Abstract
This study examined the effect of high temperature and Litsea cubeba oil with its main components against molds (Aspergillus niger, Aspergillus flavus, Penicillium sp., Penicillium cyclopium, Rhizopus sp., Fusarium sp., and Cladosporium sp.) on bamboo food packaging. Response surface methodology (RSM) with X1 (concentration of L. cubeba oil at 100, 300, and 500 mg g-1), X2 (temperature at 60, 80, and 100 °C), and X3 (time at 12, 14, and 16 h) was used to find the inhibitory periods of natural mold on packaging plates. The physical properties and the change of chemical components on the bamboo packaging plate before and after temperature treatment were determined to find the mode of action using gas chromatography–mass spectrometry (GC-MS). High temperature (at 100 °C) was a good inhibitor of all mold growth with the MIC (minimum inhibitory concentration) of 300 mg g-1; without heat treatment, no MIC was found. In addition, it was found that by using 300 mg g-1 of L. cubeba oil at 100 °C with an exposure time of 12 h, spore germination on the bamboo surfaces was completely inhibited for at least 290 days. After using high temperature, citral was detected on the surface of the packaging plates. Therefore, these components could be the key factors for inhibiting molds.
Download PDF
Full Article
Effect of High Temperature with Litsea cubeba Pers. to Control Mold Growth on Bamboo Food Packaging and Its Possible Modes of Action
Kitiya Suhem,a Narumol Matan,a,* and Nirundorn Matan b
This study examined the effect of high temperature and Litsea cubeba oil with its main components against molds (Aspergillus niger, Aspergillus flavus, Penicillium sp., Penicillium cyclopium, Rhizopus sp., Fusarium sp., and Cladosporium sp.) on bamboo food packaging. Response surface methodology (RSM) with X1 (concentration of L. cubeba oil at 100, 300, and 500 mg g-1), X2 (temperature at 60, 80, and 100 °C), and X3 (time at 12, 14, and 16 h) was used to find the inhibitory periods of natural mold on packaging plates. The physical properties and the change of chemical components on the bamboo packaging plate before and after temperature treatment were determined to find the mode of action using gas chromatography–mass spectrometry (GC-MS). High temperature (at 100 °C) was a good inhibitor of all mold growth with the MIC (minimum inhibitory concentration) of 300 mg g-1; without heat treatment, no MIC was found. In addition, it was found that by using 300 mg g-1 of L. cubeba oil at 100 °C with an exposure time of 12 h, spore germination on the bamboo surfaces was completely inhibited for at least 290 days. After using high temperature, citral was detected on the surface of the packaging plates. Therefore, these components could be the key factors for inhibiting molds.
Keywords: Litsea cubeba oil; Mold; Bamboo; Packaging plate; Heat
Contact information: a: Food Science and Technology, School of Agricultural Technology, Walailak University, Nakhon Si Thammarat 80160, Thailand; b: Materials Science and Engineering, School of Engineering and Resources, Walailak University, Nakhon Si Thammarat 80160, Thailand;
*Corresponding author: nnarumol@wu.ac.th; nnarumol@yahoo.com
INTRODUCTION
Petroleum-based food packaging types such as plastic and foam are having a major long-term impact on the environment because they are non-biodegradable. These kinds of packaging are hard to degrade naturally, and they may take 100 years or more to decompose (Satyanarayana et al. 2009). As an alternative to solve this problem, there is presently great interest in developing renewable raw materials (Cao et al. 2013). Cellulose has been identified as a suitable packaging material (Shahroze et al. 2018).
Bamboo is a lignocellulose material that is an abundant natural resource in Asia (Zakikhani et al. 2016). Generally, bamboo takes a short time to grow and has high productivity (Hu et al.2016). The bamboo plate is one of the newest packaging innovations due to its light weight, high strength, and ability to form many shapes (Kumar et al. 2017). Bamboo fiber has a higher Young’s modulus over density than wood and steel. Paper and foam made from bamboo are available in the worldwide market (Win et al. 2012; Liu and Wang 2013). However, due to its degradation properties, bamboo packaging plates are easily destroyed by natural mold when they are kept in wet conditions or high relative humidity, such as tropical rainforest areas (Matan et al. 2011; Theapparat et al. 2015; Sequeira et al. 2017). Normally, this packaging must be stored in dry conditions within a large container to provide moisture control, and such a system uses more energy. In this research, the application of an essential oil (Litsea cubeba) to protect against molds on bamboo packaging plates was selected.
Litsea cubeba is found in southern China, Japan, and Southeast Asian countries (Yang et al. 2014). Citral is the main component (78.7 to 87.4%) of L. cubeba oil (Si et al. 2012), which is similar to citrus fruit (Lante and Tinello 2015). L. cubeba oil has shown good antifungal and antimicrobial activity (Liu and Yang 2012; Suhem et al. 2015) in both in vitro (Gogoi et al. 1997) and in vivo tests (Luo et al. 2004). However, L. cubeba has its own distinct flavor and aroma that could have a sensory effect if it is directly added into foods. Within this research, to reduce such an effect, L. cubeba was instead added into the packaging rather than being adding directly into the foods. Therefore, the main objective of this study was to assess mold growth on bamboo packaging plates using high temperature.
EXPERIMENTAL
Chemicals Used in the Study
L. cubeba oil, derived by steam distillation from the fruits, was purchased from the Thai China Flavors & Fragrance Industry Company of Thailand (Nonthaburi, Thailand). Citral, citronellal, 4-hydroxybenzaldehyde, and linalool oxide were purchased from Sigma-Aldrich of Singapore (Nucleos, Singapore).
Culture Preparation
Seven strains of molds (Aspergillus niger, Aspergillus flavus, Penicillium sp., Penicillium cyclopium, Rhizopus sp., Fusarium sp., and Cladosporium sp.), isolated from the bamboo packaging plate, were obtained from the Innovation of Essential Oil for Food Safety and Packaging laboratory of Walailak University in Nakhon Si Thammarat, Thailand. All molds were grown on malt extract agar (MEA; Merck Ltd., Songkhla, Thailand) at 25 °C for 7 days. Suspensions were collected by flooding the surface of the MEA tube with 9 mL of sterile water. Viable molds were counted using MEA (7 log10 CFU ml-1).
Effect of High Temperature on Mold Growth on the Bamboo Packaging Plate
The bamboo packaging composites was first prepared by mixing 5.0 g of bamboo fiber, 1.5 g of corn starch (Thai Holdings, Bangkok, Thailand), and 1.5 g of tapioca starch (Thai Wai Food Products, Bangkok, Thailand) into 20 g of distilled water and then it was boiled for 10 min. The bamboo solution was sterilized at 121 °C for 15 min in an autoclave (Suhem et al. 2017). The solution was held for approximately 5 min or until the temperature dropped to 50 °C, and L. cubeba oil, citral, citronellal, 4-hydroxy-benzaldehyde and linalool oxide were added at concentrations from 50 to 500 mg g-1 into the paste. Next, the paste was poured into the round stainless plates, with a diameter of 5 cm. The bamboo plates were dried in an electric oven (FD23, Binder, Tuttlingen, Germany) at 100 °C for 14 h. Controls were carried out using the same procedure but drying with low temperature (30 °C). All packaging was kept at 27 ± 2 °C with 65 ± 5% relative humidity (RH) in an environmental chamber until the moisture content fell below 12%.
One mL of each mold strain suspension was inoculated on a surface of treated bamboo packaging and kept at 25 °C and 100% RH for 7 days. Next, 25 g of each specimen (n = 3) was used for visible cell counts. Compared to the control, the ones with the lowest essential oil concentration showing no visible mycelium growth were regarded as the minimum inhibitory concentration. Three replicates were prepared for each treatment.
Optimization of Drying Process Parameters for Control (Natural Mold) Using Response Surface Methodology
Experimental design
A central composite face-centered design (CCF) with three factors, X1 (concentrations of L. cubeba oil at 100, 300, and 500 mg g-1), X2 (temperatures at 60, 80, and 100 °C), and X3(times at 12, 14, and 16 h) was employed for this study. The second-order polynomial equation for a 3-factor system is shown in Eq. 1,
(1)
where Y is the predicted response, ![]() o is the interception coefficient,
o is the interception coefficient, ![]() i are the linear terms,
i are the linear terms, ![]() ii are the quadratic terms,
ii are the quadratic terms, ![]() ij are the interaction terms, and xi and xj are the coded levels of the independent variables. Models of the two responses were expressed in terms of coded variables without considering the statistically insignificant terms. The quality of the fit was checked with the coefficient of determination R2 and its statistical significance was found by the F-test. Statistical analysis was performed by using Statistica software (StatSoft, Oklahoma, USA).
ij are the interaction terms, and xi and xj are the coded levels of the independent variables. Models of the two responses were expressed in terms of coded variables without considering the statistically insignificant terms. The quality of the fit was checked with the coefficient of determination R2 and its statistical significance was found by the F-test. Statistical analysis was performed by using Statistica software (StatSoft, Oklahoma, USA).
Mold test on the bamboo packaging plates
Bamboo paste containing L. cubeba oil at 100, 300, and 500 mg g-1 was prepared according to the process described above. After preparation, the paste was poured into a molding and dried at different temperatures (60, 80, and 100 °C) for 12, 14, and 16 h. The average moisture content of the packaging specimens after conditioning at 20 °C and 65% RH for 48 h was 12 ± 1% (n = 10).
To evaluate the mold growth on the bamboo packaging plates, all plates were kept at 25 °C and 100% RH. Each plate (n = 5) was individually rated for mold growth (ASTM D4445 1998). When evaluated with the naked eye, the inhibitory period in days was determined by visualization using a microscope and then recorded. The time periods needed for the initiation of mold growth on bamboo packaging plates were recorded.
Properties of the Packaging Plate and Mold Morphology
A bamboo packaging plate containing 300 mg g-1 of L. cubeba oil after heat treatment at 100 °C for 12 h was selected for this study. The control (a plate containing 300 mg g-1 of L. cubeba oil and 300 mg g-1 of vegetable oil /or from Morakot Industries, Bangkok, Thailand) was carried out using the same method but without heat treatment (drying at 30 °C for 16 h). The plate properties and gas chromatography-mass spectrometry (GC-MS) were then measured.
Wettability measurement
A contact angle goniometer (Kyowa Interface Science, Niiza, Japan) was used to measure the spreading of the distilled water on the bamboo packaging plate. Drops of distilled water were made by a micro-syringe. Five seconds after dropping, five images were captured for every second of contact angle analysis. Each sample was subjected to 8 4-angle position measurements: vertical left, vertical right, horizontal left, and horizontal right. Finally, the images of the distilled water drops were analyzed with a computer contact angle software (FAMAS, Niiza, Japan).
Water absorption
The water absorption was done according to the work of Alamri and Low (2013). The samples were prepared at dimensions of 10 mm × 10 mm × 3.5 mm. The water absorption test was carried out by immersing the specimens in the deionized water for 10 min. The packaging weight was measured after 10 s. Then, to remove the excess water from their surfaces, each sample was dried for 30 min at 100 °C before being weighed. The water absorption (%) was calculated according to Eq. 2,
Absorption (%) = (W2–W1)/W1×100 (2)
where W1 was the initial weight of the bamboo tray and W2 was the weight of the bamboo tray.
Mold morphology
Mold morphology on selected packaging plates was examined after incubation by using a Leica DVM6 digital microscope (Leica Microsystems AG, Heerbrugg, Switzerland).
GC-MS Analysis
The bamboo packaging plates with 300 mg g-1 of L. cubeba oil that were heated at 100 °C for 12 h and then dried at 30 °C for 16 h were subjected to GC-MS. Using a method adapted from Friedman et al. (2000) the essential oil components were extracted from 10 g of the bamboo packaging plates having five replicate specimens. First, the pieces of the packaging plates were added to a glass tube with 4 mL of ethyl acetate. The tube was sealed with a Teflon-lined cap and mixed by gentle shaking. Next, the specimen was held for 30 min before being extracted with ethyl acetate twice more. The combined ethyl acetate extracts were reduced to dryness under a stream of nitrogen at room temperature. Finally, the residue of each was dissolved in 1 mL of ethyl acetate in 1 µL aliquots.
Analyses of L. cubeba oil and the extract solutions (1 µL) were carried out on a gas chromatograph (Hewlett Packard Model 7890A, California, USA) equipped with a DB-5 (J&W Scientific, California, USA) column (30 cm × 0.25 mm ID) and a film thickness of 0.25 µm. The average helium carrier gas flow rate was 1 mL min-1. The split ratio of the column oven was 50:1, and the injector and detector temperatures were set at 250 °C and 260 °C, respectively, with the temperature held at 60 °C for 30 s, increased to 150 °C at 40 °C min-1, and then maxed to 260 °C at 2 °C min-1. The solution was manually injected (1.0 µL). The identification of the constituents was based on a comparison with authentic samples, their Kovats indices, and computer matching with the NIST 0.8 L (Database/ ChemStation data system).
Statistical Analysis
All results were expressed as mean ± standard deviation (n = 3). Data were statistically treated by one-way ANOVA and Duncan’s post hoc test, with P < 0.05 being considered to be statistically significant. Statistical analysis was performed using Statistica software (StatSoft, Oklahoma, USA).
RESULTS AND DISCUSSION
Effect of High Temperature on Mold Growth
The antifungal activity of L. cubeba oil and its main components (citral, citronellal, 4-hydroxybenzaldehyde, and linalool oxide) against mold on bamboo packaging plates is summarized in Table 1. The results showed that citral had the highest antifungal activity. The MICs for citral ranged from 100 to 140 mg g-1 when the bamboo plates were dried at 100 °C for 14 h, while the MICs ranged from 300 to 350 mg g-1 for L. cubeba oil. On the other hand, when the plates were dried at 30 °C, the MIC values of the plates containing citral increased to 300 to 400 mg g-1. Furthermore, no MICs were found when L. cubeba oil and vegetable oil (control) were incorporated into the tray. Although high temperature could reduce the antifungal activity of essential oil, results from this study confirm that it could enhance antifungal activity of L. cubeba oil. From this study, using high temperature for drying plates with citral (main component of L. cubeba oil) showed greater inhibition of all mold with lower MIC values. Therefore, citral might be the key factor for inhibiting mold growth after heat drying.
Table 1. MICs of L. cubeba and its Main Components
L. cubeba oil has been used to address issues of medical concern such as inflammation and headaches; it has also been used for food flavoring (Wang et al. 2009). For the purpose of food safety, Suhem et al. (2015) showed that L. cubeba vapor could prevent the growth of Aspergillus flavus on snack bars and also could strongly inhibit the accumulation of aflatoxin B1 in licorice after being inoculated and incubated with A. flavus for 20 days (Li et al. 2016). The results from this experiment have confirmed that L. cubeba in bamboo trays could prevent the growth of A. flavus and other mold strains but only after drying at a high temperature.
Table 2. Qualitative GC-MS Analysis of Chemical Compositions in L. cubeba Oil Treated Bamboo Fiber Packaging Before and After Heat Drying at 100 °C for 10 min
The possible biosynthetic pathway of citral during heat treatment is shown in Fig. 3. High temperature treatment could cause citral to degrade into various compounds. Two components, i.e. hydroxybenzaldehyde (Wang et al. 2004; Weerawatanakorn et al. 2015) and linalool oxide (King and Dickinson 2000; Serra et al. 2017), were detected after the heat treatment at 100 °C for 10 min. However, citral alone or a combination of it with other essential oil components may be recommended as a natural preservative according to its highly antifungal activity against a food-infesting mold such as A. flavus and aflatoxin secretions (Miron et al. 2014). Citral has a high boiling point of around 229 °C (Lide 2005); this is nearly the same as L. cubeba oil, which is around 232 °C (ChemicalBook, 2017).. Therefore, setting the temperature of bamboo plate drying at 100 °C might change the form of this main component rather than destroying mold. From the GC-MS results (Table 2), higher peak areas of both E-citral and Z-citral after heat drying at 100 °C were detected. In addition, citronellal peak areas were greater after using high temperature and were more effective against molds with MIC values from 450 mg g-1, except for A. flavus and P. cyclopium (MIC > 500 mg g-1). Although, hydroxybenzaldehyde and linalool oxide were found after using high temperature, no MIC values were found for either component. Moreover, limonene (Negro et al. 2016) and geraniol (Miron et al. 2014) showed good properties against microorganisms and using low temperature was recommended (Negro et al. 2016).
Optimization of Bamboo Tray Drying and Natural Protection
The inhibitory periods of treated bamboo trays using different concentrations of L. cubeba oil, temperature, and drying times are shown in Table 3. The constants and the coefficients of the regression equation obtained after ANOVA are presented in Table 3. The concentration of essential oil, time, and temperature (P < 0.05) were significant factors in extending the shelf-life of bamboo trays. The inhibitory period (Y) fit by the polynomial equation (Eq. 3) was fit to a polynomial expression (R2 = 0.83) as follows,
Y = 237.65+16.39X1 – 10.78X2 + 9.56X3 – 33.54X12 -11.75 X1X2 – 26.75 X1X3 (3)
where Y is the inhibitory period (days), X1 is the concentration of essential oil, X2 is the time, and X3 is the temperature.
Response surface plots are also shown in Fig. 1 (a-c). The best condition against natural mold on trays for at least 290 days was L. cubeba oil at 100 mg g-1 and then drying at 300 °C for 12 h (see Tables 3 and 4). The results confirmed that using high temperature (100 °C) for drying could enhance antifungal activity of L. cubeba and extend the shelf-life of the bamboo trays. Lower temperatures (60 °C and 80 °C) showed lower inhibitory periods. Nevertheless, the control (trays without L. cubeba oil) found mold growth on the surface after 1 week of storage in 100% relative humidity at 25 °C.
Fig. 1. Response surface plots showing the effect of drying time (h) and temperature (°C) (a), time (h) and concentration (mg g-1) (b), and temperature (°C) and time (h) (C) on the growth on the mold of bamboo packaging
Table 3. Central Composite Face-centered Design (X1 = Concentrations; mg g-1, X2= Temperatures; °C; X3= Times; hours) and Responses for the Growth of Natural Mold (RSM) on Surface of Bamboo Fiber Packaging with L. cubeba oil
Table 4. Analysis of Variance (ANOVA) for the Response Surface Quadratic Model for the Inhibition Time of the Mold Growth Value of Bamboo Fiber Packaging with L. cubeba Oil
This study agreed with scientific reports about the effect of temperature (mild heat to high) on controlling microorganisms. Haberbeck et al. (2012) reported that using oregano oil with temperatures at 90 °C to 100 °C inhibits Bacillus coagulans spores. Using lime oil with heat curing at 70 °C enhances the inhibition of A. niger on sedge (Lepironia articulata) after 18 weeks of storage time (Matan et al. 2013). Using similar techniques, Matan et al. (2012) showed that garlic oil with heat curing at 100 °C for 24 h inhibits the growth of A. niger on rubberwood for at least 40 weeks. Therefore, high temperature asserts positive influences on the antifungal activity of L. cubeba. High temperature does not decompose citral but the formation of cintronellal could be observed. These compounds might protect packaging from molds for long periods of storage in accelerated conditions.
Possible Modes of Action
The results from the wettability test suggested that there were significant changes in the contact angle between the treated trays (100 ![]() ) with L. cubeba oil at 300 mg g-1 (109
) with L. cubeba oil at 300 mg g-1 (109 ![]()
![]()
![]() ), the trays with L. cubeba oil at 300 mg g-1 (93
), the trays with L. cubeba oil at 300 mg g-1 (93![]()
![]() 2
2![]() ) dried at lower temperatures (30
) dried at lower temperatures (30 ![]() ) and the control trays without essential oil (68
) and the control trays without essential oil (68![]()
![]() 2
2![]() ). The water absorption of treated bamboo trays showed a significant decrease (P<0.05) of 75% from the control (without essential oil). Hydrophobicity is an important characteristic of bamboo trays, and the results from this study demonstrated that water could not be easily adsorbed into the treated specimens. Therefore, after drying at 100
). The water absorption of treated bamboo trays showed a significant decrease (P<0.05) of 75% from the control (without essential oil). Hydrophobicity is an important characteristic of bamboo trays, and the results from this study demonstrated that water could not be easily adsorbed into the treated specimens. Therefore, after drying at 100 ![]() L. cubeba oil had a hydrophobic effect on the trays. Furthermore, the effect of L. cubeba with high temperature drying for inhibiting mold spores on bamboo trays was observed in this study. Figure 2a shows A. nigerspores on bamboo trays were covered by essential oil components and could not be germinated for at least 290 days of storage. This result agreed with the work of Tzortzakis and Economakis (2007), which found that fungal spore production inhibited up to 70% at 25 ppm of lemongrass oil concentration when compared with equivalent plates stored in ambient air. Furthermore, with the highest oil concentration (500 ppm) employed, fungal sporulation was completely retarded. In contrast, without essential oil and high temperature, no spore covering was found on the packaging surfaces (Fig. 2b). After drying, citral might be the main active component against all mold.
L. cubeba oil had a hydrophobic effect on the trays. Furthermore, the effect of L. cubeba with high temperature drying for inhibiting mold spores on bamboo trays was observed in this study. Figure 2a shows A. nigerspores on bamboo trays were covered by essential oil components and could not be germinated for at least 290 days of storage. This result agreed with the work of Tzortzakis and Economakis (2007), which found that fungal spore production inhibited up to 70% at 25 ppm of lemongrass oil concentration when compared with equivalent plates stored in ambient air. Furthermore, with the highest oil concentration (500 ppm) employed, fungal sporulation was completely retarded. In contrast, without essential oil and high temperature, no spore covering was found on the packaging surfaces (Fig. 2b). After drying, citral might be the main active component against all mold.
Table 5. Physical Properties of Bamboo Fiber Packaging With and Without L. cubeba Oil
Fig. 2. Spore of Aspergillus niger on the control packaging for 1 day (a) and on the packaging with L. cubeba oil at concentration 300 mg g-1 for 10 days (b) after incubation at 25 °C with 100% RH
Fig. 3. Possible pathway of citral during heat treatment (King and Dickinson 2000; Wang et al. 2004; Vilella et al. 2005; Weerawatanakorn et al. 2015; Serra et al. 2017)
Li et al. (2014) found that citral at the concentration of 400 µg mL-1 completely inhibits spore germination of Magnaporthe grisea on the potato dextrose agar (PDA) after 24 h. The antifungal activity of citral has also been explained by Tao et al. (2014) who stated that citral disrupts the cell membrane integrity and membrane permeability of P. italicum. When combined with another technique such as high hydrostatic pressure, citral has a greater effect on mold spore inactivation (Palhano et al. 2004). Thus, a combination of citral with a thermal treatment at 95![]() can inhibit the germination or outgrowth of Alicyclobacillus acidoterrestris spores (Huertas et al. 2014). The results from this study are in agreement with previous results.
can inhibit the germination or outgrowth of Alicyclobacillus acidoterrestris spores (Huertas et al. 2014). The results from this study are in agreement with previous results.
CONCLUSIONS
- Overall, drying a bamboo tray containing L. cubeba at 300 mg g-1 at a high temperature (100
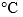 ) for 12 h can enhance the antifungal activity of Litsea cubeba on a bamboo packaging tray. Litsea cubeba components after heat treatment can be released and coated on the surface of a bamboo tray and prevent mold spore germination.
) for 12 h can enhance the antifungal activity of Litsea cubeba on a bamboo packaging tray. Litsea cubeba components after heat treatment can be released and coated on the surface of a bamboo tray and prevent mold spore germination. - After drying at a high temperature with Litsea cubeba, the shelf-life of a bamboo tray increased from 7 days to at least 290 days.
- Lower water adsorption and lower wettability properties of a bamboo tray were detected after heat treatment with Litsea cubeba oil.
- The use of high temperature for drying bamboo packaging trays is normally used in the packaging industry. Also, the application of Litsea cubeba for food flavor and medicine is increasing. Presently, the combined use of essential oil and high temperature drying could limit fungal infections on bamboo trays during long-term storage. This process is interesting and easy to apply in large-scale production of the trays.
ACKNOWLEDGMENTS
This study was supported by the Thailand Research Fund (TRF) through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0057/2556) and the Walailak University Fund (Grant No. WU58105).
REFERENCES CITED
Alamri, H., and Low, I. M. (2013). “Effect of water absorption on the mechanical properties of nanoclay filled recycled cellulose fibre reinforced epoxy hybrid nanocomposites,” Compos. Part A Appl. Sci. 44, 23-31. DOI: 10.1016/j.compositesa.2012.08.026
ASTM D4445 (1998). “Standard test method for fungi-cides for controlling sap stain and mold on unseasoned lumber,” ASTM International, West Conshohocken, PA.
Cao, Y., Wang, W., Wang, Q., and Wang, H. (2013). “Application of mechanical models to flax fiber/wood fiber/plastic composites,” BioResources 8(3), 3276-3288. DOI: 10.15376/biores.8.3.3276-3288.
ChemicalBook. (2017). “Litsea cubeba oil property,” (https://www.chemicalbook.com/ChemicalProductProperty_US_CB5157013.aspx), Accessed 18 December 2018.
Friedman, M., Kozukue, N., and Harden, L. A. (2000). “Cinnamaldehyde content in foods determined by gas chromatography mass spectrometry,” J. Agr. Food Chem. 48(11), 5702-5709. DOI: 10.1021/jf000585g
Gogoi, P., Baruah, P., and Nath, S. C. (1997). “Antifungal activity of the essential oil of Litsea cubeba Pers,” J. Essent. Oil. Res. 9(2), 213-215. DOI: 10.1080/10412905.1997 .9699462
Haberbeck, L. U., da Silva Riehl, C. A., de Cássia Martins Salomão, B., and de Aragão, G. M. F. (2012). “Bacillus coagulans spore inactivation through the application of oregano essential oil and heat,” LWT-Food Sci. Technol. 46(1), 267-273. DOI: 10.1016/j.lwt.2011. 09.021
Huertas, J. P., Esteban, M. D., Antolinos, V., and Palop, A. (2014). “Combined effect of natural antimicrobials and thermal treatments on Alicyclobacillus acidoterrestris spores,” Food Control 35(1), 73-78. DOI: 10.1016/j.foodcont.2013.06.051
Hu, Y. A., He, M., Zhu, R. X., Zhang, Y. H., Lu, Y. L., and Yu, W. J. (2016). “Influence of dyeing treatment on the performance of bamboo-based fibre composites,” J. Trop. For. Sci.28(2), 112-120.
King, A., and Dickinson J. R. (2000). “Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis,” Yeast 16(6), 499-506. DOI: 10.1002/(SICI)1097-0061(200004)16:6<499
Kumar, N., Mireja, S., Khandelwal, V., Arun, B., and Manik, G. (2017). “Light-weight high-strength hollow glass microspheres and bamboo fiber based hybrid polypropylene composite: A strength analysis and morphological study,” Compos. Part B-Eng. 109, 277-285. DOI: 10.1016/j.compositesb.2016.10.052
Lante, A., and Tinello, F. (2015). “Citrus hydrosols as useful by-products for tyrosinase inhibition,” Innov. Food Sci. Emerg. 27, 154-159. DOI: 10.1016/j.ifset.2014.11.001
Li, R. Y., Wu, X. M., Yin, X. H., Liang, J. N., and Li, M. (2014). “The natural product citral can cause significant damage to the hyphal cell walls of Magnaporthe grisea,” Molecules19(7), 10279-10290. DOI: 10.3390/molecules190710279
Li, Y., Kong, W., Li, M., Liu, H., Zhao, X., Yang, S., and Yang, M. (2016). “Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice,” Ind. Crops. Prod. 80, 186-193. DOI: 10.1016/j.indcrop.2015.11.008
Lide, D. R. (2005). CRC Handbook of Chemistry and Physics, CRC Press, Florida, USA, pp. 3-204.
Liu, T. T., and Yang, T. S. (2012). “Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems,” Int. J. Food Microbiol. 156(1), 68-75. DOI: 10.1016/j.ijfoodmicro.2012.03.005
Liu, Y., and Wang, S. H. (2013). “Experimental study of bamboo fiber cushion packaging materials for the express packaging,” Appl. Mech. Mater. 300-301, 1348-1351. DOI: 10.4028/www.scientific.net/AMM.300-301.1348
Luo, M., Jiang, L. K., Huang, Y. X., Xiao, M., Li, B., and Zou, G. L. (2004). “Effects of citral on Aspergillus flavus spores by quasi-elastic light scattering and multiplex microanalysis techniques,” Acta Bioch. Bioph. Sin. 36(4), 277-283. DOI: 10.1093/abbs/36.4.277
Matan, N., Matan, N., and Ketsa, S. (2012). “Effect of heat curing on antifungal activities of anise oil and garlic oil against Aspergillus niger on rubberwood,” Int. Biodeter. Biodegr. 75, 150-157. DOI: 10.1016/j.ibiod.2012.03.012
Matan, N., Matan, N., and Ketsa, S. (2013). “Enhanced inhibition of Aspergillus niger on sedge (Lepironia articulata) treated with heat cured lime oil,” J. Appl. Microbiol. 115(2), 376-381. DOI: 10.1111/jam.12236
Matan, N., Saengkrajang, W., and Matan, N. (2011). “Antifungal activities of essential oils applied by dip-treatment on areca palm (Areca catechu) leaf sheath and persistence of their potency upon storage,” Int. Biodeter. Biodegr. 65(1), 212-216. DOI: 10.1016/j.ibiod.2010.09.009
Miron, D., Battisti, F., Silva, F. K., Lana, A. D., Pippi, B., Casanova, B., Gnoatto, S., Fuentefria, A., Mayorga, P., and Schapoval, E. E. S. (2014). “Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts,” Rev. Bras. Farmacogn. 24, 660-667. DOI: 10.1016/j.bjp.2014.10.014
Negro, V., Mancini, G., Ruggeri, B., and Fino, D. (2016). “Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization,” Bioresource. Technol. 214, 806-815. DOI: 10.1016/j.biortech.2016.05.006
Satyanarayana, K. G., Arizaga, G. G. C., and Wypych, F. (2009). “Biodegradable composites based on lignocellulosic fibers – An overview,” Prog. Mater. Sci. 34(9), 982-1021. DOI: 10.1016/j.progpolymsci.2008.12.002
Sequeirq, S. O., Laia, C. A. T., Phillips, A. J. L., Cabrita E. J., and Macedo, M. F. (2017). “Clotrimazole and calcium hydroxide nanoparticles: A low toxicity antifungal alternative for paper conservation,” J. Cult. Herit. 24, 45-52. DOI: 10.1016/j.culher.2016.12.004
Serra, S., Simeis, D. D., and Brenna, E. (2017). “Lipase mediated resolution of cis– and trans-linalool oxide (pyranoid),” J. Mol. Catal. B-Enzym. 133(1), S420-S425. DOI: 10.1016/j.molcatb.2017.03.004
Shahroze, R. M., Ishak, M. R., Satil, M. S., Leman, Z., Asim, M., and Chandrasekar, M. (2018). “Effect of organo-modified nanoclay on the mechanical properties of sugar plam fiber-reinforced polyester composites,” BioResources 13(4), 7430-4777. DOI: 10.15376/biores.13.4.7430-7444
Si, L., Chen, Y., Han, X., Zhan, Z., Tian, S., Cui, Q., and Wang, Y. (2012). “Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China,” Molecules 17(6), 7057-7066. DOI: 10.3390/molecules17067057
Suhem, K., Matan, N., Matan, N., Danworaphong, S., and Aewsiri, T. (2015). “Improvement of the antifungal activity of Litsea cubeba vapor by using a helium–neon (He–Ne) laser against Aspergillus flavus on brown rice snack bars,” Int. J. Food Microbiol. 215, 157-160. DOI: 10.1016/j.ijfoodmicro.2015.09.008
Suhem, K., Matan, N., Matan, N., Danworaphong, S., and Aewsiri, T. (2017). “Enhanced antifungal activity of michelia oil on the surface of bamboo paper packaging boxes using helium-neon (HeNe) laser and its application to brown rice snack bar,” Food Control 37, 939-945. DOI: 10.1016/j.foodcont.2016.10.006
Tao, N., Ou Yang, Q., and Jia, L. (2014). “Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism,” Food Control 41(1), 116-121. DOI: 10.1016/j.foodcont.2014.01.010
Theapparat, Y., Chandumpa, A., Leelasuphakul, W., and Laemsak, N. (2015). “Pyroligneous acids from carbonisation of wood and bamboo: Their components and antifungal activity,” J. Trop. For. Sci. 27(4), 517-526.
Tzortzakis, N. G., and Economakis, C. D. (2007). “Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens,” Innov. Food Sci. Emerg.8(2), 253-258. DOI: 10.1016/j.ifset.2007.01.002
Wang, F., Yang, G., Zhang, W., Wu, W., and Xu, J. (2004). “Oxidation of p-cresol to p-hydroxybenzaldehyde with molecular oxygen in the presence of CuMn-oxide heterogeneous catalyst,” Adv. Synth. Catal. 346(6), 633-638. DOI: 10.1002/adsc.200303226
Wang, Y., Jiang, Z. T., and Li, R. (2009). “Complexation and molecular microcapsules of Litsea cubeba essential oil with β-cyclodextrin and its derivatives,” Eur. Food Res. Technol.228(6), 865-873. DOI: 10.1007/s00217-008-0999-3
Weerawatanakorn, M., Wu, J. C., Pan, M. H., and Ho, C. T. (2015). “Reactivity and stability of selected flavor compounds,” J. Food. Drug Anal. 23(2), 176-190. DOI: 10.1016/j.jfda.2015.02.001
Win, K. K., Ariyoshi, M., Seki, M., and Okayama, T. (2012). “Effect of pulping conduction on the properties of bamboo paper,” Sen’i Gakkaishi 64(11), 290-295. DOI: 10.2115/fiber.68.290
Yang, K., Wang, F. C., You, C. X., Geng, Z. F., Sun, R. Q., Guo, S. S., Du, S. S., Liu, Z. L., and Deng, Z. W. (2014). “Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects,” J. Asia-Pac. Entomol. 17(3), 459-466. DOI: 10.1016/j.aspen.2014.03.011
Zakikhani, P., Zahari, R., Sutan, M. T. H., and Majud, D. L. (2016). “Thermal degradation of four bamboo species,” BioResources 11(1), 414-425. DOI: 10.15376/biores.11.1.414-425
Article submitted: October 16, 2018; Peer review completed: November 26, 2018; Revised version received and accepted: December 20, 2018; Published: December 21, 2018.
DOI: 10.15376/biores.14.1.1289-1302
