Abstract
Pre-hydrolysis is an important step in the poplar residual slab dissolving pulp production process, as it aids in removing as much hemicellulose as possible from the cellulose fibers. In the pre-hydrolysis process, a portion of lignin also dissolves, along with the hemicelluloses. The presence of lignin in prehydrolysis liquor (PHL) is detrimental to the separation of xylo-oligosaccharide from the prehydrolysis liquor. This study researched lignin removal from PHL with three coagulating agents, namely aluminum sulfate (alum), polyaluminum chloride (PAC), and polydiallyldimethyl ammonium chloride (pDADMAC). It was found that the removal of lignin increased as the dosage of the alum, PAC, and pDADMAC increased. Additionally, the highest retention of xylo-oligosaccharide in the PHL occurred at dosages of 120 mg/L for alum and 160 mg/L for PAC and pDADMAC. The contents of the other oligosaccharides in PHL fluctuated irregularly with increasing dosages of the alum, PAC, and pDADMAC.
Download PDF
Full Article
Effect of Coagulating Agents on Lignin and Oligosaccharide Contents in Pre-Hydrolysis Liquor Obtained in the Production of Dissolving Pulp from Poplar Residual Slabs
Chaojun Wu,* Lijuan Bing, Shuping Li, Dongmei Yu, and Daiqi Wang
Pre-hydrolysis is an important step in the poplar residual slab dissolving pulp production process, as it aids in removing as much hemicellulose as possible from the cellulose fibers. In the pre-hydrolysis process, a portion of lignin also dissolves, along with the hemicelluloses. The presence of lignin in prehydrolysis liquor (PHL) is detrimental to the separation of xylo-oligosaccharide from the prehydrolysis liquor. This study researched lignin removal from PHL with three coagulating agents, namely aluminum sulfate (alum), polyaluminum chloride (PAC), and polydiallyldimethyl ammonium chloride (pDADMAC). It was found that the removal of lignin increased as the dosage of the alum, PAC, and pDADMAC increased. Additionally, the highest retention of xylo-oligosaccharide in the PHL occurred at dosages of 120 mg/L for alum and 160 mg/L for PAC and pDADMAC. The contents of the other oligosaccharides in PHL fluctuated irregularly with increasing dosages of the alum, PAC, and pDADMAC.
Keywords: Pre-hydrolysis; Poplar residual slab dissolving pulp; Oligosaccharide content; Removal of lignin; Coagulating agents
Contact information: Key Lab of Paper Science and Technology of Ministry of Education, Qilu University of Technology, Ji’nan, China; *Corresponding author: chaojunwu2007@163.com
INTRODUCTION
Traditionally, there are two processes used in the production of dissolving pulp. These processes include extensive acid sulfite cooking (Sixta et al. 2004) and prehydrolysis-sulfate (kraft) cooking (Saeed et al. 2012). Prehydrolysis is an important step in the kraft-based dissolving pulp production process, as it helps remove as much hemicellulose as possible from cellulose fibers before the material is subjected to the main delignification operation, i.e. the pulping process.
Earlier publications have shown that the main components of the pre-hydrolysis liquor (PHL) are mono- and oligo-saccharides, lignin, furfural, and acetic acid (Liu et al. 2011; Saeed et al. 2012; Yang et al. 2012; Tang et al. 2013). Currently, the PHL from the pre-hydrolysis stage is either mixed with black liquor and burned in the recovery boiler of the mill, or discharged to the wastewater treatment plant. The dissolved lignocellulosic materials in PHL could potentially be used for value-added bio-products such as oligosaccharides, furfural, and acetic acid. However, the presence of lignin fragments is detrimental for subsequent fermentation purposes, and so it is advantageous to remove it.
In the literature, the efficiencies of activated carbon, polyethylene oxide, surfactant/calcium oxide, acidification/PEO, chitosan, and laccase treatments for removing various components of the PHL have been comprehensively investigated (Saeed et al. 2011; Shi et al. 2011, 2012; Liu et al. 2013; Shen et al. 2013; Wang et al. 2014, 2015). This study considers the effects of alum, polyaluminum chloride (PAC), and polydimethyldiallylammonium chloride (pDADMAC) on the oligosaccharide, total sugar, and lignin contents in pre-hydrolysis liquors (PHL) obtained in the production of dissolving pulp from poplar residual slabs. Due to their strongly positive charged nature and relatively low molecular mass, the three additives considered in this work can be regarded as coagulants. The removal of lignin and the retention of various xylo-oligosaccharides in the PHL were assessed at various dosages of alum, PAC, and pDADMAC.
The objective of the present study was to simultaneously obtain a high removal of lignin and high retention of oligosaccharides from PHL. It is expected that this study can provide an efficient method of selective lignin removal in PHL during the dissolving pulp process.
EXPERIMENTAL
Pre-hydrolysis Procedure
Pre-hydrolysis liquor (PHL, pH=4.64) was collected from the pre-hydrolysis stage of a dissolving pulp made from poplar residual slabs, which included 24.9% pentosan, 76.8% holocellulose, 41.8% α-cellulose, 16.7% acid-insoluble lignin, and 3.8% acid-soluble lignin. This raw material has been used to produce high-yield pulp, bleached chemical pulp, or dissolving pulp (Gong 2007; Deng et al. 2010; Wu et al. 2015) in China. Water pre-hydrolysis was carried out in an electrically heated stainless steel digester (15 L) that rotated at a rate of 1 rpm in our laboratory. The pre-hydrolysis was carried out at a constant cooking temperature of 160 °C, and it was heated from room temperature at a heating rate of 8 °C/10 min. The time at cooking temperature was 120 min, with a liquor-to-wood ratio of 6:1.
Materials
Alum and PAC were chemically pure agents and dissolved in distilled water of 10.0 g/L prior to being used. Polydiallyldimethyl ammonium chloride (pDADMAC), 200 to 350 kDa, was purchased from Aladdin Co., Ltd., Shanghai, China and applied without further purification.
Addition of Coagulating Agents to PHL
The PHL was filtered using a 0.45-μm nylon syringe filter prior to the coagulating agent addition. Various amounts of alum, PAC, and pDADMAC were subsequently added to 100 mL of the PHL at a temperature of 28 °C , unadjusted pH value of 4.64 and stirring at 500 rpm for 60 min. Afterwards, the complexes were isolated by centrifuging at 3000 rpm for 5 min. The lignin and sugar contents in the PHL supernatant (before and after alum), PAC, and pDADMAC addition were determined.
Analytical Methods
The concentration of sugars in the PHL was determined using ion chromatography with a pulse amperometric detector and CarboPacTM PA20 column (ICS-5000, MA, USA). A mixture of 80% ultrapure de-ionized water and 20% of 10 mM NaOH was used as an eluant with a flow rate of 0.5 mL/min. To measure the oligomeric sugar in original and treated PHL, an additional acid hydrolysis was conducted on the PHL, under conditions of 4% sulfuric acid at 121 °C for 1 h in an oil bath (Saeed et al. 2012). The samples were filtered (0.22 μm) and diluted prior to analysis. The monomeric sugar contents in the post-acid hydrolysate represented the total saccharides in the pre-hydrolysis liquor. The sugar contents in oligomeric form in the prehydrolysis liquor were calculated by taking the difference in the monomeric sugar contents, both with and without the post-acid hydrolysis. The acid-soluble lignin content in the PHL was measured a UV/Vis detector (Agilent 8453, USA) at a wavelength of 205 nm (Liu et al. 2014) (TAPPI UM 250).
RESULTS AND DISCUSSION
Properties of PHL
The various PHL properties, such as sugar and acid-soluble lignin contents, are listed in Table 1. The contents of oligomeric arabinose, galactose, glucose, xylose, and mannose were 0.27, 0.63, 1.02, 11.21, and 0.22 g/L, respectively. It was obvious that xylo-oligosaccharide, which accounted for around 84% of the total oligomeric sugars and more than 75% of the total sugars, was the main sugar in the PHL. The xylo-oligosaccharide percentages of total sugars and total oligomeric sugars present in the tested PHL were more than that (74% and 61%, respectively) of the PHL from the dissolving pulp industries in Canada (Yang et al. 2012). This indicated that the PHL has the potential to be used as a recovery source of high-value xylo-oligosaccharide, as it possesses an acceptable odor, is non-carcinogenic, and has low calorie content, allowing its utilization in anti-obesity diets (Vazquez et al. 2000). The lignin content (9.16 g/L) in the PHL was relatively lower than that (10.2 g/L) of the PHL from the dissolving pulp industries in Canada (Yang et al. 2012). The isolation of lignin in PHL is a problem that remains largely unsolved and obstructs the recovery and utilization of xylo-oligosaccharide from the PHL.
Table 1. Dissolved Sugars and Lignin Concentration in PHL

Optimization of Alum Dosages
The lignin and oligomeric sugar contents in the PHL treated with various dosages of alum are presented in Table 2. As can be seen, as the alum dosage increased, a downward trend in the lignin content of PHL appeared, indicating that the removal of lignin increased with the increased alum dosage. After increasing the alum dosage, the oligomeric arabinose content in the PHL reached a maximum at 40 mg/L alum, oligomeric galactose content reached a maximum at 80 mg/L alum, oligomeric glucose content reached a maximum at 80 mg/L alum, oligomeric xylose content reached a maximum at 120 mg/L alum, and oligomeric mannose content reached a maximum at 160 mg/L alum. These results show that the highest retention (near 98%) of xylo-oligosaccharide occurred at dosages of 120 mg/L alum, and the removal of lignin could reach 43.89%, as shown in Table 3.
Table 2. Effect of Alum Dosage on Oligomeric Sugars and Lignin Concentration in PHL
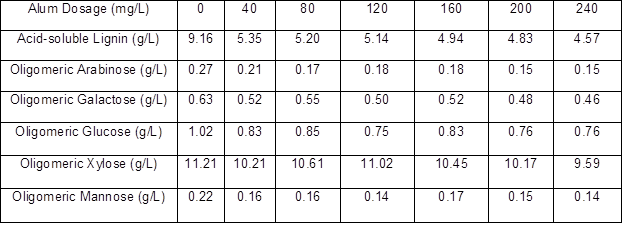
Table 3. Effect of Alum Dosage on Oligomeric Sugars Retention and Lignin Removal of PHL
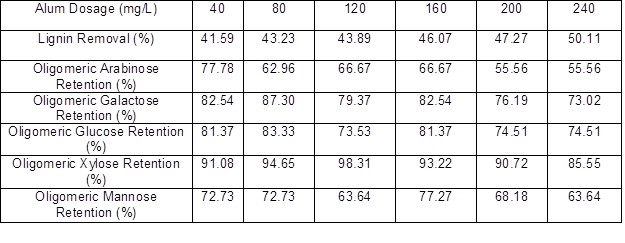
Optimization of PAC Dosages
The lignin and oligomeric sugar contents in the PHL treated with various dosages of PAC are presented in Table 4. As can be seen, the lignin content in the PHL decreased with an increase in PAC dosage. With increasing PAC dosage, oligomeric arabinose content in the PHL reached a maximum at 80 mg/L PAC, oligomeric galactose content reached a maximum at 40 mg/L PAC, oligomeric glucose content reached a maximum at 200 mg/L PAC, oligomeric xylose content reached a maximum at 160 mg/L PAC, and oligomeric mannose content fluctuated irregularly after the increase of PAC dosage. The irregular fluctuations were taken as an indication that the apparent changes in concentrations of the oligosaccharides, as a function of changes in coagulant dosage, were not large relative to the repeatability of the measurements. However, the highest retention (near 99%) of xylo-oligosaccharide occurred at dosages of 160 mg/L for PAC, and the lignin removal could reach 51.64%, as illustrated by the data in Table 5.
Table 4. Effect of PAC Dosage on Oligomeric Sugars and Lignin Concentration in PHL
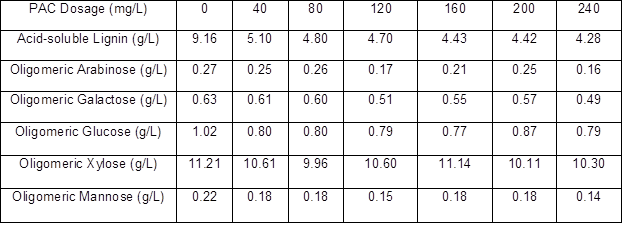
Table 5. Effect of PAC Dosage on Oligomeric Sugars Retention and Lignin Removal of PHL
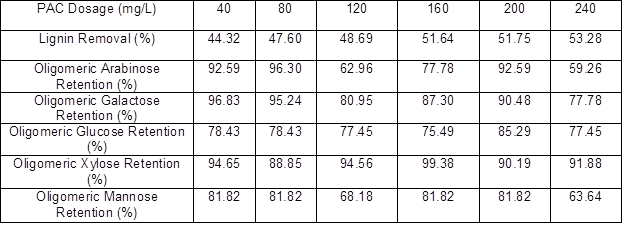
Optimization of pDADMAC Dosages
The lignin and oligomeric sugar contents in the PHL treated with various dosages of pDADMAC are presented in Table 6. As shown, the lignin content in the PHL decreased with an increase in pDADMAC dosage. After increasing the pDADMAC dosage, the oligomeric arabinose content in PHL reached a maximum of 200 mg/L pDADMAC, oligomeric galactose content fluctuated irregularly with the increase in pDADMAC dosage, oligomeric glucose content reached a maximum of 40 mg/L pDADMAC, oligomeric xylose content reached a maximum of 160 mg/L pDADMAC, and oligomeric mannose content also fluctuated irregularly after the increase in pDADMAC dosage. The results showed that the highest retention (near 94%) of xylo-oligosaccharide occurred at dosages of 160 mg/L for pDADMAC, and the removal of lignin reached 50.44% at pH=4.64 as similar with the best results working at pH=4.5 when using pDADMAC (Duarte et al. 2010) , as listed in Table 7.
Table 6. Effect of pDADMAC Dosage on Oligomeric Sugars and Lignin Concentration in PHL
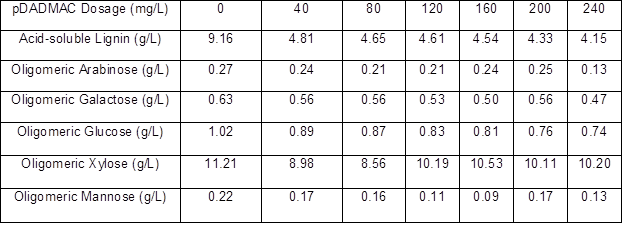
Table 7. Effect of pDADMAC Dosage on Oligomeric Sugars Retention and Lignin Removal of PHL
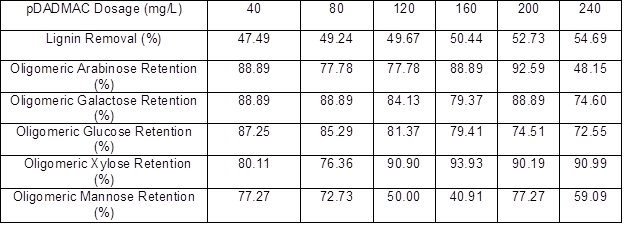
CONCLUSIONS
- The PHL from the pre-hydrolysis stage of dissolving pulp, made from poplar residual slabs, could potentially be a recovery source of high-value xylo-oligosaccharides because of its high xylo-oligosaccharide percentages of total sugars and total oligomeric sugars, as well as its relatively low lignin content.
- The removal of lignin all increased with increasing dosages of alum, PAC, and pDADMAC. However, the lowest loss (highest retention) of xylo-oligosaccharide occurred at dosages of 120 mg/L for alum and 160 mg/L for PAC and pDADMAC, respectively.
ACKNOWLEDGMENTS
The authors wish to thank the Shandong Province Department of Education Fund (No. J14LD01) and the Project of Scientific Development Program in Shandong Province (No. 2014GGX108003) for financially supporting this study, and the Shan Dong Sun Paper Industry Joint Stock Co., Ltd., for supplying the poplar residual slab.
REFERENCES CITED
Deng, Y. J., Fang, G. G., Han, S. M., Jiao, J., Li, H. B., Li, P., Liang, F. M., and Hu, J. M. (2010). “Studies on bleached chemimechanical pulps from poplar wood residues as furnishes for munken paper,” China Pulp Paper Ind. 31(8), 15-19. DOI: 10.3969/j.issn.1007-9211.2010.08.003
Duarte, G. V., Ramarao , B. V., and Amidon, T. E.(2010). “Polymer induced flocculation and separation of particulates from extracts of lignocellulosic materials,” Bioresour. Technol. 101(22), 8526–8534. DOI:10.1016/j.biortech.2010.05.079
Gong, H. R. (2007). “The mill production of poplar wood residue bleached chemical pulp,” China Pulp and Paper 26(1), 59-59.
Liu, H. T., Hu, H. R., Baktash, M. M., Jahan, M. S., Ahsan, L., and Ni, Y. H. (2014). “Kinetics of furfural production from pre-hydrolysis liquor (PHL) of a kraft-based hardwood dissolving pulp production process,” Biomass Bioenerg. 66, 320-327. DOI: 10.1016/j.biombioe.2014.02.003
Liu, Z. H., Ni, Y. H., Fatehi, P., and Saeed, A. (2011). “Isolation and cationization of hemicelluloses from pre-hydrolysis liquor of kraft-based dissolving pulp production process,” Biomass Bioenerg. 35(5), 1789-796. DOI: 10.1016/j.biombioe.2011.01.008
Liu, G., Shi, H. Q., Ping, Q. W., Zhou, J. H., Zhang, J., Li, N., Niu, M. H., Fatehi, P., Xiao, H. N., and Ni, Y. H. (2013). “Complex formation of PEO and lignin in prehydrolysis liquor and its enhancing effect on lignin removal,” BioResources 8(3), 4004-4015. DOI: 10.15376/biores.8.3.4004-4015
Saeed, A., Fatehi, P., and Ni, Y. H. (2011). “Chitosan as a flocculant for pre-hydrolysis liquor of kraft-based dissolving pulp production process,” Carbohydr. Polym. 86(4), 1630-1636. DOI: 10.1016/j.carbpol.2011.06.075
Saeed, A., Jahan, M. S., Li, H. M., Liu, Z. H., Ni, Y. H., and van Heiningen, A. (2012). “Mass balances of components dissolved in the pre-hydrolysis liquor of kraft-based dissolving pulp production process from Canadian hardwoods,” Biomass Bioenerg. 39, 14-19. DOI: 10.1016/j.biombioe.2010.08.039
Shen, J., Kaur, I., Baktash, M. M., He, Z. B., and Ni, Y. H. (2013). “A combined process of activated carbon adsorption, ion exchange resin treatment and membrane concentration for recovery of dissolved organics in pre-hydrolysis liquor of the kraft-based dissolving pulp production process,” Bioresour. Technol. 127, 59-65. DOI: 10.1016/j.biortech.2012.10.031
Shi, H. Q., Fatehi, P., Xiao, H. N., and Ni, Y. H. (2011). “A combined acidification/PEO flocculation process to improve the lignin removal from the pre-hydrolysis liquor of kraft-based dissolving pulp production process,” Bioresour. Technol. 102(8), 5177-5182. DOI: 10.1016/j.biortech.2011.01.073
Shi, H. Q., Fatehi, P., Xiao, H. N., and Ni, Y. H. (2012). “A process for isolating lignin of pre-hydrolysis liquor of kraft pulping process based on surfactant and calcium oxide treatments,” Biochem. Eng. J. 68, 19-24. DOI: 10.1016/j.bej.2012.06.017
Sixta, H., Promberger, A., Koch, G., Gradinger, C., and Messner, K. (2004). “Influence of beech wood quality on bisulfite dissolving pulp manufacture. Part 1: Influence of log storage on pulping and bleaching,” Holzforschung 58(1), 14-21. DOI: 10.1515/HF.2004.003
Tang, J. B., Chen, K. F., Huang, F., Xu, J., and Li, J. (2013). “Characterization of the pretreatment liquor of biomass from the perennial grass, Eulaliopsis binata, for the production of dissolving pulp,” Bioresour. Technol. 129, 548-552. DOI: 10.1016/j.biortech.2012.11.096
Vazquez, M. J., Alonso, J. L., Dominguez, H., and Parajo, J. C. (2000). “Xylooligosaccharides: Manufacture and applications,” Trends Food Sci. Technol. 11(11), 387-393. DOI: 10.1016/S0924-2244(01)00031-0
Wang, Q., Jahan, M. S., Liu, S. S., Miao, Q. X., and Ni, Y. H. (2014). “Lignin removal enhancement from prehydrolysis liquor of kraft-based dissolving pulp production by laccase-induced polymerization,” Bioresour. Technol. 164, 380-385. DOI: 10.1016/j.biortech.2014.05.005
Wang, Q., Liu, S. S., Yang, G. H., and Chen, J. C. (2015). “Modeling laccase-induced lignin removal in prehydrolysis liquor from kraft-based dissolving pulp production,” Bioresour. Technol. 175, 638-641. DOI: 10.1016/j.biortech.2014.10.149
Wu, C. J., Bing, L. J., Yu, D. M., Li, R. G., and Zhou, S. F. (2015). “Preparation of dissolving pulp made from poplar residual slabs and effect of xylanase post-treatment on its reactivity,” BioResources 10(1), 423-431. DOI: 10.15376/biores.10.1.423-431
Yang, G. H., Jahan, M. S., Liu, H. T., and Ni, Y. H. (2012). “Acid hydrolysis of prehydrolysis liquor produced from the kraft-based dissolving pulp production process,” Ind. Eng. Chem. Res. 51(43), 13902-13907. DOI: 10.1021/ie3023059
Article submitted: September 1, 2015; Peer review completed: October 16, 2015; Revised version received and accepted: October 23, 2015; Published: November 9, 2015.
DOI: 10.15376/biores.11.1.87-94
