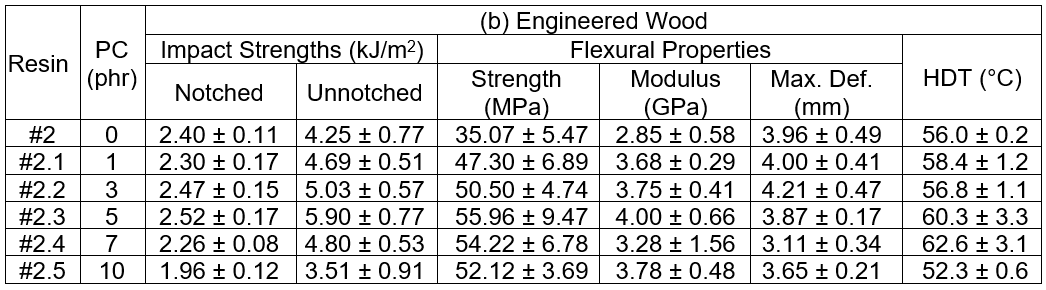
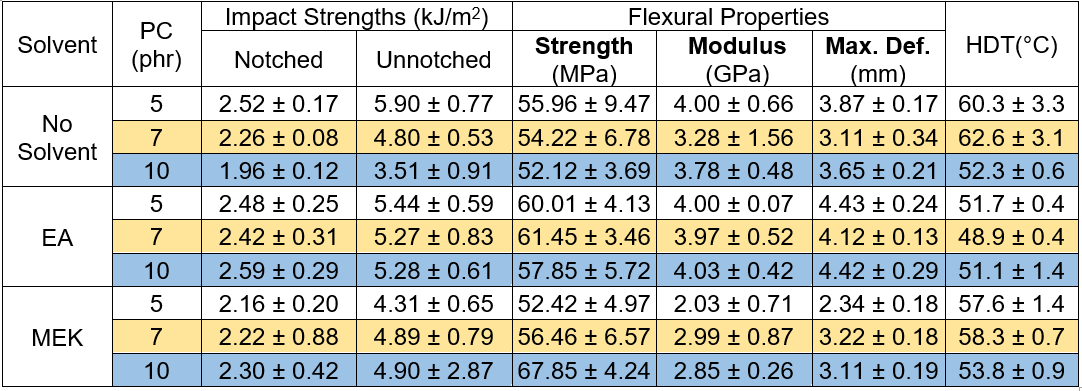
For the prepreg GFRP samples, it was seen that the notched impact strength initially decreased when the PC loading increased from 0 phr to 3 phr. At a 5 phr of PC blending, the notched impact strength noticeably increased, but it declined again at 7 phr PC. A similar trend was found for the flexural properties. There are two contradicting clarifications to be made. The addition of PC as the thermoplastic toughener resulted in a superior toughness of the epoxy matrix with the PC content at 5 phr. A limitation of the resin impregnation due to a dramatic increase in the resin viscosity was experienced for a 7 phr PC loading, and accordingly, poor fiber/matrix wettability and weak fiber adhesion resulted. Hence, the fracture toughness suffered. For the service temperature of the GFRP, it was seen that the HDT values were relatively constant at 63 °C, regardless of the PC loading, except at a PC of 7 phr, where the tested value was remarkably low. Again, this was caused by insufficient resin impregnation due to the extremely high viscosity of the resin. Finally, the weight loss of GFRP by CH2Cl2 etching revealed that the weight loss increased with increasing PC content, which was expected because CH2Cl2 is a good solvent for PC. Therefore, the PC was dissolved during the solvent etching test.
The performances of the PC blended epoxy formulation used as the high performance adhesive in the engineered wood production samples were determined. They were tested on the 1.10 g/cm3 panel wood, and the results are shown in Table 10. The impact strengths, both notched and unnotched, were more or less constant, regardless of the PC loading. However, above 7 phr PC blending, the impact strengths clearly decreased. The flexural test results included the strength, modulus, and maximum deformation. The deformation at break gradually increased as the PC content increased from 0 phr to 7 phr. At 10 phr of PC, the flexural characteristics started to decline. With the impact and flexural test results taken into account, it was determined that the toughness of the engineered wood gradually increased with increased PC loading in the epoxy adhesive. The improvement in the toughness due to the addition of PC should be taken into account. However, at 10 phr of PC loading, the deprived fiber/adhesive coating, or wetting, due to the enormously high viscosity of the PC blended epoxy adhesive, affected the final mechanical properties. Homogenous coating of the adhesive with PC loadings of 10 phr on the fiber during the mixing step would become extremely difficult. The uneven adhesive and fiber-rich phases could be visibly seen, as shown in Fig. 2. Consequently, the obtained wood had weak fracture toughness. The consequences not only affected the mechanical properties, but also influenced the HDT of the wood. The service temperature of the panel engineered wood increased when the epoxy adhesives were blended with high PC contents, but at 10 phr of PC blended epoxy, the HDT decreased.

Fig. 2. Photographs of engineered wood produced with the PC blended adhesives
Table 11 summarizes the durability evaluation, which was done by means of water immersion, for the MDF-type engineered woods using the PC blended epoxy as an adhesive. It can be clearly seen that the WAi, TSi, and TSdried, i for 1 d and 7 d of immersion decreased as the PC content increased from 0 phr to 5 phr. Above 7 phr, the durability values increased. It was interpreted from these results that the wood has better water resistance with PC loadings from 0 phr to 5 phr. However, above 7 phr, the water durability was inferior. Again, poor fiber/adhesive coating due to the enormously high viscosity of the adhesive at high PC loadings was the most likely explanation for the decreased durability, and caused excess amounts of water to be easily absorbed into the fiber-rich phase.
Table 11. Durability Test Results of the Engineered Wood Samples
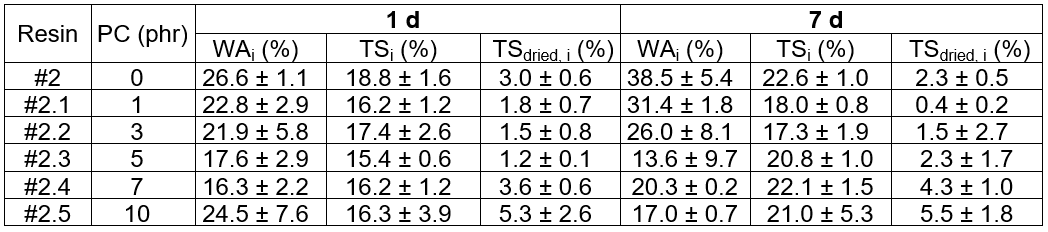
With regards to the obtained values for the properties, it could be concluded that by using the PC blended epoxy/adduct hardener, Hard#4.3.2, as an adhesive for manufacturing the eucalyptus-based engineered wood, the thermoplastic PC effectively increased toughness for the blending fractions from 1 phr to 5 phr. However, for loadings above 7 phr, the toughness was abysmal.
The limitation of resin/fiber infusion due to the drastically high viscosity of the PC blended epoxy resin was the prime concern. For the next step of the investigation, active solvents were used to reduce the viscosity of the epoxy adhesive.
Solvent Dilution of the Epoxy Adhesives
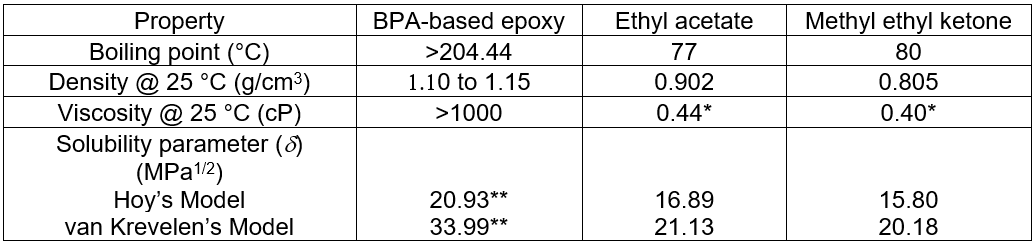
The active solvents MEK and EA are the most common used in solvent-based prepreg formulation. The typical physical properties of these solvents and the epoxy resin are summarized in Table 12. The solubility parameters (d) of the solvents and epoxy resin were calculated by the Small, Hoy, and van Krevelen methods (Koenhen and Smolders 1975; Ravindra et al. 1998; Miller-Chou and Koenig 2003). The PC blended epoxy resins with PC contents of 5 phr, 7 phr, and 10 phr were diluted with either EA or MEK at 10 phr. The solvent diluted epoxy resins were then used as adhesives for manufacturing the engineered wood. The diluted solvents were removed from the engineered wood by placing them in a vacuum oven for approximately 10 min, and then hot compression was performed at 180 °C.
Table 12. General Physical Properties of EA, MEK, and BPA-based Epoxy Resin
*Data from MSDS; **The epoxide end-group is included for calculation.
Table 13. Viscosity of Solvent Diluted PC Blended Epoxy Resin
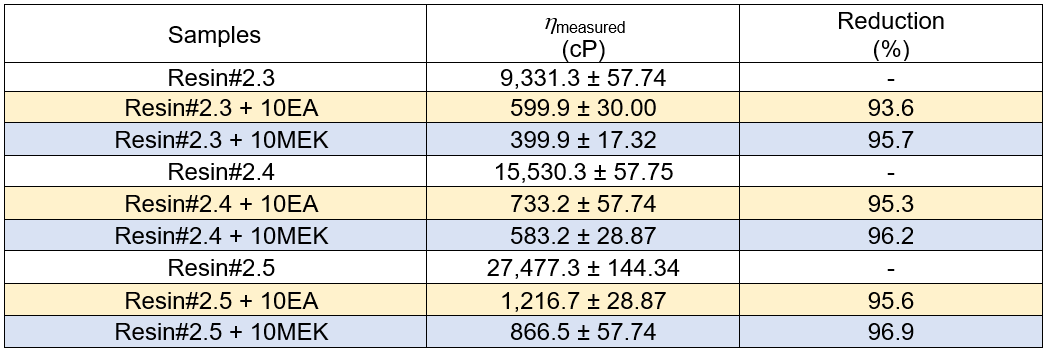
The measured viscosity of the solvent diluted PC blended epoxy resins are presented in Table 13. It was seen that the viscosity of the diluted epoxy were more than 90% lower than the non-diluted epoxy resins for all of the mixtures, regardless of the type of solvent. The viscosity of the diluted resins was well below 1000 cP. They were used well as wood adhesives and had excellent adhesive/fiber wettability during the high-speed mixing step.
Table 14 summarizes the results obtained for the engineered woods manufactured by non-diluted and solvent diluted PC blended epoxy adhesive formulations. According to the results, at the given PC content, and especially for the high PC loadings, it can be clearly seen that all of the mechanical properties, including impact strengths and flexural tests, of the wood using the solvent diluted adhesives were superior to the samples manufactured from the non-diluted adhesives. There was no doubt that poor resin infusion due to the higher viscosity of the non-diluted formulation, especially for the higher PC loadings, was the cause for the poor performance. Within the assigned solvents and disregarding the viscosity constraint, it was clearly apparent that the mechanical properties of the engineered woods increased with increased PC blending. This meant that the PC was able to improve the toughness. After careful comparison within the low viscosity diluted adhesive systems at any given PC concentration, the mechanical test results showed that the EA diluted system had superior properties to the MEK dilution formulation. There was a two-fold explanation for this superiority, the boiling point and the thermodynamics of mixing. In the production process of the wood samples, the diluted solvent was removed via vacuum evaporation at room temperature for 10 min, and therefore, the solvent with the lower boiling point solvent could be more easily removed. As shown in Table 11, the boiling point of EA was lower than that of MEK, and as such, the EA was easier to remove from the adhesive/fiber mixture than the MEK. Consequently, it was unlikely for there to be residual EA in the adhesive matrix. Thus, during the compression process at 180 °C, the generation of gas voids by solvent evaporation would be unlikely to occur within the wood. Typically, one of the main causes of brittle composite materials is the presence of voids. Using MEK, which has a higher boiling point, the adhesive dilution would create more voids than using EA. Another cause for the mechanical superiority of the EA diluted epoxy resin over the MEK diluted epoxy resin was the thermodynamics of mixing. According to the calculated solubility parameters (d) of the epoxy, EA, and MEK reported in Table 11, it was found that the dEA was closer to the depoxy than dMEK. This thermodynamically meant that the EA was a better solvent for the epoxy than MEK after the Flory-Huggins interaction, or Chi parameter (χ12), was taken into account, which can be seen in Eq. 1,
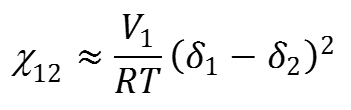 (1)
(1)
where χ12 is the Flory-Huggins interaction parameter, V1 is the Molar volume of component 1 (cm3/mol), d1 is the solubility of component 1 (Solvent; EA and MEK), d2 is the solubility of component 2 (Bisphenol A-based epoxy resin), R is the gas constant (8.3114 J/K·mol), and T is the temperature (K).
The required energy of mixing the EA/epoxy was lower than the required energy for the MEK/epoxy system. Accordingly, the latent heat of evaporation for the EA/epoxy solution would be lower than for the diluted MEK/epoxy. This means that the removal of the MEK solvent from the solution requires a high amount of energy. Hence, removing the MEK from the epoxy solution via evaporation would take longer than removing the EA. As stated earlier, the diluted solvents were removed by vacuum evaporation before processing by compression molding at 180 °C. As such, it would be more likely for there to be residual MEK in the epoxy adhesive than EA. Thus, there would be more generated gas voids present in the wood texture of the wood samples made with the MEK/epoxy.
Within the standard deviation of error testing, it was discovered that the HDT of the wood samples derived from the EA diluted adhesive were marginally lower than the non-diluted and MEK wood samples. Also, it seemed that there was not much difference in the HDT between the non-diluted and MEK systems. Commonly, the Tg or HDT of the polymer blend, especially in a miscible system, would obey the rule of mixture, or Fox’s Equation. The PC has an HDT of approximately 150 °C. In this work, only small fractions of PC were blended with the epoxy. Regarding the measured HDT, there were minute HDT differences among the wood samples. This meant that the effect of PC blending on the HDT of the wood was insignificant.
Table 14. Properties of Wood Produced from the Solvent Diluted Epoxy Adhesives
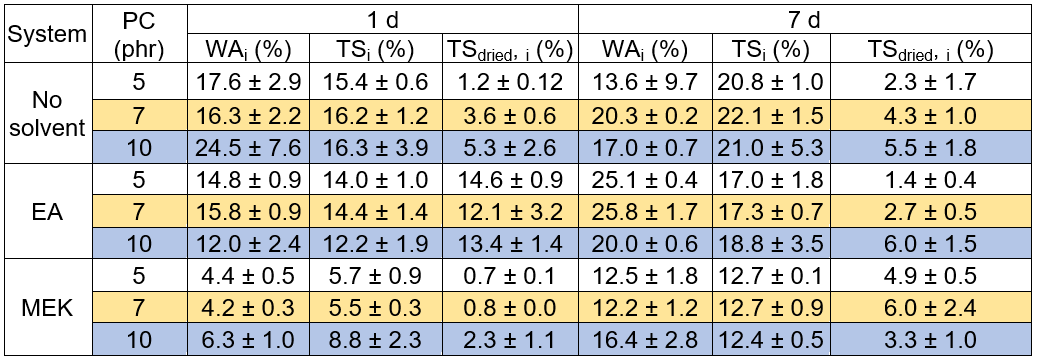
The durability properties, WAi, TSi, and TSdried, i, of the engineered wood after immersion in water for 1 d and 7 d are reported in Table 15. Typically, it was visibly established that the woods fabricated from the non-diluted adhesive demonstrated higher WAi, TSi, and TSdried, i values than the samples made by the diluted systems, regardless of the PC contents and immersion times. It was interpreted that the non-diluted woods were less resistant to water or moisture absorption than the diluted adhesives. This strengthened the conclusion that the low viscosity of the solvent-diluted epoxy adhesive can be easily infused with fiber and result in a homogenous coating. Consequently, low moisture penetration and high dimension stability, due to the hydro-static expansion, resulted from superior adhesive/fiber bonding. Moreover, it was also evidenced that the water resistivity of the wood produced from the MEK diluted adhesive was noticeably better than the wood using EA diluted adhesive, regardless of the immersion time and PC contents. These results can be explained by the lower adhesive viscosity of the MEK/epoxy than the EA/epoxy, as shown in Table 13, for all of the PC loadings.
Table 15. Durability of Engineered Wood Produced by the Diluted Adhesive
Termite Resistance Testing
Figure 3 shows weight (wt.%) versus buried time for the termite resistance testing.
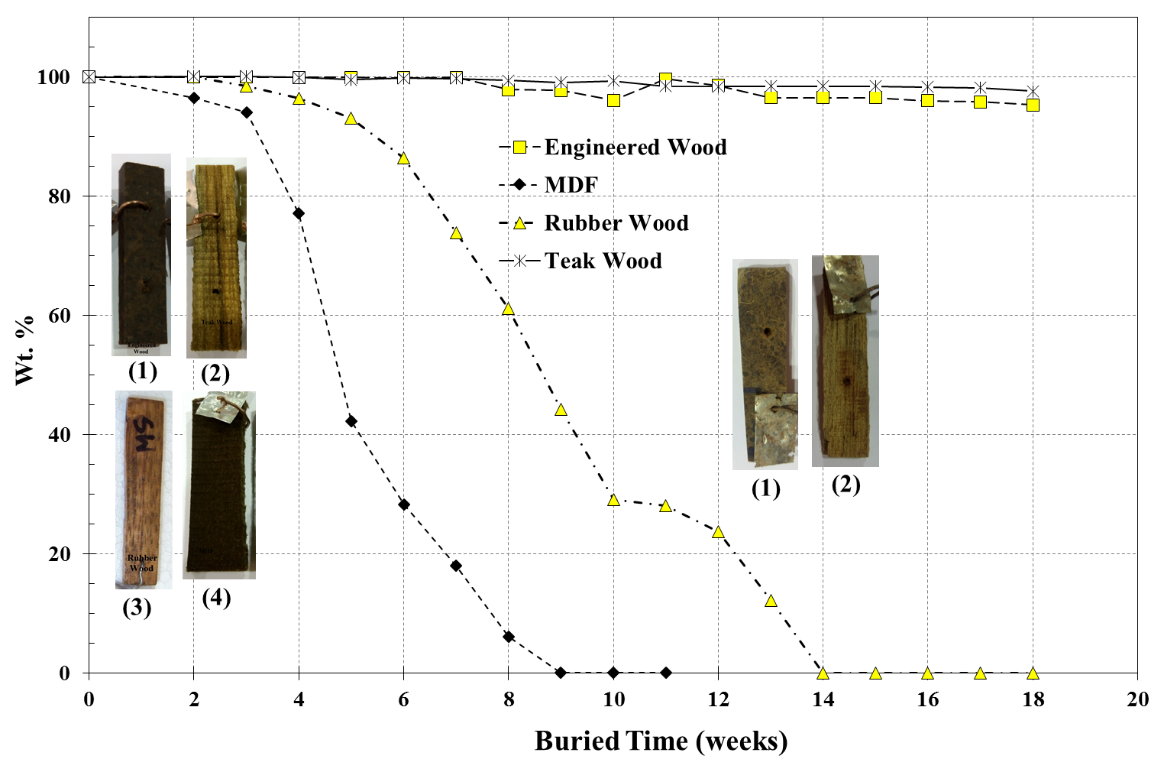
Fig. 3. The remaining wt.% vs. buried time for the termite testing; (1) is 1.1 g/cm3 Engineered Wood, (2) is Teak, (3) is Para-rubber, and (4) is MDF wood
The test samples were buried in a termite nest for 18 weeks. Teak, Para-rubber, and commercialized urea-based adhesive MDF were used as references. Teak is known to be the most termite resistant wood. Para-rubber is a soft wood and less resistant to termite attack. According to the results, it was clearly seen that the commercialized MDF was completely consumed by the termites within 9 weeks, followed by the Para-rubber within 14 weeks. However, the teak and engineered wood manufactured using the designed epoxy adhesive were in good shape after burial in the termite net for more than 18 weeks. This showed that the engineered wood had a resistance to termite attack that was comparable to teak wood.
CONCLUSIONS
- From the 2K DOE results, it was found that for all of the assigned parameters, the amount of IPDA, DDS, and BPA in the TETA-based hardener did not have a major effect on the cure and mechanical properties of epoxy formulation. Only the amount of BPA had a considerably negative effect on the HDT of the cured epoxy.
- The DICY addition into the hardener of the epoxy formulation revealed that high DICY loadings were not only retarding the curing reaction time at room temperature, but also they caused an incomplete curing process. The high temperatures, 110 °C for curing and 150 °C for post curing, were established as the optimal cure conditions for the liquidized DICY hardener and epoxy resin. The prepreg epoxy formula was obtained. This formula was tested on the neat cured epoxy resin and on the prepreg GFRP samples, and it was concluded that 20 phr of DICY with 50 g of TETA, was the optimal content for preparing the hardener.
- The results from the investigation into the GFRP and engineered wood specimens manufactured from the adduct hardener having an aliphatic epoxy (RD108) pre-reacted with TETA as the adduct ingredient showed that the incomplete crosslinking reaction was one of the main causes for the inferior properties, especially for high RD108 dosages. A weight ratio for RD108 to TETA of 14.63 g to 7.71 g for synthesizing the adduct was chosen for mechanical properties enhancement.
- The PC hardener was blended with the epoxy base resin to improve the toughness of the epoxy formulation. These epoxy formulations were tested on prepreg GFRP and the panel-type engineered woods, and the results showed that improvement to the properties were found with the PC blending fraction from 1 phr to 5 phr. Above 5 phr of PC, the toughness was abysmal. It was concluded that the limitation of resin/fiber infusion, or impregnation, was due to the high viscosity of the PC blended epoxy resin for the high PC loadings.
- The viscosity reduction of the PC blended epoxy formulations was accomplished by adding solvents (EA and MEK) to dilute the epoxy resins, which were then used for manufacturing the engineered wood samples. It was concluded that the all of the mechanical properties of the wood samples made from the EA diluted adhesive were superior to the MEK diluted samples. The advantages of EA having a lower boiling point and being a better solvent than MEK were determined to be the cause for the better properties. The HDT of the wood produced from the MEK diluted adhesive was marginally higher than that of the EA diluted adhesive. The lower viscosity of the MEK diluted epoxy adhesive than the EA system led to a more homogenous coating of the fiber/adhesive. This explanation also accounted for the durability characteristics of the manufactured wood.
- The panel type engineered wood manufactured using the designed epoxy adhesive showed an excellent resistance to termite attack.
REFERENCES CITED
Ahmad, Z., Ansell, M. P., and Smedley, D. (2010). “Effect of nano- and micro-particle additions on moisture absorption in thixotropic room temperature cure epoxy-based adhesives for bonded-in timber connections,” Int. J. Adhes. Adhes. 30(6), 448-455. DOI: 10.1016/j.ijadhadh.2010.04.001
ASTM D256-10 (2010). “Standard test methods for determining the izod pendulum impact resistance of plastics,” ASTM International, West Conshohocken, PA.
ASTM D570-98 (2010). “Standard test method for water absorption of plastics,” ASTM International, West Conshohocken, PA.
ASTM D648-07 (2007). “Standard test method for deflection temperature of plastics under flexural load in the edgewise position (withdrawn 2016),” ASTM International, West Conshohocken, PA.
ASTM D790-10 (2010). “Standard test methods for flexural properties of unreinforced and reinforced plastics and electrical insulating materials,” ASTM International, West Conshohocken, PA.
Francis, B., Rao, V. L., Vanden Poel, G., Posada, F., Groeninckx, G., Ramaswamy, R., and Thomas, S. (2006). “Cure kinetics, morphological and dynamic mechanical analysis of diglycidyl ether of bisphenol-A epoxy resin modified with hydroxyl terminated poly (ether ether ketone) containing pendent tertiary butyl groups,” Polymer 47(15), 5411-5419. DOI: 10.1016/j.polymer.2006.05.029
Jang, Y., Huang, J., and Li, K. (2011). “A new formaldehyde-free wood adhesive from renewable materials,” Int. J. Adhes. Adhes. 31(7), 754-759. DOI: 10.1016/j.ijadhadh.2011.07.003
Kim, S. (2009). “Environment-friendly adhesives for surface bonding of wood-based flooring using natural tannin to reduce formaldehyde and TVOC emission,” Bioresource Technol. 100(2), 744-748. DOI: 10.1016/j.biortech.2008.06.062
Koenhen, D. M., and Smolders, C. A. (1975). “The determination of solubility parameters of solvents and polymers by means of correlations with other physical quantities,” J. Appl. Polym. Sci. 19(4), 1163-1179. DOI: 10.1002/app.1975.070190423
Kranthi, G., and Satapathy, A. (2010). “Evaluation and prediction of wear response of pine wood dust filled epoxy composites using neural computation,” Comp. Mater. Sci. 49(3), 609-614. DOI: 10.1016/j.commatsci.2010.06.001
Kumar, R., Kumar, K., Sahoo, P., and Bhowmik, S. (2014). “Study of mechanical properties of wood dust reinforced epoxy composite,” Procedia Materials Science 6, 551-556. DOI: 10.1016/j.mspro.2014.07.070
Lei, H., Du, G., Wu, Z., Xi, X., and Dong, Z. (2014). “Cross-linked soy-based wood adhesives for plywood,” Int. J. Adhes. Adhes. 50, 199-203. DOI: 10.1016/j.ijadhadh.2014.01.026
Liu, Y., Yang, G., Xiao, H. -M., Feng, Q. -P., and Fu, S. -Y. (2013). “Mechanical properties of cryogenic epoxy adhesives: Effects of mixed curing agent content,” Int. J. Adhes. Adhes. 41, 113-118. DOI: 10.1016/j.ijadhadh.2012.10.006
Luo, J., Li, X., Zhang, H., Gao, Q., and Li, J. (2016). “Properties of a soybean meal-based plywood adhesive modified by a commercial epoxy resin,” Int. J. Adhes. Adhes. 71, 99-104. DOI: 10.1016/j.ijadhadh.2016.09.002
Miller-Chou, B. A., and Koenig, J. L. (2003). “A review of polymer dissolution,” Prog. Polym. Sci. 28(8), 1223-1270. DOI: 10.1016/S0079-6700(03)00045-5
Mimura, K., Ito, H., and Fujioka, H. (2000). “Improvement of thermal and mechanical properties by control of morphologies in PES-modified epoxy resins,” Polymer 41(12), 4451-4459. DOI: 10.1016/S0032-3861(99)00700-4
Ormondroyd, G. A. (2015). “3- Adhesives for wood composites” in: Wood Composites, M. A. Ansell (ed.), Woodhead Publishing, Sawston, Cambridge, pp. 47-66.
Osmannezhad, S., Faezipour, M., and Ebrahimi, G. (2014). “Effects of GFRP on bending strength of glulam made of poplar (Populus deltoids) and beech (Fagus orientalis),” Constr. Build. Mater. 51, 34-39. DOI: 10.1016/j.conbuildmat.2013.10.035
Raftery, G. M., Harte, A. M., and Rodd, P. D. (2009). “Bonding of FRP materials to wood using thin epoxy gluelines,” Int. J. Adhes. Adhes. 29(5), 580-588. DOI: 10.1016/j.ijadhadh.2009.01.004
Ravindra, R., Krovvidi, K. R., and Khan, A. A. (1998). “Solubility parameter of chitin and chitosan,” Carbohyd. Polym. 36(2-3), 121-127. DOI: 10.1016/S0144-8617(98)00020-4
Verrey, J., Winkler, Y., Michaud, V., and Månson, J. -A. E. (2005). “Interlaminar fracture toughness improvement in composites with hyperbranched polymer modified resin,” Compos. Sci. Technol. 65(10), 1527-1536. DOI: 10.1016/j.compscitech.2005.01.005
Wan, J., Li, C., Bu, Z. -Y., Xu, C. -J., Li, B. -G., and Fan, H. (2012). “A comparative study of epoxy resin cured with a linear diamine and a branched polyamine,” Chem. Eng. J. 188, 160-172. DOI: 10.1016/j.cej.2012.01.134
Wangkheeree, W., and Meekum, U. (2015). “Epoxy adhesive formulations for engineered wood manufacturing: Design of Experiment (DOE) and hardener modification,” in: 31st International Conference of the POLYMER PROCESSING SOCIETY, Jeju Island, Korea.
Article submitted: January 9, 2017; Peer review completed: March 12, 2017; Revised version received: March 13, 2017; Accepted: March 14, 2017; Published: March 20, 2017.
DOI: 10.15376/biores.12.2.3351-3370
