Abstract
Polypropylene/wood flour/organoclay hybrid nanocomposites were melt-compounded in an internal mixer at 190 oC and 60 rpm rotor speed. Then samples were fabricated by injection molding. Effects of immersion temperature on the water uptake of hybrid nanocomposite were investigated. To meet this objective, water absorption of samples was determined after 24 h immersion in distilled water at different temperatures (25, 50, 75, and 100 °C). Results indicated that immersion temperature had a significant influence on the water absorption of composites. By increasing the temperature, water absorption increases as well. The maximum water absorption of composite is decreased by increasing the nanoclay and compatibilizer content. The morphology of nanoclay was determined by X-ray diffraction (XRD) and transmission electron microscopy. The effect of morphology on water absorption was also evaluated. Due to inadequate compatibilizer, exfoliated morphology of nanoclay was not obtained, but there was evidence of intercalation. The order of intercalation for samples containing 3 phc was higher than that of 6 phc at the same PP-g-MA content due to some agglomerations of organoclay.
Download PDF
Full Article
EFFECT OF IMMERSION TEMPERATURE ON THE WATER UPTAKE OF POLYPROPYLENE/WOOD FLOUR/ORGANOCLAY HYBRID NANOCOMPOSITE
Behzad Kord,a,* Saeed Ismaeilimoghadam,b and Behrouz Malekian c
Polypropylene/wood flour/organoclay hybrid nanocomposites were melt-compounded in an internal mixer at 190 oC and 60 rpm rotor speed. Then samples were fabricated by injection molding. Effects of immersion temperature on the water uptake of hybrid nanocomposite were investigated. To meet this objective, water absorption of samples was determined after 24 h immersion in distilled water at different temperatures (25, 50, 75, and 100 °C). Results indicated that immersion temperature had a significant influence on the water absorption of composites. By increasing the temperature, water absorption increases as well. The maximum water absorption of composite is decreased by increasing the nanoclay and compatibilizer content. The morphology of nanoclay was determined by X-ray diffraction (XRD) and transmission electron microscopy. The effect of morphology on water absorption was also evaluated. Due to inadequate compatibilizer, exfoliated morphology of nanoclay was not obtained, but there was evidence of intercalation. The order of intercalation for samples containing 3 phc was higher than that of 6 phc at the same PP-g-MA content due to some agglomerations of organoclay.
Keywords: Immersion Temperature; Water Uptake; Organoclay; Morphology
Contact information: a: Assistant Professor, Department of Wood Science and Paper Technology, Islamic Azad University, Chalous Branch, P.O. Box: 46615/397, Mazandaran, Iran, b: Member of Young Researchers Club of Islamic Azad University, Chalous Branch, c: Bachelor Student of Islamic Azad University, Chalous Branch; * Corresponding author:behzad_k8498@yahoo.com
INTRODUCTION
In the last decade, natural organic reinforcements such as cellulose fibers have penetrated slowly into this market because they offer many advantages over most common inorganic fillers. Cellulose fibers are abundantly available and have lower costs and density. They lead to a reduced wear of processing equipment and are renewable, recyclable, and non-hazardous and biodegradable (Rowell et al. 1997). The replacement of inorganic fillers with comparable cellulose fibers provides weight savings and decreases the cost without reducing the rigidity of the composites. Wood fibers are used most extensively among the cellulose fibers used as fillers (Lee 2008).
Wood fiber/plastic composites (WPCs) can be a cost-effective alternative to many plastic composites or metals in terms of bending stiffness or weight (Woodhams et al. 1984). The wood fibers are non-abrasive so that relatively large concentrations can be incorporated into plastics without causing serious machine wear during blending and processing. The main applications of WPCs are in building products, such as fencing, rails, decking, door and window profiles, decorative trims, and so on. These composites are also gaining acceptance in automotive, industrial and marine applications (Bledzki and Gassan 1999; Rowell et al. 1997).
New applications and end uses of wood plastic composites (WPCs) for decking, flooring and outdoor facilities, window frames, various construction materials, and bathroom parts for example, and their exposure to atmosphere or contact with aqueous media has made it necessary to evaluate the water uptake characteristics of these materials. Water absorption is one of the most important characteristics of WPCs exposed to environmental conditions that determine their end use applications. Therefore, as a limiting parameter, water absorption has to be taken into account in the design of WPCs for final applications (Khan et al. 2003; Adhikary et al. 2008).
Besides moisture, most applications also expose the material to a wide range of temperatures, which may influence water absorption mechanism (distribution, amount and rate of water absorption), and its subsequent irreversible effects and environmental aging are of major practical repercussion. Temperature may influence water absorption in composites in a complex manner (Bao and Yee 2002; Kazemi Najafi et al. 2007).
Nanocomposite technology with layered silicate clays as in situ reinforcement has been intensively investigated. Essential improvements of physical and mechanical prop-erties, thermal stability, flame resistance, and barrier resistance have been observed for various thermoplastic and thermoset nanocomposites at low silicate content (Tjong 2006; Viswanathan et al. 2006; Utracki et al. 2007). Using nanoclay filler in WPC composite has been reported in the literatures (Zhao et al. 2006; Wu et al. 2007; Lei et al. 2007; Han et al. 2008; Hetzer and Kee 2008; Ghasemi and Kord 2009; Hemmasi et al. 2010; Kord et al. 2010; Kord 2010). Many efforts have been made in the formation of wood polymer composite to improve such properties so as to meet specific end-use requirements. The aim of this study was to investigate the effect of immersion temperature on the water uptake of polypropylene/wood flour/organoclay hybrid nanocomposite.
EXPERIMENTAL
Materials
Polypropylene, V30S (MFI=18 g/10min, density=0.92g/cm3) was supplied by Arak Petrochemical Co (Iran). Beech wood-flour (WF) was from Cellulose Aria Co (Iran). The particle size of wood flour was 100 meshes. PP-g-MA provided by Solvay with trade name of Priex 20070 (MFI=64 gr/10min, grafted maleic anhydride 0.1 Wt. %) was used as coupling agent. Montmorillonite modified with a methyl, tallow, bis-2-hydroxyethyl, quaternary ammonium with Cationic Exchange Capacity (CEC) of 90 meq/100gclay, density of 1.98 g/cc, and a d-spacing (d001) of 18.5Ao was obtained from Southern clay Products Co., USA, with trade name Cloisite 30B.
Methods
Composite preparation
Before preparation of samples, wood flour was dried in an oven at 65 ± 2 °C for 24 h. Then polypropylene, wood flour, and nanoclay were weighed and bagged according to formulations given in Table 1. The mixing was carried out at 180 oC and 60 rpm using a HAAKE internal mixer (HBI System 90, USA). First the PP was fed to the mixing chamber; after it was melted, the coupling agent and montmorillonite was added. At the fifth minute, wood flour was added, and the total mixing time was 13 min. The compounded materials were then ground using a pilot scale grinder (WIESER, WGLS 200/200 Model). The resulted granules were dried at 105°C for 4 h. Test specimens were prepared by injection molding (Eman machine, Iran). Finally, specimens were conditioned at a temperature of 23°C and relative humidity of 50% for at least 40 h according to ASTM D618-99 prior to testing.
Table 1. Composition of the Studied Formulations
*Per hundred compound
Measurements
Water absorption and thickness swelling tests were carried out according to ASTM D-7031-04 specification after 24 h immersion in distilled water at 25 °C (room temperature), 50 °C, 75 °C and 100 °C. The specimens of each formulation were dried in an oven for 24 h at 105 ± 2 °C. The weight and thickness of dried specimens were measured to a precision of 0.001 g and 0.001 mm, respectively. The specimens were then placed in distilled water in different temperatures. For each measurement, specimens were removed from the water and the surface water was wiped off using blotting paper. The values of the water absorption in percentage were calculated using the following equation:
(1)
where WA (t) is the water absorption at time t, Wo is the oven dried weight and W(t) is the weight of specimen at a given immersion time t.
Wide angle X-ray diffraction (XRD) analysis was carried out with a Seifert-3003 PTS (Germany) with CuKα radiation (λ=1.54 nm, 50 kV, 50 mA) at room temperature; the scanning rate was 1o/min. The morphology structure of the nanocomposites was investigated by a Philips (Model EM 208, Netherlands) transmission electron microscope (TEM) with an acceleration voltage of 100 kV. The ultrathin slides were obtained with a Leica Ultracut UCT device (Germany).
The statistical analysis was conducted using SPSS programming (Version 13) method in conjunction with the analysis of variance (ANOVA) techniques. Duncan multiply range test (DMRT) was used to test the statistical significance at α = 0.05 level. For each treatment level, five replicate samples were tested.
RESULTS AND DISCUSSION
Statistical analysis indicated that immersion temperature had a significant influence on the water uptake of polypropylene/wood flour/organoclay hybrid nanocomposites. From Fig. 1, it can be concluded that the temperature increased the water absorption of wood plastic nanocomposites (Bao and Yee 2002; Kazemi Najafi et al. 2007). By the increasing of temperature the chemical components of wood changes; this means that some chemical components such as hemi-cellulose, carbohydrate, extractives, and mineral component are extracted from the wood as a result of higher temperature (component soluble in hot water). Therefore, the OH groups in the wood flour would be accessible and react with water. Yang et al. (2005) reported that hemicelluloses are the most vulnerable components of wood towards high temperatures. Cellulose and lignin structures will change under the harsh conditions which exist at very high temperatures, such that they are decomposed.
Figure 1 also shows that the water absorption decreased with increase of nanoclay loading. It seems that the barrier properties of nanoclay fillers inhibit the water permeation in the polymer matrix. Two mechanisms have been reported for this phenomenon. The first is based on the hydrophilic nature of the clay surface that tends to immobilize some of the moisture (Rana et al. 2005); second, surfactant-covered clay platelets form a tortuous path for water transport (Alexandre et al. 2006; Bharadwaj et al. 2002). This barrier property hinders water from going into the inner part of the nanocomposite. It seems that both of aforesaid mechanisms could be more efficient when the morphology is exfoliated. In other words, in the exfoliated morphology there is more available surface of organoclay (with hydrophilic nature) and surfactant (tortuous path), so the water transport goes down under the severe conditions. The reason for less water uptake could be existence of nanoclay as a nucleating agent (Ghasemi and Kord 2009). Due to such nucleation the crystallinity of the hybrid composite can be improved by the presence of the nanofiller as a nucleating agent. As the crystalline regions are impermeable, the water absorption is less in the composites.
Figure 2 shows that the water absorption decreased with the addition of coupling agent. This means that it is the interfacial region which influences the water uptake of the composite. Because uncompatibilized wood flour composite has weak fiber/matrix adhesion in nature, the interface is enhanced in the presence of the compatibilizer. Generally it is necessary to use compatibilizers or coupling agents in order to improve the polymer/fiber bonding and in turn to enhance water resistance. The PP-g-MA chemically bonds with the OH groups in the lignocellulosic filler and limits the water absorption of the composites. As a result, it is important to use coupling agents to improve the quality of adhesion between plastics and fibers, to reduce the gaps in interfacial region, and to block the hydrophilic groups (Kokta et al. 1990; Raj and Kokta 1989; Gauthier et al. 1998; George J, Sreekala and Thomas 2001; Guduri and Luyt AS 2007).
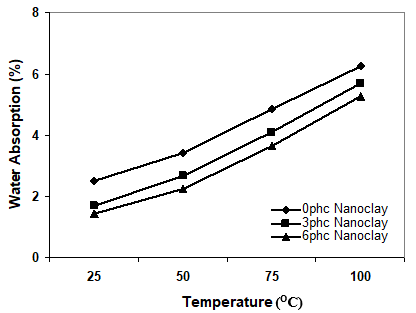
Figure 1. Effect of immersion temperature on the water uptake of samples at different levels of nanoclay
According to a review of the literature the incompatibility between phases results in a poor interfacial adhesion between hydrophilic wood and the hydrophobic polymer matrix, which results in poor adhesion and therefore in poor ability to transfer stress from the matrix to the fiber reducing the composite properties. So, the use of coupling agents improves the quality of adhesion between polymer and wood flour to reduce the gaps in interfacial region and to block the hydrophilic groups. Also, the clay is naturally a hydrophilic material, which makes it difficult to exfoliate in a polymer matrix. Therefore, the surface treatment of silicate layers is necessary to render its surface more hydrophobic, which facilitates exfoliation. Generally, this can be done by ion-exchange reactions with cationic surfactants, including primary, secondary, tertiary, and quaternary alkylammonium cations. This modification also leads to expansion of the basal spacing between the silicate layers due to the presence of alkyl chains intercalated in the interlayer. For polymers containing polar functional groups, an alkyl ammonium surfactant is adequate to promote nanocomposite formation. However, in the case of polypropylene, it is frequently necessary to use a coupling agent, such as maleic anhydride modified polypropylene (PP-g-MA).
As a conclusion we can expected that there are four important functions for a coupling agent: (1) the coupling agent can improve the interfacial region between hydrophilic wood and the hydrophobic polymer matrix; (2) the compatibilizer should be miscible with the polypropylene matrix; (3) it should include a certain amount of polar functional groups in a molecule; and (4) the coupling agent reacts with nanoclay, polymer, and wood flour.
According to Fig. 2, the addition of coupling agent to the composites boosted the thermal stability of the composite system. It seems that the addition of coupling agent improves the nucleation efficiency and the crystallinity of the composite.
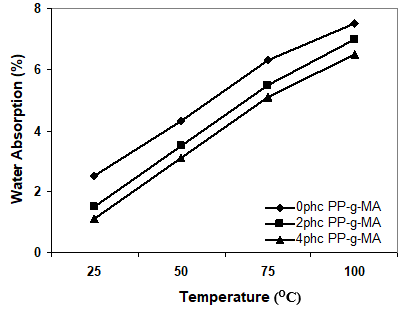
Figure 2. Effect of immersion temperature on the water uptake of samples at different levels of PP-g-MA
Dispersion Behavior
It is useful to start by describing the morphology of the nanocomposite. Characterization of morphological state of the hybrid composite was accomplished using X-ray diffraction and transmission electron microscopy (TEM). The X-ray scattering intensities for modified organoclay and hybrid composites with different levels of nanoclay are demonstrated in Fig. 3, where the quantity 2θ (equal to 4.76º) is related to neat clay with a basal spacing of 18.5nm. In the sample with 3 phc concentration of clay, the peak was shifted to a lower angle (2θ = 4.48º, d-spacing = 19.70nm), which implies the formation of the intercalation morphology. The (001) peak of 6 phc nanoclay appeared at 2θ=4.54º, with a d-spacing of 19.44 nm. These data show that the order of intercalation is higher for 3 phc of nanocaly than the 6 phc of nanoclay concentration. By increasing the nanoclay loading, the water absorption decreased, because when the naoclay loading increased the agglomeration of clay caused the accessibility of water absorption to decrease (this phenomenon called a zigzag effect).
The same trend was observed in Fig. 4, which shows the X-ray diffraction of samples with the different levels of PP-g-MA at the same concentration of nanoclay. As can be seen with increase of PP-g-MA in the composite, the d-spacing (doo1) was increased. Because of the strong interaction between clay layers and coupling agent, PP-g-MA molecules could enter and penetrate the gallery between clay layers when the clay was mixed with 4phc of PP-g-MA.
Figure 3. XRD patterns of composites with different levels of nanoclay
Figure 4. XRD patterns of composites with different levels of coupling agent
Figure (5) shows the dispersion state of the nanocomposites, as it was made evident by TEM. The dark line represents the intersection of the silicate layers, while the white background corresponds to polypropylene matrix. When the loading level of nanoclay into the PP/WF composite was as low as 3 phc (Fig. 5a), nanoclay exhibited better dispersion of the clay layers within the polymer matrix than at 6 phc of nanoclay content (Fig. 5b). Increasing the level of nanoclay to 6 phc, the size of dispersed nanoclay became larger or even aggregated in part (as confirmed by decreased d-spacing from XRD in Fig. 3). It is also clear from the TEM images in Fig. 5c that the presence of coupling agent improved the dispersion of the nanoclay, as the clay aggregates were broken down into smaller stacks. With increase of PP-g-MA, the inter-gallery spacing of composite increased (as confirmed by increased d-spacing from XRD in Fig. 4).
Figure 5. TEM micrographs of polypropylene/wood flour/organoclay hybrid nanocomposite: (a) 3phc clay (b) 6phc clay (c) 3phc clay with 2phc PP-g-MA
CONCLUSIONS
The following conclusions could be drawn from the results of the present study:
1. Immersion temperature had a significant influence on the water uptake of polypropyl-ene/wood flour/organoclay hybrid nanocomposites. By increasing the temperature, water absorption increased as well.
2. With increase of the nanoclay and compatibilizer content in composite, water absorption decreased.
3. Morphological findings of nanocomposites by X-ray diffraction patterns (XRD) and transmission electron microscopy (TEM) showed that samples containing 3 phc of nanoclay had higher order of intercalation than those at 6 phc. Also, the clay dispersion can be improved in the PP matrix in the presence of coupling agent.
REFERENCES CITED
Adhikary, K. B., Pang, S., and Staiger, M. P. (2008). “Long-term moisture absorption and thickness swelling behaviour of recycled thermoplastics reinforced with Pinus radiata sawdust,” Chemi Engineer J. 142, 190-198.
Alexandre, B., Marais, S., Langevin, S., Médéric, P., and Aubry, T. (2006). “Nanocomposite-based polyamide 12/montmorillonite: Relationships between structures and transport properties,” Desalin. J. 99, 164-166.
Bao, L. R., AND Yee, A. F. (2002). “Effect of temperature on moisture absorption in bismaleimide resin and its carbon fiber composites,” Polym. J. 43, 3987-3997.
Bharadwaj, R. K., Mehrabi, A. R., Hamilton, C., Trujillo, C., Murga, M., Fan, R., Chavira, A., and Thompson A. K. (2002). “Structural property relationship crosslinked polyester clay nanocomposites,” Polym. J. 43, 3699-3705.
Bledzki, A. K., and Gassan, J. (1999). “Composites reinforced with cellulose based fibers,” J. Polym. Sci. 24, 221-274.
Gauthier, T. C., Joly, A. C., Coupas, H., and Escoubes, M. (1998). “Interfaces in polyolefin-cellulose fiber composites; chemical coupling, morphology, correlation with adhesion and aging in moisture,” Journal of Polymer Composite 19, 287-300.
George, J., Sreekala, M. S., and Thomas, S. (2001). “A review on interface modification and characterization of natural fiber reinforced plastic composites,” Journal of PolymerEngineering Science 41, 1471-1485.
Ghasemi, I., Kord, B. (2009). “Longterm water absorption behavior of polypropylene/ wood flour/organoclay hybrid nanocomposite,” Irani. Polym. J. 18(9), 683-691.
Guduri, B. R., and Luyt, A. S. (2007). “Comparison of the influence of different compatabillizers on the structures and properties of ethylene vinyl acetate copolymer/modified clay nanocomposites,” Journal of Applied Polymer Science 103, 1268-1274.
Han, G., Lei, Y., Wu, Q., Kojima, Y., and Suzuki, S. (2008). “Bamboo-fiber filled high density polyethylene composites; effect of coupling treatmentand nanoclay,” J. Polym. Enviro. 21, 1567-1582.
Hemmasi, A., Khademi-Eslam, H., Talaiepoor, M., Ghasemi, I., and Kord, B. (2010). “Effect of nanoclay on the mechanical and morphological properties of wood polymer nanocomposite,” J. Reinf. Plas. Comp. 29(7), 964-971.
Hetzer, M., Kee, D. (2008). Wood/polymer/nanoclay composites, environmentally friendly sustainable technology; A review. Chemi Engineer J, 16, 1016-1027.
Kazemi Najafi, S., Tajvidi, M., and Hamidinia, E. (2007). “Effect of temperature, plastic type and virginity on the water uptake of sawdust/plastic composites,” Holz Roh Werks. 65, 377-382.
Khan, M. A., Islam, M. N., Alam, M. K., and Zaman, M.A (2003). “Study of water absorption behavior in wood plastic composites by using neutron radiography techniques,” Polymer, J. Plast. Tech. and Engineer. 42, 925-934.
Kokta, B. V., Beland, P., and Maldas, D. (1990). “Improving adhesion of wood fiber with polystyce by chemical treatment of fiber with coupling agent and influence on mechanical properties,” Journal of Composite 3, 529-539.
Kord, B., Hemmasi, A., and Ghasemi, I. (2010). “Properties of PP/wood flour/ organomodified montmorillonite nanocomposite,” Wood Sci and Tech J., in press.
Kord, B. (2010). “Effect of organo-modified layered silicates on flammability performance of HDPE/ Rice husk flour nanocomposite,” J. Appl. Polym. Sci., in press.
Lee, H.Y. (2008). “Foaming of wood flour/polyolefin/layered silicate composite,” PhD Thesis in Faculty of Forestry, Toronto University, 198.
Lei, Y., Wu, Q., Clemons, C. M., Yao, F., and Xu, Y. (2007). “Influence of nanoclay on properties of HDPE/wood composites,” J. Appl. Polym. Sci. 18, 1425-1433.
Raj, R. G., and Kokta, B. V. (1989). “Effect of chemical treatment of fiber composites,” Journal of Adhesive Science Technology 3, 55-64.
Rana, H. T., Gupta, R. K., Ganga Rao, H.V.S., and Sridhar, L. N. (2005). “Measurement of moisture diffusivity through layered-silicate nanocomposites,” AIChE J. 51, 3249-3256.
Rowell, R. M., Sandi, A. R., Gatenholm, D. F., and Jacobson, R. E. (1997). “Utilization of natural fibers in plastic composites: Problem and opportunities in lignocellulosic composites,” J. Compos. 18, 23-51.
Tjong, S. C. (2006). “Structural and mechanical properties of polymer nanocomposites; A review,” J. Mater. Sci. Engineer. 53, 73-197.
Utracki, L. A., Sepehr, M., and Boccaleri, E. (2007). “Synthetic layered nanoparticles for polymeric nanocomposites (PNCs); A review,” Polym. Adva. Tech. J. 18, 1-37.
Viswanathan, V., Laha, T., Balani, K., Agarwal, A., and Seal, S. (2006). “Challenges and advances in nanocomposite processing techniques; A review,” J. Mater. Sci. Engineer. 54, 121-285.
Woodhams, R. T., Thomas, G., and Rodgers, D. K. (1984). “Wood fibers as reinforcing fillers for polyoefins,” J. Polym. Engineer. Sci. 24, 1166-1171.
Wu, Q., Lei, Y., Clemons, C. M., Yao, F., Xu, Y., and Lian, K. (2007). “Properties of HDPE/clay/wood nanocomposites,” J. Plast. Tech. 27, 108-115.
Yang, H. S., Wolcott, M. P., Kim, H. S., and Kim, H. J. (2005). “Thermal properties of lignocellulosic fillers thermoplastic polymer bio-composites,” J. Therm. Analy. Calorim. 82, 157-160.
Zhao, Y., Wang, K., Zhu, F., Xue, P., and Jia, M. (2006). “Properties of poly (vinylchloride)/ woodflour/montmorillonite composites: Effects of coupling agents and layered silicate,” J. Polym. Degrad. Stabil. 91, 2874-2883.
Article submitted: October 29, 2010; Peer review completed: November 20, 2010; Revised version received: Dec. 16, 2010; Accepted: January 1, 2011; Published: January 3, 2011.
