Abstract
Surveys of indigenous weeds in six provinces located in the low northern part of Thailand were undertaken to determine the potential of weed biomass for bio-energy and bio-ethanol. The results reveal that most of the weed samples had low moisture contents and high lower heating values (LHVs). The LHVs at the highest level, ranging from 17.7 to 18.9 Mg/kg, and at the second highest level, ranging from 16.4 to 17.6 Mg/kg, were obtained from 11 and 31 weed species, respectively. It was found that most of the collected weed samples contained high cellulose and low lignin contents. Additionally, an estimate of the theoretical ethanol yields based on the amount of cellulose and hemicellulose in each weed species indicated that a high ethanol yield resulted from weed biomasses with high cellulose and hemicellulose contents. Among the collected weed species, the highest level of ethanol yield, ranging from 478.9 to 548.5 L/ton (substrate), was achieved from 11 weed species. It was demonstrated that most of the collected weed species tested have the potential for thermal conversion and can be used as substrates for ethanol production.
Download PDF
Full Article
Determining the Potential of Inedible Weed Biomass for Bio-Energy and Ethanol Production
Siripong Premjet,a,* Boondarick Pumira,a and Duangporn Premjet b
Surveys of indigenous weeds in six provinces located in the low northern part of Thailand were undertaken to determine the potential of weed biomass for bio-energy and bio-ethanol. The results reveal that most of the weed samples had low moisture contents and high lower heating values (LHVs). The LHVs at the highest level, ranging from 17.7 to 18.9 Mg/kg, and at the second highest level, ranging from 16.4 to 17.6 Mg/kg, were obtained from 11 and 31 weed species, respectively. It was found that most of the collected weed samples contained high cellulose and low lignin contents. Additionally, an estimate of the theoretical ethanol
yields based on the amount of cellulose and hemicellulose in each weed species indicated that a high ethanol yield resulted from weed biomasses with high cellulose and hemicellulose contents. Among the collected weed species, the highest level of ethanol yield, ranging from 478.9 to 548.5 L/ton (substrate), was achieved from 11 weed species. It was demonstrated that most of the collected weed species tested have the potential for thermal conversion and can be used as substrates for ethanol production.
Keywords: Weed; Biomass; Bioenergy; Lower heating values (LHVs); Theoretical ethanol yield
Contact information: a:Department of Biology, Faculty of Science, Naresuan University Pitsanulok, 65000, Thailand; b: Center for Agricultural Biotechnology, Faculty of Agriculture Natural Resources and Environment, Naresuan University Pitsanulok, 65000, Thailand;
* Corresponding author: siripongp@nu.ac.th
INTRODUCTION
The use of fossil energy (e.g., gasoline and diesel) in the transportation sectors results in the emission of greenhouse gases (GHGs) such as carbon dioxide and carbon monoxide, which potentially affect climate change (Tan et al. 2008). There is a high global level of concern regarding several problems related to GHGs emissions, energy security, and reduced energy consumption. This concern is a driving force for the Thai government to find a renewable energy alternative. Consequently, bioethanol has been supported and promoted by the Thai government. Currently two gasoline blends are produced, 10% and 20% (E10 and E20), which are available for gasoline engine cars (Goedecke et al. 2007; Nguyen et al. 2007). In Thailand, sugarcane, cassava starch, and molasses are the main feedstocks used for producing bioethanol (Nguyen and Gheewala 2008a; Nguyen et al. 2008b). Edible food crops, such as sugarcane, sugar beet, maize, sorghum, and wheat have been used as feedstocks for the first generation of bioethanol production on the large scale in the United States, Brazil, European countries, and South America (Charles et al. 2007; Mojović et al. 2009). As a result, the increase in demand for bioethanol has caused a rise in food prices from these crops and the land for cultivation. First-generation biofuels are a hot current topic because using food crops as a main energy source is unsustainable in the long term, as this has the potential to cause competition between food and energy (Tan et al. 2008). To avoid using food crops for the production of biofuels, second-generation bioethanol is being considered as a better alternative; this approach should generate fewer GHG emissions and be more environmentally friendly than the previous generation (Charles et al. 2007). The feedstock used for producing bioethanol is lignocellulosic biomass that mainly consists of cellulose, hemicellulose, and lignin (Tan et al. 2008). The three main sources of lignocellulosic biomass are: 1) agriculture residues (e.g., corn fiber, corn stover, sugarcane, wheat straw, rice hull, and forest residues); 2) lignocellulosic waste from industrial and agricultural processes (e.g., paper mill sludge, municipal solid waste, industrial waste, paper pulp, sawdust, and citrus peel waste); and 3) lignocellulosic perennial crops (e.g., short rotation cops and inedible grasses). The advantages of these feedstocks include their inedibility, low cost, sustainability, and the fact that a wide range of lignocellulose feedstocks are available (Balat et al. 2008; Tan et al. 2008; Maki et al. 2009). Moreover, several types of weed biomass such as Chromolaena odorata,Saccharum spontaneum, Lantana camara, and Prosopis juliflora, which are common in Thailand, have been considered for use as feedstocks for bioethanol production (Chandel and Singh 2011). This indicates that any biomass has a high potential as a substrate for bioethanol, but other considerations must be considered for final usage, such as carbohydrate availability, availability of substrate, and competition with other applications. However, research on the composition of inedible grasses for future bioenergy application has been limited because most of the research was focused on evaluating the composition of biomass from agricultural residues and woody plants.
Thailand is a tropical country in southeast Asia that has a rich diversity of both valuable plants and presumably non-valuable weeds (Zimdahl 1993). Previous researchers have observed and recorded common weeds found in Thailand, such as Barleria strigosa, Chloris barbata, and Mimosa pudica (Radanachaless and Maxwell 1997). Weeds that are annual and perennial plants can be observed in any area and in every season in Thailand. Although it can be observed that weeds are a kind of plant that can produce a large amount of biomass, no data has ever been collected on weed biomass in Thailand with respect to use as a source of renewable energy. Data for estimating of weed biomass yields are essential for predicting ethanol yields, calculating conversion efficiency, and conducting economic analyses. Therefore, the objectives of this research are to assess the amount of each lignocellulosic component (i.e., cellulose, hemicellulose, lignin, and ash), and to estimate the theoretical ethanol yields based on the amount of cellulose and hemicellulose in weed species found in Thailand.
EXPERIMENTAL
Materials
Field experiments were carried out for two consecutive dry seasons in the following provinces; Phisanulok (latitude 16°49´N, longitude 100°16´E), Phichit (latitude 16°26´N, longitude 100°21´E), and Nakornsawan (latitude 15°42´N, longitude 100°4´E), from November 2008 to February 2009. The next three provinces were Tak (latitude 16°52´N, longitude 99°7´E), Uttaradit (latitude 17°37´N, longitude 100°6´E), and Sukhothai (latitude 17°0´N, longitude 99°45´E). The survey was conducted from November 2009 to February 2010. Surveys of weed vegetation were performed on five different area types in 162 villages within 6 provinces (27 villages per province) located in the lower northern part of Thailand. Of the 162 different areas surveyed, 101 were vacant fields, 27 were paddy fields, 13 were roadside areas, 12 were residential areas, and 9 were gardens. The experiment was performed in a randomized complete block design. Three quadrants (1 x 1 m2) were randomly designated in each sampling site. On site, weeds as high or higher than 0.5 m inside of each quadrant were counted, recorded, up-rooted, cleaned, collected, and tagged.
Identification
The weed samples collected from the field studies were classified and identified on the basis of plant taxonomy, morphological features, including flowers, with the help of suitable literature sources (Harada et al. 1987; Noda et al. 1994; Radanachaless and Maxwell 1997) and the aid of plant taxonomists at the Department of Biology, Faculty of Science, Narasuan University.
Sample Preparation
The fresh weight of the weed samples obtained from all quadrants were measured and separated into narrow leaves and broad leaves. All weed samples were chopped and air dried under a shaded area for a week. Subsequently, the samples were ground with a wood milling machine into a weed meal, which was sifted through a 1.0 µm sieve. To determine the moisture content, 1.0 g of each sample meal was dried in a hot air oven at 105°C until constant weight was achieved.
Determination of Composition of Weed Biomass
Cellulose, hemicellulose, lignin, and ash contents of the above ground weed biomass samples were determined by the Animal Nutrition Research and Development Center, Nakhornratchasima, using a Forage fiber analysis or the Detergent method (Goering and Van Soest, 1970). The experiment was duplicated, and the data were expressed as mean values.
Determination of Lower Heating Value
The lower heating value content all of weed samples was determined by the bomb calorimetric method according to standard method DIN 51900-3 (1997). The heat capacity of the calorimeter was determined by using benzoic acid as a reference substance. The unit of lower heating value was expressed as MJ/kg dry basis. The experiment was duplicated and the data were expressed as mean values.
Estimation of Theoretical Ethanol Yields from Each Weed
For the conversion of lignocelluloses to ethanol, cellulose and hemicellulose that are composed of long chain of hexose for cellulose and pentose for hemicellulose have to be hydrolyzed in order to release monomer sugar or fermentable sugar (Kumar et al. 2009). The theoretical ethanol yields from each weed were calculated by the following equations that were developed by Vogelet al. (2011). The theoretical hexose (HEXT) and pentose (PENT) sugar yield from biomass were achieved by dilute sulfuric acid hydrolysis and assuming 100% conversion. Therefore, the amount of cellulose and hemicellulose of each weed species were used to represent hexose and pentose sugar for the calculation.
HEXT = b x Yh x HEX Kg/Ton (substrate) (1)
PENT = b x Yp x PEN Kg/Ton (2)
Theoretical ethanol yields from hexose (HEXTEL) and pentose (PENTEL) sugar from simultaneous saccharification and fermentation assuming 100% fermentation yield were obtained from,
HEXTEL = HEXT x Ye.h Kg/Ton (3)
PENTEL = PENTEL x Ye.p Kg/Ton (4)
ETOHTLT = (HEXTEL + PENTEL)/d L/Ton (5)
where HEX is the theoretical hexose (D-glucose) yield upon saccharification from hexosan (C6 = 1.111), PEN is the theoretical pentose (D-xylose) yield upon saccharification from pentosan (C5= 1.136), b is the weight of substrate (1,000 kg), Yh is the fraction of hexosan (% cellulose in sample), Yp is the fraction of pentosan (% hemicellulose in sample), Ye.h is the theoretical ethanol yield from hexose (D-glucose) (0.511), Ye.p is the theoretical ethanol yield from pentose (D-xylose) (0.511), and d is the ethanol density (0.789 kg/L).
Data Analysis
The determinations of cellulose, hemicellulose, lignin, ash, lower heating value (LHV), and moisture content of the weed samples were conducted at least two times for each test sample. The data were expressed in terms of statistical mean and standard deviation, as shown in Table 1. Each weed species was divided and placed into 5 different levels (highest-level, high-level, moderate-level, low-level, and lowest-level) by using class intervals ([maximum–minimum]/5) of the amounts of cellulose, hemicellulose, lignin, ash, LHV, moisture content, and ethanol yields. The range of each level is shown in Table 2.
The significant variations between groups of data sets were determined by one-way analysis of variance (ANOVA) by using SPSS Version 11. Means were considered significant at P 0.05, as shown in Table 2.
RESULTS AND DISCUSSION
Amount of Weed Flora
Surveys of weeds were conducted within six provinces located in the low northern part of Thailand. A total of 162 different areas consisting of 101 vacant fields, 27 paddy fields, 13 roadside areas, 12 residential areas, and nine gardens were surveyed. The distribution of weeds in each province varied. A total of 62 genera containing 74 known species and three unknown species belonging to 25 families were collected from different natural sites (Table 1). The results showed that the major families of collected weed samples were Gramineae and Compositae with 12 genera each, followed by Leguminosae:Papilionoideae with seven genera, Amaranthaceae with six genera, and Malvaceae with five genera. The families of Cyperaceae, Euphorbiaceae, andLeguminosae:Mimosoideae contained four genera each. The family Capparaceae contained only three genera. Only two genera each were observed in the families Acanthaceae, Labiatae, Leguminosae:Caesalpinioideae, Scrophulariaceae, Sterculiaceae, and Verbenaceae. Finally, only one genus was observed in the families Boraginaceae, Moraceae, Nyctaginaceae, Onagraceae, Pedaliaceae, Solanaceae, Tiliaceae, and Typhaceae. The observations revealed that 22.08% (17 species) and 77.92% (60 species) of weeds were narrowleaf and broadleaf weed, respectively. Of the collected weeds, 35.06% (27 species) were perennial and 64.94% (50 species) were annual plants. Six species of weeds (Imperata cylindrical, Pennisetum polystachyon, Rottboellia cochinchinensis, Sorghum halepense, Mimosa pigra, and
Leucaena leucocephala) were invasive weeds and five species (Ageratum conyzoides, Rottboellia cochinchinensis, Hyptis suaveolens, Mimosa invisa and Typha angustifolia) were on the list of the noxious weeds of Thailand (Zungsontiporn 2006). Additionally, Amaranthus spinosus, Imperata cylindrical and Sorghum halepense were on the list of the world’s noxious weeds (Holm 1969; Anderson 1983). Most of the collected weeds from the low northern part of Thailand are common weeds that had been previously recorded and observed in Thailand (Holm 1969; Haradaet al. 1987; Noda et al. 1994; Radanachaless and Maxwell 1997).
Determination of Moisture Content of Weed Biomass
The results showed that 13, 34, and 18 weed species presented moisture contents at moderate-level (9.2±0.5%), low-level (7.8±0.4%) and the lowest-level (6.5±0.4%), respectively, as shown in Table 2. The analysis of variance of moisture content is summarized in Table 2. However, the lowest moisture content (5.6±0.1%) was derived from Sorghum halepense (Table 1). The high-level (10.5±0.5%) and the highest-level (12.0±0.5%) of moisture contents occurred in seven and five weed species, respectively (Table 2). The greatest moisture content was found in Hibiscus sabdariffa (12.8±0.0%) followed by Heliotropium indicum(12.1±0.0%). The moisture contents of 64 collected samples were less than 10%, ranging from 5.6±0.1 to 9.9±0.1%. However, another 13 weed species contained moisture contents ranging from 10.0±0.0 to 12.8±0.0% (Table 1). The moisture content of the biomass was dependent on plant species, weather, harvesting and processing conditions (McKendry 2002a). The moisture content of the biomass is a factor to determine if the biomass should be used in direct combustion or in co-firing with other fuels (Sheng and Azevedo 2005). High moisture content in lignocellulose affects its combustion efficiency. Our results indicated that most collected weed samples had moisture contents of less than 10%. Biomass with moisture content less than 50% is suitable as a combustion fuel (McKendry 2002b; Ogden et al. 2010).
Determination of Lower Heating Value (LHV) of Weed Biomass
The interval value of LHV was 1.2, and analysis of variance results for LHV are summarized in Table 2. The LHVs of collected weed specimens were compared to those of various lignocellulosic biomasses being used as combustion fuels in Thailand (Biomass Clearing House 2008) and to those in the literature (McKendry 2002a). The results showed that 25 weed samples yielded LHVs at moderate-levels (15.8±0.4 MJ/kg), which were similar to those of sugarcane leaves, and 31 weed samples yielded LHVs at high-levels (16.9±0.4 MJ/kg), which were equal to those of observed from palm shell. However, the highest-level of LHV (18.0±0.3 MJ/kg), which was equivalent to those of wheat straw, was observed for 11 weed species (Table 2). Among the collected weed samples, Aeschynomene americana gave the maximum LHV (18.5±0.1 MJ/kg), followed by Sphaeranthus senegalensis(18.4±0.2 MJ/kg), Crotalaria striata (18.2±0.3 MJ/kg), and Crotalaria goreensis (18.1±0.0 MJ/kg), which was similar to those observed from wood. Furthermore, low-level (14.4±0.5 MJ/kg) and the lowest-level (12.7±0.3 MJ/kg) LHVs were obtained from seven and two weed samples, respectively (Table 1). The LHVs of Synedrella sp. (12.8±0.4MJ/kg) and Physalis minima(12.5±0.2MJ/kg) were comparable to those obtained from rice husks. However, it was determined that 67 collected weed species gave LHVs greater than 15 MJ/kg, ranging from 15.2±0.1 to 18.5±0.1 MJ/kg (Table 1). The variation in LHVs of biomass resulted from the different chemical compositions of each plant species (Senelwa and Sims 1999). It was reported that the amount of lignin in lignocellulosic materials is highly correlated with heating values (Demirbaş 2001). However, our study did not find a correlation between the LHV and the lignin content (Fig. 1). Our results demonstrated that the LHV of most weed biomasses were greater than those of various types of biomasses that have been used as combustion fuels in Thailand (Biomass Clearing House 2008). These results indicated that most of the dry weed biomasses in Thailand exhibit low moisture contents and high LHVs; these raw material attributes are highly desirable for producing fuel briquettes.
Determination of Ash, Lignin, Hemicelluloses, and Cellulose Content in Weed Biomass
The chemical composition of all collected weed species is presented in Table 1. The amount of cellulose (%), hemicelluloses (%), lignin (%), and ash (%) of weed species was divided into five groups, highest-level, high-level, moderate-level, low-level, and lowest-level, by using class intervals (Table 2). The interval values of ash, lignin, hemicelluloses, and cellulose were 4.3, 3.1, 6.1, and 7.9, respectively. Additionally, an analysis of variance of the amount of ash, lignin, hemicelluloses, and cellulose of weed lignocellulose in each weed sample were significant (P 0.05) when compared to various types of lignocellulosic biomass from common agricultural wastes, residues, and several weeds reported in the literature (Balat and Balat 2009; Abbasi and Abbasi 2010; Chandel and Singh 2011).
The present results revealed that the amount of ash from most weed samples was at the low-level (9.00±1.3%) or the lowest-level (5.0±1.10), which were derived from 31 and 21 weed species, respectively (Table 2). The minimum amount of ash content was obtained from Leucaena leucocephala (2.60±0.6%) followed by Clerodendrum sp. (2.6±0.4%) and Phyllanthus reticulates(3.62±0.3%) (Table 1). Moreover, it was found that 21 weed samples gave a moderate-level of ash (13.0±1.3%). The highest-level of ash (23.8±0.2%) was observed only from Synedrella sp.; however, the high-level was obtained from three weed species, Boerhavia diffusa (15.9±0.1%), Amaranthus viridis (16.9±0.2%) and Blumea napifolia (17.5±0.0%), as shown in Table 1. Ash is a solid inorganic residue that occurs from burning lignocellulosic biomass in the air. Consequently, a high amount of ash in biomass causes an increase in the cost of energy conversion, handling, and processing (McKendry 2002a) and lowers the efficiency of its combustion (Sarenbo 2009). The results indicated the amount of ash in most collected weed species ranged from the low-level to the lowest-level.
The lowest-level of lignin content out of 25 weed samples was 6.2 ± 1.0%. The lowest amounts of lignin contents were observed from Echinochloa crus-galli (4.6 ± 0.0 %), followed by Cyperus compactus (4.6 ± 0.5%) and Cyperus imbricatus (4.7 ± 0.3%), which were slightly greater than Eichlornia crassipes that was reported by (Chandel and Singh 2011). Low-levels of lignin content (9.3 ± 1.0%) were found in 27 weed samples. However, 17 weed samples showed moderate levels of lignin content (12.1±0.9%), similar to those of switch grass (12.00%). However, lignin content at the high-level (15.7 ± 0.6%), which equals those obtained from barley straw and corn cob, was derived from five weed species. Amounts of lignin content of Leucaena leucocephala (16.1±0.1) and Chloris barbata (16.0±0.3) were similar to that of
Eupatorium adenophorum (Chandel and Singh 2011). The highest-level of lignin content (19.6 ± 0.6%) is similar to those of oat straw and rye straw. Among the collected weed species only Mimosa invisa had the highest lignin content (20.41±0.24%), comparable to those of sorted refuses. However, it was obvious that the lignin content of 46 weed species (Table 1) ranged from 5.0±0.0 to 10.1±0.1%, similarly to those of waste papers from chemical pulps. These results indicated that the amount of lignin content in most weed species was less than several weed species (L. camara, P. juliflora, and S. spontaneum) and various types of lignocellulosic biomasses, which were reported in the literature (Balat and Balat 2009; Abbasi and Abbasi 2010; Chandel and Singh 2011).
The low-level (12.3±20%) and lowest-level (7.5±1.8%) amounts of hemicellulose were produced by 33 and 14 weed species, respectively. Hemicellulose at the moderate-level (17.6 ±1.5%), similar to that of black locust, was provided by 13 weed samples (Table 2). The amount of hemicellulose at the high-level (24.5±1.1%) was equal to that obtained from rice straw. However, it was observed that amounts of hemicellulose from seven weeds species (Celosia argentea, Cyathula prostrate, Typha angustifolia, Ludwigia hyssopifolia, Hyptis capitata, Ruellia tuberosa, and Clerodendrum sp.) were equal to those obtained from L. camara (Chandel and Singh 2011). The other two species, Pentapetes phoenicea and Dicliptera roxburghiana were similar to those of Miscanthus spp. and alfalfa (Chandel and Singh 2011). The amount of hemicellulose at the highest-level (30.5±1.6%), which is similar to that of rye straw, was derived from three weed species, Amaranthus viridis (34.2±0.0%), Cyperus imbricatus (32.3±0.3%), and Cenchrus echinatus (31.8±0.4%). Among the collected weed, A. viridis provided the greatest amount of hemicelluloses (Table 1). However, amount of hemicelluloses is still lower than E. crassipes.Moreover, it was observed that the amounts of hemicellulose from Cleome viscosa (4.7±0.1) and Acalypha indica (3.3± 0.1) were greater than that from solid cattle manure which was reported in the literature (Balat and Balat 2009; Abbasi and Abbasi 2010).
The amount of cellulose at the moderate-level (36.3±2.1%) was observed from 34 weed species, equivalent to those of oat straw, birch, willow, Miscanthus spp., and crofton weed stem. Sixteen weed specimens presented cellulose contents at the low-level (27.9±2.6%), which was comparable to those of nut shells, swine waste, and switch grass. The lowest-level of cellulose content (18.8±2.7) was obtained from seven weed specimens and was equal to those obtained from leaves and E. crassipes. The cellulose
content at the high-level (42.5±2.1%) was obtained from 13 weed species; this value was comparable to bamboo, pine, hemlocks, andseveral weeds (Chromolaena odorata, S. spontaneum, L. camara and P. juliflora). Seven weed species had the highest-level of cellulose content (51.8±3.0%), equaling those attained from the bast fibers of jute and hardwood. Additionally, among the collected weeds species, Sida acuta had the highest cellulose content (56.0±0.3%) followed by Leucaena leucocephala(55.2±0.0%) and Achyranthes aspera (53.7±0.1%); these values were greater than several weeds that were reported in the literature (Chandel and Singh 2011). On the other hand, the lowest cellulose contents (Table 1) were observed for Chloris barbata (16.06±0.0%), Ruellia tuberose (16.22±0.1%), and Broussonetia papyrifera (16.06±0.2%) which were equals to those obtained from rye grass. No correlation between cellulose and hemicelluloses, cellulose and lignin, or lignin and hemicelluloses were found in this study, as shown in Figs. 2 to 4. The present results revealed that the amounts of cellulose, hemicelluloses, lignin, and ash were variable for the weed species examined. The diversity of the composition of the biomasses was dependent on the plant species, soil nutrients, climate, and competition (McKendry 2002a). In terms of bioethanol production from biomass materials, the amount of cellulose and lignin are of the most interest. The proportion of cellulose and lignin contents in biomass is one of the factors for selecting the appropriate plant biomass. Moreover, the maximum theoretical ethanol yield was provided by a biomass that had higher cellulose and lower lignin content since it is the cellulose that is hydrolyzed into glucose by acids and enzymes (McKendry 2002a). The fermentation process converts monosaccharides into ethanol, with the highest ethanol yields obtained from glucose. Lignin is a highly complex biopolymer composed of phenylpropane monomer units and is found in the plant’s cell wall; this non-cellulosic material acts as an inhibitor in enzymatic hydrolysis (Adler et al. 2006; Binod et al. 2010; Hamelinck et al. 2005). Hemicellulose is composed of a mixture of monosaccharides, such as xylose and other five-carbon sugars, which can be removed during the pretreatment process to improve enzyme hydrolysis (Taherzadeh and Karimi 2008). Additionally, they are decomposed into furfurals, which inhibit the fermentation process (Zhu and Pan 2010).
Estimation of Theoretical Ethanol Yields from Each Weed
The total theoretical ethanol yields from cellulose and hemicellulose (ETOHTLT) are shown in Table 1. The determination of ethanol yields from all collected weeds samples are summarized in Table 1, and the analysis of variance of theoretical ethanol yields are summarized in Table 2. Moreover, the theoretical ethanol yields from these weeds are compared to theoretical ethanol yields from other biomass materials reported in the Alternative Fuels Data Center (AFDC) report (AFDC 2012)
In this study, the lowest-level of ethanol yields (239.2±21.8 L/ton) were estimated from 11 weed samples, which was equivalent to those of cotton gin trash, but the lowest yield (200±2.2 L/ton) was calculated from Ageratum conyzoides. Twenty weed samples are predicted to yield ethanol at the low-level (319.8±19.5 L/ton), which were slightly higher than those of forest thinnings. However, it was found that ethanol yield of S. indica (307.4±4.6 L/ton), H. capitata (306.0±4.6 L/ton), and S. senegalensis (304.2±3.4 L/ton) (Table 1) were comparable to those of forest thinnings. A moderate-level of ethanol yield (374.2±18.9 L/ton) which was slightly lower than those obtained from hardwood sawdust, was predicted for 22 weed samples. It was observed that the ethanol yield of C. odoratum (382.2±1.6 L/ton) was similar to that of hardwood sawdust.
In addition, ethanol yields at the high-level (455.9±16.1 L/ton) were expected from 13 weed samples. This value was greater than those obtained from corn grain, corn stover, rice straw, bagasse, and mixed paper. However, only 11 weed species are forecasted to yield ethanol at the highest-level (504.3±20.1 L/ton).
Fig. 1. Correlation between amounts of lignin content and LHV
Fig. 2. Correlation between amounts of cellulose and hemicellulose content
Fig. 3. Correlation between amounts of cellulose and lignin content
Fig. 4. Correlation between amounts of lignin and hemicellulose content
Among the collected weed species (Table 1), the maximum ethanol yield was envisaged for Imperata cylindrical (548.4±1.4 L/ton) followed by A. viridis (521.0±0.9 L/ton), S. acuta (520.3±5.4 L/ton), R. cochinchinensis (509.7±8.1L/ton), S. halepense(508.8±2.6 L/ton), E. amabilis (502.9±4.7 L/ton), C. imbricatus(493.6±3.2 L/ton), C. echinatus (491.4±7.4 L/ton), C. prostrata(484.8±4.4 L/ton), E. procera (483.2±1.1 L/ton), and B. mutica(482.8±6.7 L/ton); these ethanol yields are greater than various types of lignocellulosic biomasses that have been reported in the literature (AFDC 2012).
The quantitative estimation revealed the direct potential of each weed species for bioethanol production. The theoretical ethanol yields indicated that high ethanol yields were derived from a high amount of both cellulose and hemicellulose. Thus, the ethanol yield gradually increased when the cellulose and hemicellulose content in the weed samples was enhanced, as shown in Table 1. The results indicated that several weed species in Thailand could be used as a potential biomass source for bioethanol production. Moreover, the utilization of weed biomass as an alternative fuel source will provide the opportunity to overcome barriers for improving low-cost ethanol production from weed biomass in the near future. On the other hand, using weeds biomass as a substrate for producing bio-ethanol will help to control invasive weeds in agriculture areas, which will also increase the lands agricultural productivity. Presently, we are investigating the optimum pretreatment processes to achieve high sugar yields from four candidate weed species that had high cellulose contents.
CONCLUSIONS
- From the present results, a total of 62 genera containing 74 known and three unknown species from 25 families were collected from various natural sources.
- All dry weed biomasses had relatively low moisture contents and high LHVs. Among the collected weed samples, Aeschynomene americana gave the maximum LHV (18.5±0.1 MJ/kg) followed by Sphaeranthus senegalensis (18.4±0.2 MJ/kg),
Crotalaria striata (18.2±0.3MJ/kg), and Crotalaria goreensis(18.1±0.0 MJ/kg). - By determining the composition of weed biomasses, it was found that most of the
collected weed samples produced high cellulose and low lignin contents. Among the collected weeds species, Sida acuta produced the greatest cellulose content (56.0±0.3%) followed by Leucaena leucocephala (55.2±0.0%) and Achyranthes aspera (53.7±0.1%). - The theoretical ethanol yields indicated that ethanol yields gradually improved when the cellulose and hemicellulose content in weed samples was greater. Various
ethanol yields were calculated, which ranged from 200.1±2.2 L/ton to 521.0±0.9 L/ton, from the collected weed samples. The highest ethanol yield (L/ton) was
predicted from I. cylindrical (548.4±1.4) followed by A. viridis (521.0±0.9), S. acuta (520.3±5.4), R. cochinchinensis(509.7±8.1), S. halepense (508.8±2.6), E. amabilis(502.9±4.7), C. imbricatus (493.6±3.2), C. echinatus (491.4±7.4), C. prostrata (484.8±4.4), E. procera(483.2±1.1), and B. mutica (482.8±6.7). - The results indicated that most of the collected weed species had the potential for thermal conversion and could be used as substrates for ethanol production.
ACKNOWLEDGMENTS
This research was funded by the National Research Council of Thailand in the fiscal year 2009-2010 (projects numbers SC-AR-070/2551 and SC-AR-034/2552). The authors express their gratitude to Dr. Pranee Nang-Ngam, Department of Biology,
Faculty of Science, Naresuan University for identification of weed species. The first author was grateful for the financial support from Naresuan University to conduct short term research about biomass in Japan (2012).
Table 1. Composition and Theoretical Ethanol Yields of Weeds Biomass Collected in the Lower Northern Part of Thailand
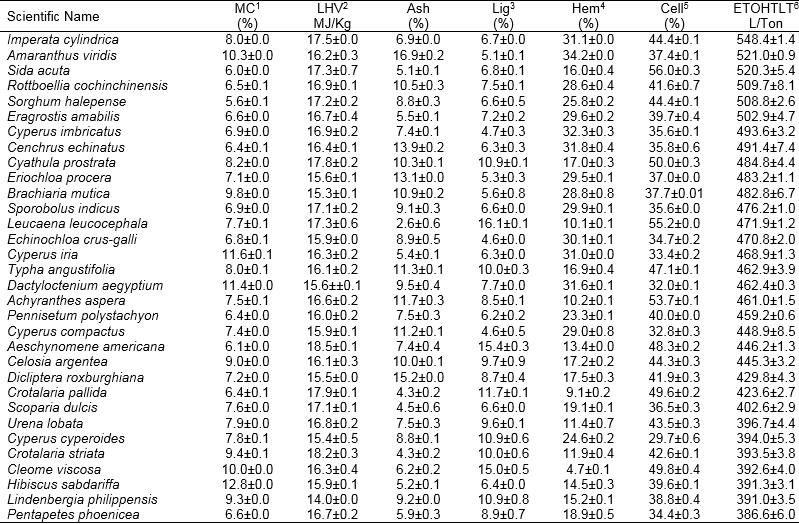
Values represent the mean, n=2, ± SD, 1 = moisture content, 2 = Lower heating value, 3 = Lignin, 4 = Hemicellulose, 5 = Cellulose, 6 = Theoretical ethanol yield.
Table 1. Continued
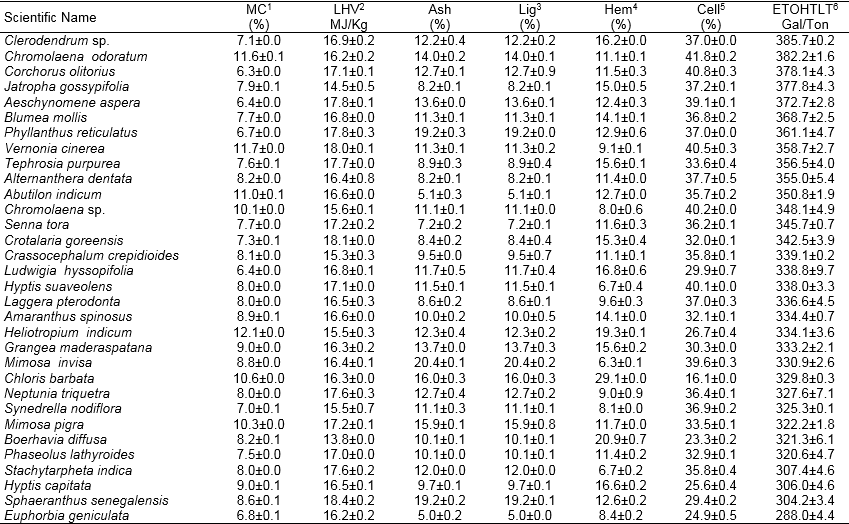
Values represent the mean, n=2, ± SD, 1 = moisture content, 2 = Lower heating value, 3 = Lignin, 4 = Hemicellulose, 5 = Cellulose, 6 = Theoretical ethanol yield.
Table 1. Continued
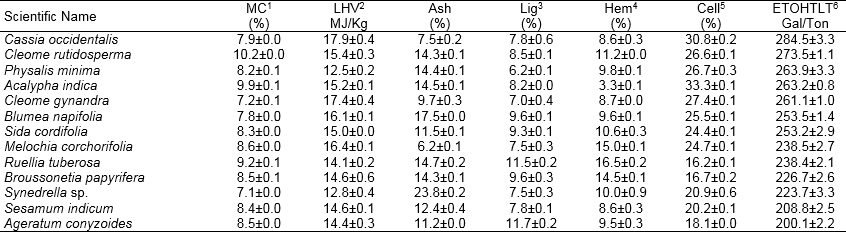
Values represent the mean, n=2, ± SD, 1 = moisture content, 2 = Lower heating value, 3 = Lignin, 4 = Hemicellulose, 5 = Cellulose, 6 = Theoretical ethanol yield.
Table 2. Amounts of Cellulose, Hemicellulose, Lignin, and Ash in Weeds from the Low Northern Area of Thailand, Their Moisture, Lower Heating Values (LHVs), and the Ethanol Yield of Weed Biomass
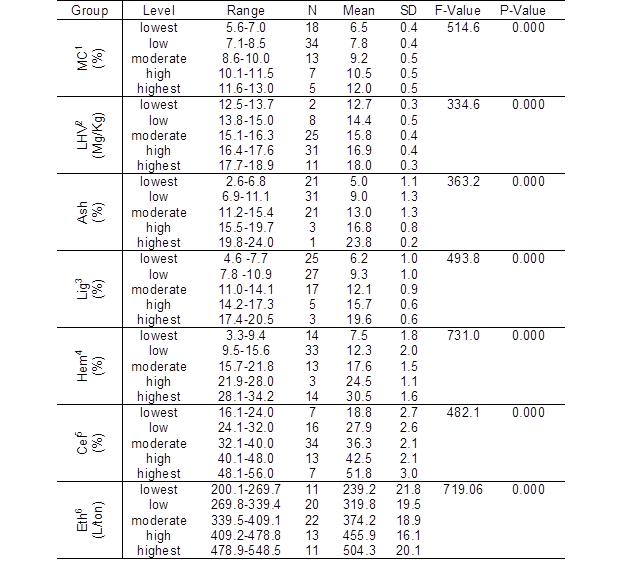
N = number of weed species (Total = 77), SD = standard deviation, P-Value 0.05,1= moisture content, 2 = lower heating value, 3 = lignin, 4 = hemicellulose,5 = cellulose, and 6 = ethanol yield.
REFERENCES CITED
Abbasi, T., and Abbasi, S. A. (2010). “Biomass energy and the environmental impacts associated with its production and utilization,” Renew Sust Energ Rev. 14(3), 919-937.
Adler, P. R., Sanderson, M. A., Boateng, A. A., Weimer, P. J., and Jung, H. J. G. (2006). “Biomass yield and biofuel quality of switchgrass harvested in fall or spring,” Agron. J. 98(6), 1518-1525.
Alternative Fuels Data Center. (2012). “Cellulosic ethanol feedstocks,” (http://www.afdc.energy.gov/fuels/ethanol_feedstocks.html), Access on September 25, 2012.
Anderson, W. P. (1983). Weeds Science; Principles, West Publishing Company, New York.
Balat, M., and Balat, H. (2009). “Recent trends in global production and utilization of bio-ethanol fuel,” Appl Energ. 86(11), 2273-2282.
Balat, M., Balat, H., and Öz, C. (2008). “Progress in bioethanol processing,” Prog Energ Combust. 34(5), 551-573.
Binod, P., Sindhu, R., Singhania, R. R., Vikram, S., Devi, L., Nagalakshmi, S., Kurien, N., Sukumaran, R. K., and Pandey, A. (2010). “Bioethanol production from rice straw: An overview,” Bioresource Technol. 101(13), 4767-4774.
Biomass Clearing House. (2008). Biomass, W. Kaewboonsong and P. Chira-adisai (Translator), Biomass Clearing House, Energy for Environment Foundation (E for E) Bangkok, Thailand.
Chandel, A., and Singh, O. (2011). “Weedy lignocellulosic feedstock and microbial metabolic engineering: Advancing the generation of biofuel,” Appl. Microbiol. Biotechnol. 89(5), 1289-1303.
Charles, M. B., Ryan, R., Ryan, N., and Oloruntoba, R. (2007). “Public policy and biofuels: The way forward?” Energ Policy. 35(11), 5737-5746.
Demirbaş, A. (2001). “Relationships between lignin contents and heating values of biomass,” Energ Convers Manage. 42(2), 183-188.
DIN 51900-3. (1997). “Testing of solid and liquid fuels. Determination of gross caloric value by the bomb calorimeter and calculation of net value; method with the adiabatic jacket.”
Goedecke, M., Therdthianwong, S., and Gheewala, S. H. (2007). “Life cycle cost analysis of alternative vehicles and fuels in Thailand,” Energ Policy. 35(6), 3236-3246.
Goering, H. K., and Van Soest, P. J. (1970). “Forage fiber analyses (apparatus, reagent, procedures, and some applications),” In: Agriculture Handbook No. 379, United States Department of Agriculture Research Service, Washington, D.C.
Hamelinck, C. N., Hooijdonk, G. V., and Faaij, A. P. C. (2005). “Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term,” Biomass Bioenerg. 28(4), 384-410.
Harada, J., Paisooksantivatana, Y., and Zungsontiporn, S. (1987). Weeds in the Highlands of Northern Thailand: Color Illustrated, National Weed Science Research Institute Project, c/o Botany and Weed Science Division, Department of Agriculture. Bangkok, Thailand.
Holm, L. (1969) “Weed problems in developing countries,” Weed Sci.17(1), 113-118.
Kumar, P., Barrett, D. M., Delwiche, M. J., and Stroeve, P. (2009). “Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production,” Ind. Eng. Chem. Res. 48(8), 3713-3729.
Maki, M., Leung, K. T., and Qin, W. (2009). “The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass,” Int. J. Biol. Sci. 5(5), 500-516.
McKendry, P. (2002a). “Energy production from biomass (part 1): Overview of biomass,” Bioresource Technol. 83(1), 37-46.
McKendry, P. (2002b). “Energy production from biomass (part 2): Conversion technologies,” Bioresource Technol. 83(1), 47-54.
Mojović, L., Pejin, D., Grujić, O., Markov, S., Pejin, J., Rakin, M., Vukašinović, M., Nikolić, S., and Savić, D. (2009). “Progress in the production of bioethanol on starch-based feedstocks,” Chem. Ind. Chem. Eng. Q. 15(4), 211-226.
Nguyen, T. L. T., and Gheewala, S. H. (2008a). “Fossil energy, environmental and cost performance of ethanol in Thailand,” J. Clean Prod. 16(16), 1814-1821.
Nguyen, T. L. T., Gheewala, S. H., and Garivait, S. (2007). “Fossil energy savings and GHG mitigation potentials of ethanol as a gasoline substitute in Thailand,” Energy Policy 35(10), 5195-5205.
Nguyen, T. L. T., Gheewala, S. H., and Garivait, S. (2008b). “Full chain energy analysis of fuel ethanol from cane molasses in Thailand,” Appl Energ. 85(8), 722-734.
Noda, K., Teerawatsakul, M., Prakongvongs, C., and Chaiwiratnukul, L. (1994). Major Weeds in Thailand: Illustrated by Color, (3ed.), National Weed Science Research Institute Project, c/o Botany and Weed Science Division, Department of Agriculture, Bangkhen, Bangkok, Thailand.
Ogden, C. A., Ileleji, K. E., Johnson, K. D., and Wang, Q. (2010). “In-field direct combustion fuel property changes of switch grass harvested from summer to fall,” Fuel Process Technol. 91(3), 266-271.
Radanachaless, T., and Maxwell, J. F. (1997). List of Weeds Reported in Thailand, (2nd Ed,), Work Press, Thailand.
Sarenbo, S. (2009). “Wood ash dilemma-reduced quality due to poor combustion performance,” Biomass Bioenerg. 33(9), 1212-1220.
Senelwa, K., and Sims, R. E. H. (1999). “Fuel characteristics of short rotation forest biomass,” Biomass Bioenerg. 17(2), 127-140.
Sheng, C., and Azevedo, J. L. T. (2005). “Estimating the higher heating value of biomass fuels from basic analysis data,” Biomass Bioenerg. 28(5), 499-507.
Taherzadeh, M., and Karimi, K. (2008). “Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A Review,” Int. J. Mol. Sci. 9(9), 1621-1651.
Tan, K. T., Lee, K. T., and Mohamed, A. R. (2008). “Role of energy policy in renewable energy accomplishment: The case of second-generation bioethanol,” Energy Policy 36(9), 3360-3365.
Vogel, K., Dien, B., Jung, H., Casler, M., Masterson, S., and Mitchell, R. (2011). “Quantifying actual and theoretical ethanol yields for switchgrass strains using NIRS analyses,” BioEnergy Research. 4(2), 96-110.
Zhu, J. Y., and Pan, X. J. (2010). “Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation,” Bioresource Technol. 101(13), 4992-5002.
Zimdahl, R. L. (1993). Fundamentals of Weed Science, Academic Press, San Diego.
Zungsontiporn, S. (2006). “Global invasive plants in Thailand and its status and a case study of Hydrocotyle umbellata L.,” Proceedings of International Workshop on Development of Database (APA5D) for Biological Invasion, Taiwan.
Article submitted: September 29, 2012; Peer review completed: November 15, 2012; Revised version received and accepted: Dec. 12, 2012; Published: Dec. 14, 2012.
