Abstract
Autohydrolysis of Eucalyptus globulus both with and without the addition of 5 g/L formic acid was explored for different liquid-to-solid (L/S) ratios at 150 ºC for 100 min. The L/S ratio has an impact on the dissolution of wood components during prehydrolysis. The extraction yield of wood components other than lignin decreased with increasing L/S ratio, while lignin removal increased with increasing L/S ratio, irrespective of acid reinforcement. The molecular weight (Mw) of dissolved hemicelluloses and lignin remained relatively constant. The average degree of polymerization of hemicelluloses isolated from the hot water extract was between 7 and 8 over a L/S ratio range from 3:1 to 50:1. The cellulose to glucose conversion yield of pretreated wood samples improved during prehydrolysis with 5 g/L formic acid. Although the conversion yield of autohydrolyzed wood meal stayed relatively constant, the conversion yield of dilute acid-pretreated wood samples increased with increasing L/S ratio.
Download PDF
Full Article
Effect of Liquid to Solid Ratio on Autohydrolysis of Eucalyptus globulus Wood Meal
Mehmet S. Tunc
Autohydrolysis of Eucalyptus globulus both with and without the addition of 5 g/L formic acid was explored for different liquid-to-solid (L/S) ratios at 150 ºC for 100 min. The L/S ratio has an impact on the dissolution of wood components during prehydrolysis. The extraction yield of wood components other than lignin decreased with increasing L/S ratio, while lignin removal increased with increasing L/S ratio, irrespective of acid reinforcement. The molecular weight (Mw) of dissolved hemicelluloses and lignin remained relatively constant. The average degree of polymerization of hemicelluloses isolated from the hot water extract was between 7 and 8 over a L/S ratio range from 3:1 to 50:1. The cellulose to glucose conversion yield of pretreated wood samples improved during prehydrolysis with 5 g/L formic acid. Although the conversion yield of autohydrolyzed wood meal stayed relatively constant, the conversion yield of dilute acid-pretreated wood samples increased with increasing L/S ratio.
Keywords: Autohydrolysis; Eucalyptus; Fractionation; Formic acid; Hemicelluloses; Liquid to solid ratio; Preextraction; Prehydrolysis; Wood; Xylan
Contact information: American Process Inc., Atlanta, GA 30308, USA;
Correspondence: stunc@americanprocess.com
INTRODUCTION
The increasing concentration of greenhouse gases in the atmosphere and the high cost of oil have once again facilitated the concept of a biorefinery (Myerly et al. 1981). This technology involves converting plant biomass into a wide range of renewable products, such as fuels, chemical intermediates, nanocellulose, and biomaterials, after fractionation of the biomass into its main components: cellulose, hemicellulose, and lignin. However, the technology to convert plant biomass to liquid fuels economically is not available at the present time and has been a subject of intense investigation for the last decade.
To remove hemicellulose in pure form from lignocellulosics, the covalent bonds (ester and ether) between hemicelluloses and lignin in the form of lignin carbohydrate complexes (LCC) must first be hydrolyzed. This process can be accomplished by alkaline, acidic, or hydrothermal prehydrolysis. Preextraction, which is also known as prehydrolysis, is the most important step for techno-economic utilization of lignocellulosic biomass; the preextraction process extends the use of biomass by decreasing the yield of degradation products, improving acidic hydrolysis or enzymatic saccharification of cellulose, reducing energy consumption, and increasing the amount of dissolution (Harris et al. 1984).
Significant amounts of xylan can be extracted from hardwood using aqueous alkaline solutions without prior delignification (Timell 1967). However, alkaline preextraction processes are not well suited for complete utilization of hemicellulose due to extensive degradation of hemicellulose and other carbohydrates into low-molecular weight (Mw) hydroxy-acids that are difficult to recover as pure constituents (Sixta and Schild 2009). In addition, extensive degradation of cellulose polymer (less than 20% of the original Mw) takes place, and no more than 75% of the cellulose that is originally present in the wood can be recovered (Young and Akhtar 1998).
Hemicelluloses, especially O-acetyl-(4-O-methylglucurono) xylan in hardwood and annual plants, are more vulnerable to hydrolysis during dilute acid pretreatment than cellulose. Therefore, the time requirement for hydrolysis of hemicelluloses is much shorter, allowing selective isolation of hemicelluloses from lignocellulosics by prehydrolysis, while both cellulose and lignin remain mostly in the solid phase (Casebier et al. 1969; Conner 1984; Conner and Lorenz 1986; Gütsch et al. 2012; Seaman 1945). The degree of xylan hydrolysis is influenced by time, temperature, acidity, liquid-to-solid (L/S) ratios, and wood species (Sixta 2006).
When lignocellulosic biomass is exposed to hydrothermal treatment, labile acetyl groups (AcG) attached to the hemicellulose backbone are released as acetic acid (AcOH), which acts as a hydrolysis catalyst (Brasch and Free 1965). This process is also known as autohydrolysis (AH) because the hydrolyzing compound is released from the substrates itself. When wood chips are treated with pure water or steam (at 165 to 180 °C), hemicelluloses are degraded into monosugars and oligomers with a degree of polymerization (DP) of 13 to 20, which is low enough for oligomers to diffuse out of chips into the reaction medium (Casebier et al. 1973; Overend et al. 1987). However, hemicelluloses with higher DP, ranging from 40 to 70, can easily diffuse out when wood meal (≤ 2 mm) is used instead of wood chips (Tunc and van Heiningen 2011) due to much better diffusion as a result of size reduction. AcG released from the xylan backbone can be found in the free form as AcOH or bound to xylooligosaccharides as OAc in the autohydrolyzate (Leschinsky et al. 2009; Testova et al. 2011; Tunc and van Heiningen 2008a; 2008b), and the ratio of free-to-bound AcOH found in the AH extract increases significantly with increasing intensity of AH (Testova et al. 2011). AH extract also contains monomeric and oligomeric sugars, lignin residues, uronic acid groups (UAG), extractives, and ash, as well as degradation/decomposition products such as furfural, hydroxymethylfurfural (HMF), formic acid (FA), and levulinic acid (Casebier et al. 1969; 1973; Garrote et al. 1999; Leschinsky et al. 2009; Lora and Wayman 1978; Testova et al. 2011; Tunc and van Heiningen 2008a; 2008b; 2009).
The effect of operating conditions such as temperature, time, and pH on the dissolution of hemicelluloses during AH of lignocellulosic biomass has been investigated in the literature. However, the effect of L/S ratio is still not fully explored, and it has been a subject of controversial discussions (Leschinsky et al. 2009). Schild (1994) reported that the xylan dissolution rate decreases significantly with decreasing L/S ratio from 5:1 to 3.5:1 and 2.1:1 during AH of Eucalyptus (E.) saligna wood chips at 170 °C. On the other hand, Garrote et al. (1999) observed only a negligible influence of L/S ratio on the dissolution of xylan during mild AH of E. globulus. Leschinsky et al. (2009) suggested that a possible reason for the contradictory results might be due to the experimental setup. The decision of whether or not the wood residues were washed extensively following AH plays an important role in the dissolution of hemicellulose. The cited researchers concluded that the strong impact of the L/S ratio on xylan dissolution observed by Schild (1994) was probably due to the high amount of the extract remaining in the wood pores, as the washing step was ignored. Therefore, the subject matter needs to be further explored. The effect of the L/S ratio on the dissolution of hemicelluloses during AH with or without dilute acid is the subject of this study.
EXPERIMENTAL
Materials
Eucalyptus globulus wood samples were used as a feedstock for AH experiments both with and without the addition of 5 g/L FA. E. globulus wood chips were air dried and ground in a Wiley mill. The fractions passing through 2-mm holes were then stored in double plastic bags for experiments. Analytical-grade FA with a purity of 99.5% was purchased from Sigma-Aldrich and used for the experiments.
Methods
Autohydrolysis of E. globulus
AH of E. globulus both with and without the addition of 5 g/L FA was conducted at 150 °C for 100 min in the extraction cell of a modified ASE100 commercial apparatus manufactured by Dionex. An expansion tank was introduced to conduct a constant volume extraction while a heat exchanger was installed to cool down the extract at the end of the preextraction (Tunc and van Heiningen 2008a). In addition three more solvent delivery bottles were adapted through a four-way valve to perform multi-solvent extraction (Tunc and van Heiningen 2013a). The volume of the extraction cell was 100 mL. The L/S ratio of AH was adjusted by varying the amount of wood (solid) loaded into the cell. A subsequent washing step with 150 mL of water following AH was conducted in the extraction cell to remove all dissolved wood components from the wood pores (Tunc and van Heiningen 2008a; 2008b; 2013b) after the AH process. The heat up and cool down time of all experiments conducted in the modified ASE100 at 150 °C for 100 min were 5 and 1 min, respectively. Therefore, all AH experiments were conducted at the same severity. The same was true for carrying out FA prehydrolysis of E. globulus.
Analysis of the solid phase
Complete compositional analysis of the solid phase (original and prehydrolyzed wood) after the washing step was performed, and details about analysis were reported elsewhere (Tunc and van Heiningen 2008a). The solid residues that were exposed to AH and 5 g/L FA pretreatment were subjected to enzymatic saccharification by Novozymes CTec2 enzyme according to NREL method NREL/TP-510-42629 (Selig et al. 2008). The concentration of glucose formed as a result of enzymatic saccharification was quantified using an YSI analyzer.
Analysis of the liquid phase
The liquid phase (extract including washing liquor) generated during autohydrolysis and 5 g/L FA prehydrolysis of wood was analyzed for mono- and oligo-saccharides, lignin, AcOH, furfural, and HMF as reported elsewhere (Tunc and van Heiningen 2008a). Size exclusion chromatography (SEC) was utilized to determine the molecular weight distribution (MWD) of dissolved wood components after freeze-drying the extract. More details about MWD analysis have been reported elsewhere (Tunc and van Heiningen 2011).
RESULTS AND DISCUSSION
The chemical composition of E. globulus used for AH and FA prehydrolysis experiments is summarized in Table 1.
Table 1. Chemical Composition of E. globulus Based on Oven Dried (od) Wood
E. globulus wood meal was treated with pure water and dilute FA (5 g/L) at 150 °C for 100 min, and the extraction yield is plotted against the L/S ratio in Fig. 1. It is clear from Fig. 1 that the AH, both with and without FA, follows the same dissolution profile. Figure 1 also shows that the extraction yield of components other than lignin (non-lignin) during AH and dilute FA prehydrolysis of E. globulusdecreased with increasing L/S ratio up to about 10:1 and 20:1 (w/w), respectively, and then remained relatively constant with increasing L/S ratio in the range of study. Since solubility of carbohydrates (non-lignin) were expected to be higher at the high L/S ratio (Sixta 2006), this cannot be explained by solubility of carbohydrates alone. On the other hand, lignin removal during AH both with and without FA addition slightly increased with increasing L/S ratio. This indicates that lignin and non-lignin components (mostly carbohydrates) undergo two different dissolution behaviors.
Fig. 1. Extraction yield versus L/S ratio of lignin and non-lignin components during AH of E. globulusat 150 °C for 100 min both with and without addition of 5 g/L FA
It is clear from Fig. 1 that the addition of 5 g/L FA did not have any effect on delignification. A similar finding was observed by Gütsch et al. (2012) when E. globulus wood was pretreated with dilute AcOH (0.02 to 0.15 g/L). Figure 1 also shows that the delignification of E. globulus wood increased with increasing L/S ratio during AH both with and without FA support, most likely due to better acid-soluble lignin dissolution with the increasing amount of liquor used to adjust the L/S ratio or due to precipitation and degradation/decomposition of dissolved lignin at relatively high acidity during AH with low L/S ratio. It is clear from Fig. 1 that dissolution of non-lignin components (mostly hemicelluloses) significantly improved with FA addition, leading to selective dissolution of hemicelluloses from E. globulus. Although it was not evaluated in this study, the dissolution of hemicelluloses was most likely improved as a result of partial breakage of bonds between lignin carbohydrates as LCC (Tunc and van Heiningen 2011) in E. globulus by acid hydrolysis due to FA addition.
Figure 2 shows the pH of the extract in the reaction cell of the extractor during AH both with and without 5 g/L FA reinforcement. It is clear from Fig. 2 that the AcG released from the hemicellulose backbone and other organic acids such as formic acid and levulinic acid formed during AH and 5 g/L FA prehydrolysis increased the acidity of the liquid phase. It is also clear from Fig. 2 that the acidity of the FA extract was much higher than that of AH extract during pretreatment. This explains the improvement in dissolution yield of hemicellulose during AH with the addition of 5 g/L FA (Fig. 1). It is well known that the release of organic acids (mostly AcOH) from wood during AH increases the acidity of the extraction medium and accelerates the dissolution of wood components, especially hemicelluloses, by acidic prehydrolysis (Brasch and Free 1965; Conner 1984; Conner and Lorenz 1986; Garrote et al. 1999; Gütsch et al. 2012; Sixta 2006; Testova et al. 2011; Tunc and van Heiningen 2008a; 2008b; 2009). Similarly, the decreasing yield of non-lignin components with increasing L/S ratio during pretreatment shown in Fig. 1 could be explained by the decreasing acidity of the extraction medium with increasing L/S ratio.
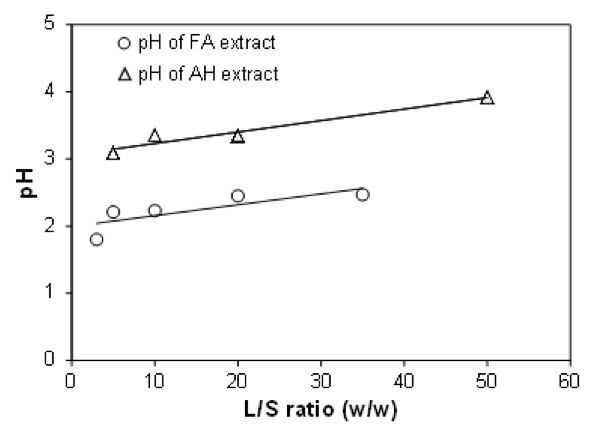
Fig. 2. The pH of the reaction medium versus L/S ratio in the extraction cell during AH of E. globulusat 150 °C for 100 min both with and without the addition of 5 g/L FA
The compositions of the extract generated during AH and 5 g/L FA prehydrolysis of E. globulus at different L/S ratios with respect to the original wood content are summarized in Tables 2 and 3, respectively. It is clear from both Tables 2 and 3 that xylan was the most abundant component in the reaction medium during AH, both with and without FA. Xylan dissolved mostly in oligomeric form during AH, and the amount of both xylo-oligosaccharides and xylose in the extract decreased with increasing L/S ratio (Table 2) due to decreasing acidity, as was discussed earlier. Although xylan dissolves mostly as xylo-oligosaccharides, the FA extract contained a relatively high xylose content that depended on the L/S ratio (Table 3). Increasing acidity of the reaction medium with FA addition further enabled the hydrolysis of xylo-oligosaccharides. The amounts of arabinan, glucan, and mannan dissolved during AH, both with and without 5 g/L FA, were minor and generally remained constant with increasing L/S ratio (Tables 2 and 3).
Table 2. Composition of AH Extract Produced at 150 °C for 100 min
Although arabinan is dissolved mostly as arabinose, glucan and mannan are dissolved mostly as gluco-oligosaccharides and manno-oligosaccharides, respectively. It is obvious from Tables 2 and 3 that the formation of sugar degradation products, furfural and HMF, were insignificant during AH of E. globulus both with and without 5 g/L FA at 150 °C for 100 min. A similar finding was reported for dilute FA prehydrolysis of a mixture of southern hardwood chips with 10 g/L FA at 150 °C for 100 min with a L/S ratio of 4:1 (Tunc et al. 2013a; 2013b). It is apparent from both Tables 2 and 3 that the majority of AcG that was dissolved during AH, both with and without dilute acid addition, was bound to xylo-oligosaccharides as OAc. A similar finding was reported by Leschinsky et al. (2009) for AH of the same wood species at mild and moderate AH intensity. The amount of OAc was almost constant with increasing L/S ratio. Tables 2 and 3 also show that AcOH in the FA extract was slightly higher than that in the AH extract. This is expected due to better acid prehydrolysis in the presence of FA.
Table 3. Composition of FA Extract Produced at 150 °C for 100 min
The molecular weight distributions of the wood components dissolved during AH, both with and without 5 g/L FA, at different L/S ratios were obtained by SEC and are shown in Fig. 3. Two Mwpeaks that depend on the L/S ratio of prehydrolysis are shown in Fig. 3. These two peaks, at low Mwand medium Mw, are assigned to lignin and hemicellulose, respectively. Figure 3 shows that the average Mw of hemicelluloses and lignin dissolved during AH, both with and without 5 g/L FA, remained relatively constant with increasing L/S ratio in the range of study. The average DP of hemicelluloses dissolved during AH of E. globulus with or without FA addition at different L/S ratios are summarized in Table 4.
Table 4. DP of Xylo-oligosaccharides Dissolved during AH and FA Prehydrolysis of E. globulus at 150 °C for 100 Min
The average DP of hemicelluloses dissolved during AH was between 7 and 8 over the L/S ratio range from 3:1 to 50:1. Similar to this study, an average DP of 11 was reported for xylo-oligosaccharides at a L/S ratio of 5:1 when E. globulus wood particles (2.5 and 3.5 mm) were subjected to mild AH (Leschinsky et al. 2009). Likewise, the average DP of hemicelluloses dissolved during 5 g/L FA prehydrolysis was around 6 for the range of L/S ratios from 3:1 to 20:1.
Fig. 3. Molecular weight distribution versus L/S ratio of wood components dissolved during AH: (a) autohydrolysis; (b) 5 g/L FA prehydrolysis
The original wood and the solid phases after autohydrolysis (with hot water) as well as prehydrolysis with 5 g/L FA at 150 °C for 100 min at different L/S ratios were subjected to enzymatic saccharification at a commercial enzyme dosage of 5 FPU per g of cellulose. The cellulose to glucose conversion yield obtained in these experiments is shown in Fig. 4. As expected, the enzymatic saccharification of the original E. globulus was minimal due to the “recalcitrance” of untreated wood. The maximum cellulose conversion yield of the original E. globulus reached about 12% after 48 h (Fig. 4, L/S ratio of 0:1). The enzymatic conversion yield of cellulose to glucose stayed relatively constant during AH of E. globulus while the conversion yield increased during 5 g/L FA prehydrolysis of E. globulus with increasing L/S ratio and it reached about 40 % after 48 h with a L/S ratio of 20:1.
Fig. 4. Cellulose to glucose conversion yield versus L/S ratio of original E. globulus and E. globuluspretreated with water and 5 g/L FA at 150 °C for 100 min
CONCLUSIONS
- The L/S ratio has an impact on the dissolution of wood components during autohydrolysis of E. globulus, both with and without the addition of 5 g/L FA.
- The acidity of the reaction medium changes during AH and FA prehydrolysis of E. globulus at different L/S ratios due to variations in the amount of liquor used and AcOH released from the hemicellulose backbone.
- Selective carbohydrate dissolution is promoted with FA addition during prehydrolysis of E globulus, while lignin removal does not depend on FA addition.
- The Mw value of dissolved hemicellulose and lignin is relatively constant during pretreatment of E. globulus with pure water and dilute acid at 150 °C for 100 min.
- The cellulose to glucose conversion yield of pretreated wood particles increases with FA addition. In addition, although the conversion yield of wood partials subjected to AH stays relatively constant, the conversion yield of dilute FA pretreated wood meal increases with increasing L/S ratio.
ACKNOWLEDGMENTS
This research has been made possible by the financial support of the J. Larcom Ober Research Chair in Chemical Engineering of the University of Maine, Orono, ME, USA.
REFERENCES CITED
Brasch, D. J., and Free, K. W. (1965). “Prehydrolysis-kraft pulping of Pinus radiata grown in New Zealand,” TAPPI J. 48(4), 245-248.
Casebier, R. L., Hamilton, J. K., and Hergert, H. L. (1969). “Chemistry and mechanism of water prehydrolysis on southern pine wood,” TAPPI J. 52(12), 2369-2377.
Casebier, R. L., Hamilton, J. K., and Hergert, H. L. (1973). “Chemistry and mechanism of water prehydrolysis on black gumwood, effect of temperature at constant time,” TAPPI J. 56(3), 135-139.
Conner, A. H. (1984). “Kinetic modeling of hardwood prehydrolysis. Part I: Xylan removal by water prehydrolysis,” Wood Fiber Sci. 16(2), 268-277.
Conner, A. H., and Lorenz, L. F. (1986). “Kinetic modeling of hardwood prehydrolysis. Part III: Water and dilute acetic acid prehydrolysis of southern red oak,” Wood Fiber Sci. 18(2), 248-263.
Garrote, G., Dominguez, H., and Parajo, J. C. (1999). “Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood,” J. Che. Techol. Biot. 74(11), 1101-1109.
Gütsch, J. S., Nousiainen, T., and Sixta, H. (2012). “Comparative evaluation of autohydrolysis and acid-catalyzed hydrolysis of Eucalyptus globulus wood,” Bioresource Technol. 109, 77-85.
Harris, J. F., Scott, R. W., Springer, E. L., and Wegner, T. H. (1984). “Factors influencing dilute sulfuric acid prehydrolysis of southern red oak wood,” In: Progress in Biomass Conversion, D. A. Tillman and E. C. Jahn (eds.), Academic Press, Orlando, FL, pp. 101-141.
Leschinsky, M., Sixta, H., and Patt, R. (2009). “Detailed mass balance of the autohydrolysis of Eucalyptus globulus at 170 ºC,” BioResources 4(2), 687-703.
Lora, J. H., and Wayman, M. (1978) “Delignification of hardwood by autohydrolysis and extraction,” TAPPI J. 61(6), 47-50.
Myerly, R. C., Nicholson, M. D., Katzen, R., and Taylor, J. M. (1981). “The forestry refinery,” Chemtech. 11(3), 186-192.
Overend, R. P., Chornet, E., and Gascoigne, J. A. (1987). “Fractionation of lignocellulosics by steam-aqueous pretreatments,” Phil. Trans. R. Soc. Lond. Ser. A 321(1561), 523-536.
Schild, G. (1994). Vergleich von Verfahren zur Herstellung von Chemiezellstoffen aus Buche- und Eucalyptusholz, Ph.D. thesis, University of Hamburg, Hamburg, Germany.
Seaman, J. F. (1945). “Kinetic of wood saccharification. Hydrolysis of cellulose and decomposition of sugar in dilute acid at high temperature,” Ind. Eng. Chem. Res. 37(1), 43-52.
Selig, M., Weiss N., and Ji, Y. (2008). “Enzymatic saccharification of lignocellulosic biomass,” Laboratory Analytical Procedure, Technical Report, NREL/TP-510-42629, National Renewable Energy Laboratory, Golden, CO.
Sixta, H. (2006). “Kinetic modeling of hardwood prehydrolysis” (Chapter 4), In: Handbook of pulp, Sixta, H. (ed.). WILEY-VCH Verlag GmbH &Co. KGaA, Weinheim, p.329-342.
Sixta, H., and Schild, G. (2009). “A new generation kraft process,” Lenzinger Berichte 87, 26-37.
Testova, L., Chong, S. L., Tenkanen, M., and Sixta, H. (2011). “Autohydrolysis of birch wood,” Holzforschung 65(4), 535-542.
Timell, T. E. (1967). “Recent progress in the chemistry of wood hemicelluloses,” Wood Sci. Technol. 1(1), 45-70.
Tunc, M. S., and van Heiningen, A. R. P. (2008a). “Hemicelluloses extraction of mixed southern hardwood with water at 150 °C: Effect of time,” Ind. Eng. Chem. Res. 47(18), 7031-7037.
Tunc, M. S., and van Heiningen, A. R. P. (2008b). “Hydrothermal dissolution of mixed southern hardwoods,” Holzforschung 62(5), 539-545.
Tunc, M. S., and van Heiningen, A. R. P. (2009). “Hydrothermal dissolution of mixed southern hardwoods: Effect of P-factor,” Nord. Pulp Pap. Sci. J. 24(1), 42-47.
Tunc, M. S., and van Heiningen, A. R. P. (2011). “Characterization and molecular weight distribution of carbohydrates isolated from the autohydrolysis extract of mixed southern hardwoods,” Carbohyd. Polym. 83(1), 8-13.
Tunc, M. S., Chheda, J., van der Heide, E., Morris, J., and van Heininge, A. (2013a). “Two-stage fractionation of hardwoods,” BioResources 8(3), 4380-4895.
Tunc, M. S., Chheda, J., van der Heide, E., Morris, J., and van Heininge, A. (2013b). “Two stage fractionation and fiber preparation of lignocellulosic biomass for liquid fuels and chemicals,” Ind. Eng. Chem. Res. 52(36), 13209-13216.
Young, R. A., and Akhtar, M. (1998). Wood: Chemistry, Ultrastructure, Reactions, Wiley, New York.
Article submitted: February 11, 2014; Peer review completed: March 23, 2014; Revised version received: March 28, 2014; Accepted: March 29, 2014; Published: April 9, 2014.
