Abstract
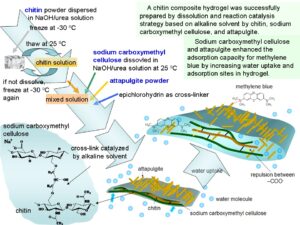
As the most abundant natural amino polysaccharide, chitin remains among the least exploited polymers due to its poor solubility, which restricts its research and utilization. In this study, a new chitin composite hydrogel was prepared by a mild process at 25 °C within a short time. To enhance the adsorption capacity, sodium carboxymethyl cellulose and attapulgite were introduced into the structure of a hydrogel by chemical reaction and physical interaction, respectively. Alkaline solution was used as a solvent to dissolve chitin and used as a catalyst to accelerate the cross-linking reaction between chitin and sodium carboxymethyl cellulose. This solvent (8 wt% NaOH/6 wt% urea solution) has dual functions: to dissolve chitin and to accelerate the cross-linking reaction of chitin with carboxymethyl cellulose by epichlorohydrin. The cross-linking reaction occurred at room temperature (25 °C) within a short time (4 h). Fourier transform infrared spectroscopy (FTIR) indicated that chitin and sodium carboxymethyl cellulose has been successfully cross-linked. X-ray diffraction results showed that the cross-linked structure was amorphous and that attapulgite kept its crystal structure in the hydrogel. Scanning electron microscopy showed the coarse surface of composite hydrogel with attapulgite. The adsorption capacity for methylene blue reached 167 mg g-1.
Download PDF
Full Article
Dissolution and Reaction Catalysis Strategy Using Alkaline Solvent for Mild Fabrication of Chitin Composite Hydrogel for Dye Adsorption
Xiaoyu Chen,* and Zhen Mao
As the most abundant natural amino polysaccharide, chitin remains among the least exploited polymers due to its poor solubility, which restricts its research and utilization. In this study, a new chitin composite hydrogel was prepared by a mild process at 25 °C within a short time. To enhance the adsorption capacity, sodium carboxymethyl cellulose and attapulgite were introduced into the structure of a hydrogel by chemical reaction and physical interaction, respectively. Alkaline solution was used as a solvent to dissolve chitin and used as a catalyst to accelerate the cross-linking reaction between chitin and sodium carboxymethyl cellulose. This solvent (8 wt% NaOH/6 wt% urea solution) has dual functions: to dissolve chitin and to accelerate the cross-linking reaction of chitin with carboxymethyl cellulose by epichlorohydrin. The cross-linking reaction occurred at room temperature (25 °C) within a short time (4 h). Fourier transform infrared spectroscopy (FTIR) indicated that chitin and sodium carboxymethyl cellulose has been successfully cross-linked. X-ray diffraction results showed that the cross-linked structure was amorphous and that attapulgite kept its crystal structure in the hydrogel. Scanning electron microscopy showed the coarse surface of composite hydrogel with attapulgite. The adsorption capacity for methylene blue reached 167 mg g-1.
DOI: 10.15376/biores.18.1.291-301
Keywords: Chitin; Sodium carboxymethyl cellulose; Hydrogel; Attapulgite; Adsorption; Composite hydrogel
Contact information: School of Material Engineering, Jinling Institute of Technology, Nanjing 211169, China; *Corresponding author: chxy@jit.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
As the most abundant amino polysaccharide, chitin exists in the shells of many animals, such as shrimp, crab, and insects (Duan et al. 2018). Chitin is nontoxic, easy to degrade (Ma et al. 2020), and renewable, and it has good adsorption ability for dye or heavy metal ions (Liao and Huang 2019; Mabel et al. 2019; Nguyen et al. 2019). These advantageous characteristics make chitin a suitable material for usage in medicine and functional materials. Similar to cellulose or lignocelluloses, chitin has a dense crystal structure with extensive hydrogen bonds (Rujiravanit et al. 2020). These hydrogen bonds prevent the dissolution of chitin in water, which greatly restrict its applications (Yang et al. 2021). Thus, chitin has not been studied or used as extensively as cellulose, chitosan, and alginate.
Main solvents that have been applied to dissolve chitin include LiCl/DMAc solution, ionic liquids, CaCl2·2H2O saturated methanol, deep eutectic solvents, mineral acids, inorganic salt aqueous solutions, and aqueous alkali/urea solution. LiCl/DMAc solution has been shown to be a suitable organic solvent for the dissolution of chitin (Truong et al. 2022). Ionic liquids are organic salts in the liquid state at room temperature. Deep eutectic solvents are composed of hydrogen bond acceptors and donors with lower melting points compared to each constituent. Another organic solvent includes calcium chloride dihydrate in methanol (CaCl2-MeOH). These organic solvents have disadvantages including toxicity, strong volatility, high cost, difficult recovery, and low biodegradability.
Mineral acids such a H3PO4, H2SO4, and HCl with high concentration can dissolve chitin. Aqueous solutions of certain inorganic salts that are capable of strong hydration, such as LiSCN, LiI, LiBr, LiCl, Ca(SCN)2, CaI2, CaBr2, CaCl2, and Ca(NO3)2, also can be used to dissolve chitin.
Alkaline solution is another important class of aqueous solvent. Alkaline solutions tend to destroy the hydrogel bonds in cellulose and lignocellulose. For example, NaOH/urea solution dissolves cellulose to prepare cellulose hydrogel, film, microspheres, and fiber. The alkali molecules destroy the hydrogen bonds in cellulose, and urea prevents the aggregation of cellulose chains.
Chitin could be dissolved in a concentrated NaOH solution mixing with ice. NaOH/urea aqueous systems with freeze/thaw cycles have been used extensively to dissolve chitin and to prepare various chitin materials. Chitin is dissolved in NaOH/urea aqueous solutions by forming a hydrogen-bonded chitin/NaOH complex, which is surrounded by the urea hydrates. This sheath-like structure leads to good solubilization of chitin. Besides NaOH, LiOH and KOH have also been used for dissolving chitin (Xu et al. 2019; Huang et al. 2021). The alkaline solvents, especially NaOH/urea aqueous solution, are a much safer and facile choice. Beyond its use as solvent to dissolve chitin, an alkaline solvent can catalyze certain reactions between the solute molecules in the solution. Recently, this solvent has been applied to dissolve chitin, enabling the production of chitin materials such as spheres (Duan et al. 2015), film (Duan et al. 2013), and hydrogel (Tang et al. 2012; Sharma et al. 2021).
Hydrogels have cross-linking polymeric network structure with hydrophilic groups (Liao et al. 2022). For preparing hydrogel, alkaline solution is a solvent and a catalyst for the reaction of some cross-linkers, such as epichlorohydrin (Chen and Hong 2022). This catalytic effect allows the cross-linking reaction to occur in a mild way at room temperature. Thus, it is possible to fabricate a hydrogel in room temperature. Such a temperature is sometimes important for preparing hydrogel containing a bioactive component that would have been destroyed by high temperature. Dissolution and reaction all happen in the same alkaline solution. This strategy can be extended to other reactions catalyzed by alkaline conditions for chitin, cellulose, or other natural polymers, but this preparation has not been reported.
In this study, an alkaline based dissolution-catalysis strategy was used to prepare a new chitin hydrogel for dye adsorption. Organic dyes such as methylene blue can be toxic and have stable structures (Seera et al. 2021). Dye wastewater effluents cause serious environmental problems (Liu et al. 2020), but hydrogels can adsorb large amounts of dye from solution. To enhance the adsorption capacity of chitin hydrogel, sodium carboxymethyl cellulose (CMC) and attapulgite were added to the chitin hydrogel. Sodium carboxymethyl cellulose is a carboxymethyl derivative of cellulose (Yang et al. 2021) with –COONa side chains. Attapulgite is a magnesium aluminum silicate with rod-like morphology (Liu et al. 2011) and negative charge. Methylene blue (MB) was selected as dye in this study.
EXPERIMENTAL
Materials
NaOH, urea, methylene blue (methylthioninium chloride C16H18N3SCl), and epichlorohydrin were all analytical grade. Chitin was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Sodium carboxymethyl cellulose (CMC) was purchased from Sinopharm Chemical Regent Co., Ltd. Attapulgite was produced by Jiangsu dianjinshi Au soil Mining Industry Co., Ltd.
Preparation of Composite Hydrogel
Chitin (2 g) was immersed into 100 g of 8 wt% NaOH/6 wt% urea solution and underwent freezing/thawing cycles until it was completely dissolved. CMC (2 g) was also dissolved in this solvent (100 g) but at 25 °C. The two solutions were mixed together at a certain weight ratio, with the addition of epichlorohydrin (14% weight ratio of above mixture) as cross-linker. After incubating the mixture at 25 °C for 4 h, the chitin-CMC hydrogel was formed. Residual NaOH and urea were removed by distilled water, and finally, chitin hydrogels were freeze-dried. Chitin-CMC hydrogels were coded as C-0%, C-16.7%, C-20%, C-25%, C-33.3%, and C-50% according to the weight percentage of CMC solution. A certain amount attapulgite was added into C-50% before cross-linking to prepare chitin composite hydrogels. Chitin composite hydrogels were coded as A-0.2%, A-0.5%, A-1%, A-2%, A-5.7%, A-9%, A-12.3%, and A-16.7% according to the weight percentage of attapulgite.
Characterization
Scanning electron microscopy (JSM-7800, JEOL, Shanghai, China), Fourier transform infrared spectroscopy (FTIR) using a spectrometer (Thermo Fisher Nicolet iS10, Nanjing, China), and wide-angle X-ray diffraction (XRD) (D8-Advance, Bruker, Nanjing, China) were used to detect the surface morphology and structure of chitin composite hydrogel(A-5.7%).
Water Uptake Ability and Dye Adsorption Capacity Measurement
Water uptake ratio (S) was calculated by Eq. 1,
(1)
where Wt is the sample weight after immersed in water 24 h, and W0 is the initial weight of freeze-dried sample.
The adsorption capacity (Q) was calculated by Eq. 2,
(2)
where C0 (200 mg L-1) is the initial dye concentration, Ce is the dye concentration after adsorption of 72 h at 25 °C, V (0.05L) is the methylene blue (MB) solution volume, and m (0.1 g) is the mass of freeze dried sample.
To measure the effect of initial concentration of MB on adsorption capacity, the adsorption capacity of A-5.7% in 100, 200, 300, 400, 500, and 600 mg L-1 MB solution was measured.
RESULTS AND DISCUSSION
Preparation of Composite Hydrogel
Chitin composite hydrogel was successfully fabricated by dissolution of chitin in alkaline solvent and subsequently cross-linking of chitin and sodium carboxymethyl cellulose catalyzed by same alkaline solvent. NaOH/urea solution as the alkaline solvent was used to dissolve chitin by repeatedly freezing/thawing process to get a viscous solution. Figure 1(a) shows a good dissolution of chitin, the solution of which climbs up the stirring rod (Weissenberg effect). After dissolution, chitin and CMC were cross-linked with epichlorohydrin in the same solvent via Williamson etherification and alkali-catalyzed oxalkylation (Lin et al. 2017). The chlorine or epoxy group of epichlorohydrin mainly reacted with the hydroxyl of chitin and CMC to form ether bonds. Both reactions need alkaline conditions to enable catalysis. In NaOH/urea solution, the reaction occurred without the addition of an extra base. As a result, chitin composite hydrogel easily formed at room temperature in a short time, which was a mild method to fabricate chitin hydrogel (Fig. 1(b)).
Fig. 1. (a) Dissolution of chitin in 8% NaOH/6% urea solution; (b) just prepared chitin composite hydrogel; (c) freeze-dried chitin composite hydrogel
Fig. 2. Schematic depiction of fabrication of composite hydrogel by dissolving and reaction catalyzing strategy based on alkaline solvent
Morphology and Structure
Sample C-50% exhibited the largest water uptake ratio among chitin-CMC hydrogels. A-5.7% had larger adsorption capacity and medium attapulgite content, which can represent other samples containing attapulgite. Freeze-dried chitin-CMC hydrogel C-50% had a porous structure and smooth surface (Fig. 3A,B), which indicates a good dissolution of chitin. Freeze-dried chitin composite hydrogel (A-5.7%) exhibited a coarse surface with rod-like attapulgite (Fig. 3C-F).
Fig. 3. SEM photos of C-50% (A, B) and A-5.7% (C, D, E, F)
Figure 4(a) shows the FTIR spectra of chitin, CMC, C-50%, and A-5.7%. In C-50%, the peaks at 1587 cm−1 and 1418 cm−1 indicate the stretching vibration of –COO– of CMC. The peak at 1649 cm−1 belongs to the amide band I of chitin; 2925 cm-1 and 2877 cm-1 belong to the stretch vibration of –CH2–, which was strengthened by adding epichlorohydrin through the cross-linking reaction. In A-5.7%, the peaks at 3611 cm-1 and 3537 cm-1 belong to the stretching of hydroxyls, coordinate crystal water in attapulgite, respectively.
Fig. 4. (a) FTIR spectra of chitin, CMC, C-50%, and A-5.7%; (b) XRD spectra of attapulgite, chitin, CMC, C-50%, and A-5.7%
In Fig. 4(b) for attapulgite, peaks at 8.4, 13.8, 16.4, 19.8, 21.4, and 35.3° were attributed to the (110), (200), (130), (040), (121), and (061) crystal faces of attapulgite (Wang et al. 2014). The (110), (040), and (061) peaks also appear in A-5.7%, reflecting the intact crystal structure of attapulgite in A-5.7%. Peaks of chitin at 9.3, 12.8, 19.3, 23.4, and 26.4° were assigned to (020), (101), (110), (130), and (013), respectively (Cárdenas et al. 2004). These results indicated the crystalline structure of α-chitin (Cárdenas et al. 2004). There was no diffraction peaks in CMC and C-50% spectra. CMC is a semi-synthesized derivative of cellulose and is amorphous. C-50% is a cross-linked structure of chitin and CMC; the dissolution and cross-linking destroyed the crystalline structure of chitin, which exists in an amorphous state.
Water Uptake Behavior and Dye Adsorption Capacity
Figure 5(a) shows water uptake ratio of hydrogels (without attapulgite). C-50% exhibited the largest water uptake ratio. Compared with other hydrogels, C-50% had the largest content of CMC. Sodium ions in CMC solution are easy to dissociate in solution. Residues of –COO– with negative charge repel each other through repulsive electrostatic forces, which expands the network and increases the water uptake. The high content of CMC enables more solution to enter the hydrogel and is beneficial for the adsorption of dye.
Hydrogels are three-dimensional network structures that are able to retain large amounts of water. Hydrogels can absorb water more than 20% of their dry weight, up to thousands of times their dry weight (superabsorbent hydrogels). Hydrogels can be classified on the basis of origin, composition, ionic charge, physical structure, or cross-linking. On the basis of origin, natural hydrogels are prepared using natural polymers such as alginate, cellulose, chitosan, and chitin, while synthetic hydrogels are of synthetic origin. On the basis of nature of electric charge on the cross-linked chains, hydrogels are classified into neutral (non-ionic) hydrogels, ionic hydrogels, ampholytic hydrogels. On the basis of nature of cross-linking, hydrogels are of two types: physical hydrogels and chemical hydrogels. Physical hydrogels are cross-linked by various physical processes such as crystallization, hydrogen bonding, and hydrophobic interactions, whereas covalent cross-linking is used to prepare chemical hydrogels. In this study, nature-based ionic hydrogel specimens were prepared with chemical cross-linking.
Figure 5(b) shows that the adsorption capacities of composite hydrogel increased to the highest value and then decreased with increased content of attapulgite. The adsorption capacities of composite hydrogel were larger than for chitin hydrogel without attapulgite.
Adding high adsorption ability clay into hydrogel to prepare composite hydrogel is a common strategy to enhance the adsorption ability of hydrogel. Clays such as montmorillonite (Shen et al. 2016), laponite (Mahdavinia and Karami 2015), and attapulgite (Chen et al. 2019) have been incorporated in hydrogels to increase their adsorption capacity. As a hydrated octahedral layered magnesium aluminum silicate, attapulgite has a negative charge and can absorb cationic dyes such as methylene blue through electrostatic attraction. A high content of attapulgite increases the adsorption sites in the hydrogel, which increases its adsorption capacity. However, too much attapulgite impedes the diffusion of MB solution into the inner part of the hydrogel, leading to decreased adsorption capacity. A-0.5% (0.5% attapulgite content) had the largest adsorption capacity.
Fig. 5. (a) Water uptake ratio of hydrogels with different sodium carboxymethyl cellulose content; (b) Adsorption capacities of chitin composite hydrogels with increased content of attapulgite in 200 mg g-1 methylene blue solution
The initial concentration of MB had a large effect on the adsorption capacity. With the increase of initial concentration, Qe increased to 167 mg g-1 at 500 mg L-1 and then it remained constant (Fig. 6). The high MB concentration increased the diffusion of MB molecules into the composite hydrogel, leading to the high adsorption capacity. Table 1 compares the adsorption capacity of this work with some reported adsorption material by nature polymers.
Fig. 6. Effect of initial methylene blue concentration on the adsorption capacity of A-5.7%
Figure 7 illustrates the water uptake and adsorption behavior of chitin composite hydrogel. When dried hydrogel is immersed into dye contaminated water, –COONa groups in CMC are dissociated to negatively charged –COO– groups, which repel each other. This repulsion expands the network structure of the hydrogel via swelling; a large amount of MB solution is taken into the hydrogel network. Positively charged MB molecules interact with the negatively charged attapulgite, leading to the adsorption of MB onto the composite hydrogel. The high swelling degree and more adsorption groups are beneficial to the adsorption for dye molecules (Chen and Zhu 2019).
Table 1. Comparison of Maximum Adsorption Capacities of Adsorbents for Methylene Blue
Fig. 7. Water uptake and adsorption behavior of chitin composite hydrogel
CONCLUSIONS
- A chitin composite hydrogel was successfully prepared by dissolving and reaction catalyzing strategy based on alkaline solvent by chitin, sodium carboxymethyl cellulose, and attapulgite. This cross-linking reaction is mild in 8%NaOH/6%urea solution.
- Fourier transform infrared (FTIR) analysis confirmed the cross-linking reaction. Scanning electron micrographs (SEM) showed a coarse surface of composite hydrogel with rod-like attapulgite located on the surface.
- Sodium carboxymethyl cellulose and attapulgite enhanced the adsorption capacity for methylene blue by increasing water uptake and adsorption sites in hydrogel.
REFERENCES CITED
Al-Futaisi, A., Jamrah, A., Al-Rawas, A., and Al-Hanai, S. (2007). “Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman),” Environmental Geology 51(8), 1317-1327. DOI:10.1007/s00254-006-0430-y
Cárdenas, G., Cabrera, G., Taboada, E., and Miranda, S. P. (2004). “Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR,” Journal of Applied Polymer Science 93(4), 1876-1885. DOI: 10.1002/app.20647
Chen, X., Chen, C., and Zhu, J. (2019). “Facile preparation of cellulose-attapulgite nanocomposite hydrogel for dye adsorption,” Iranian Polymer Journal 28(4), 347-359. DOI: 10.1007/s13726-019-00703-9
Chen, X., and Hong, L. (2022). “Preparation of chitin composite hydrogel for dye-contaminated water treatment,” Polish Journal of Environmental Studies 31(1),15-23. DOI: 10.15244/pjoes/138210
Chen, X., and Zhu, J. (2019). “Alginate composite hydrogel bead with multilayer flake structure for dye adsorptions,” Journal of Renewable Materials 7(10), 983-996. DOI: 10.32604/jrm.2019.07325
Duan, B., Chang, C., and Ding, B. (2013). “High strength films with gas-barrier fabricated from chitin solution dissolved at low temperature,” Journal of Materials Chemistry, A. Materials for Energy and Sustainability 1(5), 1867-1874. DOI: 10.1039/C2TA00068G
Duan, B., Huang, Y., Lu, A., and Zhang, L. (2018). “Recent advances in chitin based materials constructed via physical methods,” Progress in Polymer Science 82, 1-33. DOI: 10.1016/j.progpolymsci.2018.04.001
Duan, B., Zheng, X., Xia, Z., Fan, X., Guo, L., Liu, J., Wang, Y., Ye, Q., and Zhang, L. (2015). “Highly biocompatible nanofibrous microspheres self-assembled from chitin in NaOH/urea aqueous solution as cell carriers,” Angewandte Chemie International Edition 54, 1-6. DOI: 10.1002/anie.201412129
Hasan, Z., Afroz, S., Nipa, K., Rahaman, M. S., Hasnine, S. M. M., Ahmed, T., Sultana, S., Takafuji, M., and Alam, M. A. (2022). “Adsorption isotherm and kinetics of methylene blue on gamma radiation assisted starch/acrylic acid/4-styrenesulfonic acid sodium salt hydrogel,” Polymer-Plastics Technology and Materials 61(3), 306-324. DOI: 10.1080/25740881.2021.1982970
Huang, J., Zhong, Y., Wei, P., and Cai, J. (2021). “Rapid dissolution of β-chitin and hierarchical self-assembly of chitin chains in aqueous KOH/urea solution,” Green Chemistry 23(8), 3048-3060.DOI: 10.1039/d1gc00514f
Liao, J., Hou, B., and Huang, H. (2022). “Preparation, properties and drug controlled release of chitin-based hydrogels: An updated review,” Carbohydrate Polymers 283, article 119177. DOI: 10.1016/j.carbpol.2022.119177
Liao, J., and H., Huang (2019). “Magnetic chitin hydrogels prepared from Hericium erinaceus residues with tunable characteristics: A novel biosorbent for Cu2+ removal,” Carbohydrate Polymers 220, 191-201. DOI: 10.1016/j.carbpol.2019.05.074
Lin, Q., Chang, J., Gao, M., and Ma, H. (2017). “Synthesis of magnetic epichlorohydrin cross-linked carboxymethyl cellulose microspheres and their adsorption behavior for methylene blue,” Journal of Environmental Science and Health, Part A 52(2), 106-116. DOI: 10.1080/10934529.2016.1237117
Liu, Y., Wang, W., Jin, Y., and Wang, A. (2011). “Adsorption behavior of methylene blue from aqueous solution by the hydrogel composites based on attapulgite,” Separation Science and Technology 46(5), 858-868. DOI: 10.1080/01496395.2010.528502
Liu, C., Liu, H., Tang, K., Zhang, K., Zou, Z., and Gao, X. (2020). “High-strength chitin based hydrogels reinforced by tannic acid functionalized graphene for congo red adsorption,” Journal of Polymers & the Environment 3, 984-994. DOI: 10.1007/s10924-020-01663-5
Ma, M., Zhong, Y., and Jiang, X. (2020). “Thermosensitive and pH-responsive tannin-containing hydroxypropyl chitin hydrogel with long-lasting antibacterial activity for wound healing,” Carbohydrate Polymers 236, article 116096. DOI: 10.1016/j.carbpol.2020.116096
Mabel, M. M., Sundararaman, T. R., Parthasarathy, N., and Rajkumar, J. (2019). “Chitin beads from Peneaus sp. shells as a biosorbent for methylene blue dye removal,” Polish Journal of Environmental Studies 28(4), 2253-2259. DOI: 10.15244/pjoes/90359
Mahdavinia, G. R., and Karami, S. (2015). “Synthesis of magnetic carboxymethyl chitosan-g-poly(acrylamide)/laponite RD nanocomposites with enhanced dye adsorption capacity,” Polymer Bulletin 72(9), 2241-2262. DOI: 10.1007/s00289-015-1402-8.
Nguyen, K. D., Trang, T. T. C., and Kobayashi, T. (2019). “Chitin-halloysite nanoclay hydrogel composite adsorbent to aqueous heavy metal ions,” Journal of Applied Polymer Science 136(11), article 47207. DOI: 10.1002/app.47207
Rujiravanit, R., Kantakanun, M., Chokradjaroen, C., Vanichvattanadecha, C., and Saito, N. (2020). “Simultaneous deacetylation and degradation of chitin hydrogel by electrical discharge plasma using low sodium hydroxide concentrations,” Carbohydrate Polymers 228, article 115377. DOI: 10.1016/j.carbpol.2019.115377
Santoso, S. P., Angkawijaya, A. E., Bundjaja, V., Hsieh, C.-W., Go, A. W., Yuliana, M., Hsu, H.-T., Tran-Nguyen, P. L., Soetaredjo., F. E., and Ismadji, S. (2021). “TiO2/guar gum hydrogel composite for adsorption and photodegradation of methylene blue,” International Journal of Biological Macromolecules(Part A) 193, 721-733. DOI: 10.1016/j.ijbiomac.2021.10.044
Seera, S. D. K., Kundu, D., Gami, P., Naik, P. K., and Banerjee, T. (2021). “Synthesis and characterization of xylan-gelatin cross-linked reusable hydrogel for the adsorption of methylene blue,” Carbohydrate Polymers 256, article 117520. DOI: 10.1016/j.carbpol.2020.117520
Sharma, G., Kumar, A., Naushad, M., Thakur, B., Vo, D.-V. N., Gao, B., Al-Kahtani, A. A., and Stadler, F. J. (2021). “Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel,” Journal of Hazardous materials 416, article 125714. DOI: 10.1016/j.jhazmat.2021.125714
Shen, X., Shamshina, J. L., and Berton, P. (2016). “Hydrogels based on cellulose and chitin: Fabrication, properties, and applications,” Green Chemistry 18(1), 53-75. DOI: 10.1039/C5GC02396C
Tang, H., Zhou, W., and Zhang, L. (2012). “Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels,” Journal of Hazardous Materials 209-21, 218-225. DOI: 10.1016/j.jhazmat.2012.01.010
Taşdelen, B., Çifçi, D. İ., and Meri, S. (2021). “Preparation and characterization of chitosan/AMPS/kaolinite composite hydrogels for adsorption of methylene blue,” Polymer Bulletin 97, 9643-9662. DOI: 10.1007/s00289-021-03970-w
Truong, T. T. C., Kobayashi, T., and Van Tran, L. (2022). “Preparation, characterization, and application of hydrogel derived from chitin with lithium chloride for growth and development of rosemary plants,” Environmental Quality Management 32(1), 63-74. DOI: 10.1002/tqem.21841.
Wang, C., Wu, Q., Liu, F., An, J., Lu, R., Xie, H., and Cheng, R. (2014). “Synthesis and characterization of soy polyol-based polyurethane nanocomposites reinforced with silylated palygorskite,” Applied Clay Science 101, 246-252. DOI: 10.1016/j.clay.2014.08.009
Wang, F., Zhang, Y., and Wang, Y. (2021). “Recycling of waste cotton sheets into three-dimensional biodegradable carriers for removal of methylene blue,” ACS Omega 6(50), 34314-34326. DOI: 10.1021/acsomega.1c04019
Wu, Z. Y., Liao, Q. J., Chen, P. P., Zhao, D., Huo, J. Y., An, M. Z., Li, Y. H., Wu, J. H., Xu, Z.-M., Sun, B.-G., and Huang, M.-Q. (2022). “Synthesis, characterization, and methylene blue adsorption of multiple-responsive hydrogels loaded with Huangshui polysaccharides, polyvinyl alcohol, and sodium carboxyl methyl cellulose,” International Journal of Biological Macromolecules 216, 157-171. DOI: 10.1016/j.ijbiomac.2022.06.178
Xu, H., Zhang, L., and Cai, J. (2019). “Injectable, self-healing, β-chitin-based hydrogels with excellent cytocompatibility, antibacterial activity, and potential as drug/cell carriers,” ACS Applied Bio Materials 2(1), 196-204. DOI: 10.1021/acsabm.8b00548
Xu, R., Mao, J., Peng, N., Luo, X., and Chang, C. (2018). “Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes,” Carbohydrate Polymers 188, 143-150. DOI: 10.1016/j.carbpol.2018.01.073
Yang, H. R., Li, S. S., An, Q. D., Zhai, S. R., Xiao, Z. Y., and Zhang, L. P. (2021). “Facile transformation of carboxymethyl cellulose beads into hollow composites for dye adsorption,” International Journal of Biological Macromolecules 190, 919-926. DOI: 10.1016/j.ijbiomac.2021.08.229
Zhou, Y. M., Li, T., Shen, J. L., Meng, Y., Tong, S. H., Guan, Q. F., and Xia, X. X. (2021). “Core-shell structured magnetic carboxymethyl cellulose-based hydrogel nanosorbents for effective adsorption of methylene blue from aqueous solution,” Polymers 18, article 3054. DOI: 10.3390/polym13183054
Zhang, L., Zhao, D., Lua, Y., Chena, J., Lia, H., Xiea, J., Xuab, Y., Yuana, H., Liua, X., Zhuc, X. et al. (2021). “A graphene oxide modified cellulose nanocrystal/PNIPAAm IPN hydrogel for the adsorption of Congo red and methylene blue,” New Journal of Chemistry 45(36), 16679-16688. DOI: 10.1039/D1NJ01969D
Article submitted: September 4, 2022; Peer review completed: October 9, 2022; Revised version received and accepted: November 3, 2022; Published: November 9, 2022.
DOI: 10.15376/biores.18.1.291-301
