Abstract
The Fenton reaction has been widely used in the pretreatment of lignocellulose. It offers the advantages of simple operation, fast reaction speed, and low pollution. In this study, the effects of different proportions of Fenton reagents on the enzymatic hydrolysis of poplar were compared and analyzed, and the optimal ratio of Fenton reagents was obtained. The maximum yield of enzymatic hydrolysis of glucose in Fenton pretreatment samples was 406 mg/g at H2O2 and Fe2+ concentrations of 1.0 mol/L and 0.01 mol/L, respectively, which was 2.5 times that of untreated samples. Meanwhile, the composition analysis and FT-IR analysis showed that Fenton pretreatment could degrade lignin and hemicellulose effectively. X-ray diffraction (XRD) analysis showed that Fenton pretreatment can partially destroy the amorphous region of poplar. These findings will contribute to efforts to improve the viability of the Fenton pretreatment process for converting biomass into energy.
Download PDF
Full Article
Effect of Fenton Pretreatment on Enzymatic Hydrolysis of Poplar
Shujie Wang, Jin Sun, Mengjie Chen, Xianfeng Hou,* and Zhenzhong Gao *
The Fenton reaction has been widely used in the pretreatment of lignocellulose. It offers the advantages of simple operation, fast reaction speed, and low pollution. In this study, the effects of different proportions of Fenton reagents on the enzymatic hydrolysis of poplar were compared and analyzed, and the optimal ratio of Fenton reagents was obtained. The maximum yield of enzymatic hydrolysis of glucose in Fenton pretreatment samples was 406 mg/g at H2O2 and Fe2+ concentrations of 1.0 mol/L and 0.01 mol/L, respectively, which was 2.5 times that of untreated samples. Meanwhile, the composition analysis and FT-IR analysis showed that Fenton pretreatment could degrade lignin and hemicellulose effectively. X-ray diffraction (XRD) analysis showed that Fenton pretreatment can partially destroy the amorphous region of poplar. These findings will contribute to efforts to improve the viability of the Fenton pretreatment process for converting biomass into energy.
Keywords: Fenton; Pretreatment; Enzymatic Saccharification; Poplar
Contact information: Key Laboratory for Biobased Materials and Energy of Ministry of Education, College of Materials & Energy, South China Agricultural University, Guangzhou 510642, China;
* Corresponding authors: xfhou@scau.edu.cn; zzgaoscau@163.com
INTRODUCTION
Lignocellulosic biomass is an environmentally friendly renewable material that can be converted into an alternative to traditional fossil fuels (Csilla et al. 2019). Due to its physical and chemical rigidity and recalcitrance, it is difficult to produce sugars from the carbohydrates in lignocellulose (Chu et al. 2019). Lignocellulose is mainly composed of cellulose, hemicellulose, and lignin. Cellulose is a carbohydrate that can be used to produce glucose, which is an important raw material for microbial fermentation to synthesize biofuels and bio-based chemicals. Due to the protective frames of lignin and chains between cellulose and hemicellulose, sugar yield is limited. Physical, chemical, and biological pretreatment is required prior to the enzymatic hydrolysis of cellulose (Liu et al. 2019b). Although these physical and/or chemical pretreatment methods have been extensively studied, they still have problems such as high operating temperatures leading to high energy consumption and the generation of byproducts that are toxic to biomass enzymolysis (Supanchaiyamat et al. 2019). Although biological pretreatment requires less energy and chemicals, it is less attractive to researchers because of its long reaction time. Biological pretreatment with fungi may offer some hints on the development of a cost-effective and environmentally friendly pretreatment processes with less solvents and acids (Goshadrou 2019). For example, in the degradation of wood by fungi, the Fenton reaction (i.e., iron and H2O2) facilitates the chemical degradation of lignocellulose (Hyunseok et al. 2017). The Fenton reaction involves the oxidation of Fe2+ to Fe3+ by H2O2, and then the reduction of Fe3+ to Fe2+, resulting in the formation of H2O, O2, and two kinds of oxygen radicals, namely HO× and HOO× (Liu et al. 2019c). The oxygen radicals initiate chain reactions for the oxidation of the lignocellulosic components and the further formation of other reactive oxygen species (Roccotelli et al. 2020).
The Fenton reaction takes place under mild conditions, such as indoor temperature, normal atmospheric pressure, or low concentrations of chemicals. Therefore, it is considered to be an environmentally benign process (Zhang et al. 2018; Ying et al. 2019). Furthermore, the Fenton reaction is found to be effective in destroying the chemical composition of wood cell walls in nature, and it can be applied to the pretreatment of lignocellulose for increasing the enzymatic digestibility.
In this study, poplar wood was selected as a raw material for biomass conversion, and it was pretreated with Fenton reagent to improve its enzymatic hydrolysis. Then the impact of Fenton reagents on the enzymatic hydrolysis of poplar, and the optimal ratio of Fenton reagents was obtained. Later, the pretreatment mechanism for poplar with Fenton reagents were explored through analyzing the components of poplar, FTIR, and XRD. These results can offer valuable references for the pretreatment of lignocellulosic biomass.
EXPERIMENTAL
Materials
Poplar wood specimens, collected from the Woodworking Machinery Laboratory of South China Agricultural University in Guangzhou, China, were air-dried, crushed with a Wiley mill, and then separated with sieves of 40 and 80 mesh, yielding powder particles in the size range 0.18 to 0.425 mm. All chemicals were reagent grade, and they were purchased from the Damao Chemical Reagent Factory. Except as otherwise noted, batch cellulase with a 99% purity was purchased from Ruiyang, Jiangsu with an enzyme activity of 35 FPU/g. All the raw materials were dried overnight in the high-temperature oven set to 80 ℃, and then the change in moisture content was eliminated.
Poplar Pretreatment
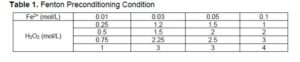
The samples were pretreated with Fenton reagents in different proportions, and the ratio of Fe2+ and H2O2 is shown in Table 1. Poplar and Fenton reagents were added to conical flask in a solid-liquid ratio of 1:20, and the pH was adjusted to 3 with oxalic acid solution. The conical flask was placed in a thermostatic oscillator for 12 h at 28 °C and 180 rpm/min. The sample was cooled to room temperature, filtered to recover the solid residue, and rinsed with distilled water. Upon filtration, the solid was dried (105 ℃) overnight.
Enzymatic Hydrolysis of Cellulose
Pretreated poplar samples weighing 0.05 g were placed in a 50 mL Erlenmeyer flask, and 20 mL of a citric acid buffer with a pH of 4.8 was added, and the cellulase content was 35 FPU/g. The sealed conical flask was then shaken on a constant temperature shaker at a temperature of 50 ℃ and a speed of 150 rpm for 48 h. The sugar reduction determination was conducted following the DNS test method based on reducing sugars.
X-ray Diffraction (XRD)
Pretreated and untreated samples were analyzed by XRD. The samples were scanned on a D8 Advance diffractometer equipped with a sealed tube Cu Kα source. The operating voltage and current were 40 kV and 40 mA, and the X-ray wavelength was 0.15406 nm. Scans were collected from 2θ = 10° to 50° with step size of 0.03 at 4 s per step. A peak deconvolution method was used to extract the crystallinity. The obtained biomass crystallinity was normalized by the cellulose content in each waste fiberboard sample to get the cellulose crystallinity.
Fourier Transform Infrared Spectroscopy (FT-IR)
FTIR was recorded from an FT-IR spectrophotometer (NICOLET6700, Thermo, Waltham, MA, USA). The samples were prepared by mixing 1 mg of material powder with 100 mg of KBr. Thirty-two scans were taken from 4000 to 400 cm-1.
RESULTS AND DISCUSSION
Effects of Different Proportions of Fenton Reagent Pretreatment on the Enzymatic Hydrolysis of Poplar
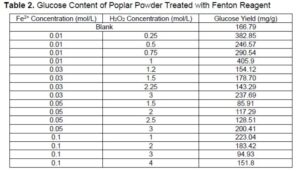
As shown in Table 2, the glucose content of poplar powder pretreated with Fenton reagents of different proportions was significantly different. Among them, when the concentration of Fe2+ was 0.01 mol/L and the concentration of H2O2 was 1.0 mol/L, the glucose content was the highest, which was about 2.5 times the untreated group. In this experiment, the concentration of Fe2+ was 0.01mol/L, and the concentration of H2O2 was 1.0 mol/L and was determined as the optimal ratio, which can be used in further experiments.
Fenton Pretreatment of Poplar Component Analysis
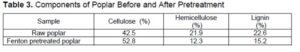
The composition analysis of the highest Fenton pretreatment enzymatic hydrolysis sample and untreated poplar was analyzed. As shown in Table 3, the hemicellulose content of poplar treated with Fenton reagent decreased from 21.9% to 12.3%, while the lignin content was reduced from 22.6% to 15.2%, which is consistent with Yu et al. (2018). This result indicates that Fenton pretreatment could effectively degrade hemicellulose and lignin, which might be the reason for the improved enzymatic hydrolysis effect of poplar samples after Fenton pretreatment.
FT-IR Analysis
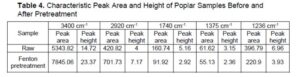
Infrared spectroscopy is a useful tool to study the physical and chemical changes of polysaccharides. The FT-IR spectra of poplar samples are shown in Fig. 1, the area and height of each characteristic peak are shown in Table 3. The regional bandwidth of 3400 to 3300 cm-1 belongs to the O-H stretch vibration of cellulose, and the region of 2920 cm-1 belongs to the C-H stretch vibration in cellulose methylene (Ferrari et al. 2019). After Fenton pretreatment, the characteristic peak strength and area of cellulose increased obviously, which indicates that the poplar samples had a higher cellulose content. This is consistent with the previous component analysis.
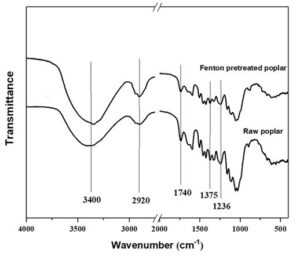
Fig. 1. FT-IR analysis of poplar before and after pretreatment
The absorbance peak at 1740 cm-1 belongs to hemicellulose carbonyl (Sakuragi et al. 2018), so the peak intensity here was positively correlated with the content of hemicellulose in the sample (Gogoi et al. 2018). Compared with untreated samples, the absorbance peak strength of poplar samples pretreated with Fenton at 1740 cm-1 significantly weakened, which was caused by the destruction of hemicellulose acetyl in the pretreatment process (Liu et al. 2019a). The acetyl in hemicellulose was one of the main obstacles in the destruction process of lignocellulose structure, and the deacetylation reaction in hemicellulose could improve the efficiency of enzymatic hydrolysis. The absorption peak at 1236 cm-1 belongs to the vibration of the C-O bond in hemicellulose or lignin, and the absorption peak at 1375 cm-1 belongs to the C-H vibration of cellulose and hemicellulose (Yao et al. 2018). The reduction of the absorption peak strength of the samples pretreated with Fenton at these two sites was partly due to the degradation of hemicellulose.
X-ray Diffraction Analysis
The XRD method was used to evaluate the structural changes of poplar. The XRD spectra of untreated and pretreated poplar are shown in Fig. 2.
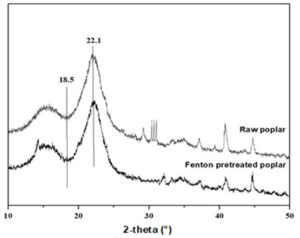
Fig. 2. XRD analysis of poplar before and after pretreatment
The main diffraction peaks of 18.5° and 22.1° exhibit the crystal structure of cellulose I (101) and (002), respectively, and the crystallinity index (CrI) of untreated poplar and poplar pretreated with Fenton were 0.502 and 0.547, respectively. The increase of CrI can be attributed to the removal of the amorphous parts. The crystal region of poplar is a rigid structure that was difficult to destroy via the Fenton reagent (Xu et al. 2018). The removal of amorphous components provided more contact sites for cellulase in the crystal region of poplar, which may be the reason for the increased sugar yield of enzymatic hydrolysis increased after Fenton pretreatment.
CONCLUSIONS
1. A new mild and green Fenton pretreatment was used to pretreat poplar, and a high sugar yield of 405.9 mg/g was obtained after 48 h.
2. Through the deep understanding of the reaction mechanism of Fenton pretreatment. Fenton pretreatment can effectively degrade hemicellulose and lignin, remove the non-crystalline area of lignocellulosic fibers, and improve the accessibility of the cellulose crystalline area. These effects are beneficial to the enzymatic hydrolysis and responsible for the high sugar yield.
3. This study demonstrates that Fenton pretreatment can make cellulose more efficient for enzymes and microbial species, which indicates that Fenton reagent is a viable option in our biomass pretreatment strategy library.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support by National Key R&D Program of China (2018YFD0600305) and Science and Technology Project of Guangzhou, China (201803030031).
REFERENCES CITED
Chu, Q.-L., Song, K., Wang, J., Hu, J.-G., and Chen, X.-Y. (2019). “Improving enzymatic saccharification of hardwood through lignin modification by carbocation scavengers and the underlying mechanisms,” Bioresource Technology 294, 122216. DOI: 10.1016/j.biortech.2019.122216.
Csilla, F., Rezessy-Szab, J. M. R., Gupta, V. K., Truong, D. H., Friedrich, L., Felfoldi, J., and Nguyen, Q. D. (2019). “Microbial saccharification of wheat bran for bioethanol fermentation,” Journal of Cleaner Production 240, 118269. DOI: 10.1016/j.jclepro.2019.118269
Ferrari, F. A., Pereira, J. F. B., Witkamp, G. J., and Forte, M. B. S. (2019). “Which variables matter for process design and scale-up? A study of sugar cane straw pretreatment using low-cost and easily synthesizable ionic liquids,” ACS Sustainable Chem. & Eng. 7(15), 12779-12788. DOI: 10.1021/acssuschemeng.9b01385
Gogoi, P., Zhang, Z., Geng, Z., Liu, W., Hu, W., and Deng, Y. (2018). “Novel low temperature, low energy and high efficiency pretreatment technology for large wood chips with a redox couple catalyst,” ChemSusChem 11(6), 1121-1131. DOI: 10.1002/cssc.201702090
Goshadrou, A. (2019). “Bioethanol production from Cogongrass by sequential recycling of black liquor and wastewater in a mild-alkali pretreatment,” Fuel 258, 116141. DOI: 10.1016/j.fuel.2019.116141.
Hyunseok, K., Bipinchandra, K. S., Iskandarani, B., Oh, W. G., and Kim, B. S. (2017). “Improved degradation of lignocellulosic biomass pretreated by Fenton-like reaction using Fe3O4 magnetic nanoparticles,” Biotechnology and Bioprocess Engineering 22(5). DOI: 10.1007/s12257-017-0225-x.
Liu, J.-L., Zhang, S., Jin, C.-D., Shuang, E., Sheng, K., and Zhang, X. (2019a). “Effect of swelling pretreatment on properties of cellulose-based hydrochar,” ACS Sustainable Chemistry & Engineering 7(12), 10821-10829. DOI: 10.1021/acssuschemeng.9b01640
Liu, T.-J., Yang, L., Liu, B., and Tan, L.-P. (2019b). “Hydroxycinnamic acids release during bioconversion of corn stover and their effects on lignocellulolytic enzymes,” Bioresource Technology 294, 122116. DOI: 10.1016/j.biortech.2019.122116
Liu, W., Wu, R.-J., Hu, Y.-Y., Ren, Q., Hou, Q-Q., and Ni, Y.-H. (2019c). “Improving enzymatic hydrolysis of mechanically refined poplar branches with assistance of hydrothermal and Fenton pretreatment,” Bioresource Technology 316, 123920. DOI: 10.1016/j.biortech.2020.123920
Roccotelli, A., Araniti, F., Tursi, A., Simeon, G. D. R., Rao, M., Lania, I., Chidichimo, G., Abenavoli, M. R., and Gelsomino, A. (2020). “Organic matter characterization and phytotoxic potential assessment of a solid anaerobic digestate following chemical stabilization by an iron-based Fenton reaction,” Journal of Agricultural and Food Chemistry 68(35), 9461-9474. DOI: 10.1021/acs.jafc.0c03570
Sakuragi, K., Igarashi, K., and Samejima, M. (2018). “Application of ammonia pretreatment to enable enzymatic hydrolysis of hardwood biomass,” Polymer Degradation & Stability 148, 19-25. DOI: 10.1016/j.polymdegradstab.2017.12.008
Supanchaiyamat, N., Jetsrisuparb, K., Knijnenburg, J. T. N., Tsang, D. C. W., and Hunt, A. J. (2019). “Lignin materials for adsorption: Current trend, perspectives and opportunities,” Bioresource Technology 272, 570-581. DOI: 10.1016/j.biortech.2018.09.139
Xu, F., Sun, J., Wehrs, M., Kim, K. H., Rau, S. S., Chan, A. M., Simmons, B. A., Mukhopadhyah, A., and Singh, S. (2018). “Biocompatible choline-based deep eutectic solvents enable one-pot production of cellulosic ethanol,” ACS Sustainable Chemistry & Engineering 6(7), 8914-8919. DOI: 10.1021/acssuschemeng.8b01271
Yao, L., Chen, C.-X., Yoo, C. G., Meng, X.-Z., Li, M., Pu, Y. Q., Ragauskas, A. J., Dong, C., and Yang, H. (2018). “Insights of ethanol organosolv pretreatment on lignin properties of Broussonetia papyrifera,” ACS Sustainable Chemistry & Engineering 6(11), 14767-14773. DOI: 10.1021/acssuschemeng.8b03290
Ying, W.-J., Xu, G.-F., Yang, H.-Y., Shi, Z.-J., and Yang, J. (2019). “The sequential Fenton oxidation and sulfomethylation pretreatment for alleviating the negative effects of lignin in enzymatic saccharification of sugarcane bagasse,” Bioresource Technology 286, 121392. DOI: 10.1016/j.biortech.2019.121392
Yu, H.-T., Chen, G.-Y., Li, B.-Y., Tseng, M.-C., Han, C.-C., and Shyu, S.-G. (2018). “Efficient pretreatment of lignocellulosic biomass with high recovery of solid lignin and fermentable sugars using Fenton reaction in a mixed solvent,” Biotechnology for Biofuels 11, 287. DOI: 10.1186/s13068-018-1288-4
Zhang, K.-J., Si, M.-Y., Liu, D., Zhuo, S.-N., Liu, M.-R., Liu, H., Yan, X., and Shi, Y. (2018). “A bionic system with Fenton reaction and bacteria as a model for bioprocessing lignocellulosic biomass,” Biotechnology for Biofuels 11, 31. DOI: 10.1186/s13068-018-1035-x
Article submitted: October 7, 2020; Peer review completed: January 2, 2021; Revised version received and accepted: January 12, 2021; Published: January 28, 2021.
DOI: 10.15376/biores.16.1.1980-1987
