Abstract
The structure and properties of ethanol organosolv lignin (EOL) extracted from bamboo under various conditions were characterized. EOL yield increased at high temperatures of 160 to 200 °C and a reaction time of 1 to 3 h. The nitrogen content in lignin was low, with a maximum of 0.62%. The carbon content increased with increasing temperature and prolonged time, whereas oxygen content showed an inverse trend. EOL extracted from bamboo showed high purity levels (more than 95.5% Klason lignin) with low impurity contents (carbohydrate and ash). The severity of the process increased the carboxylic acid and phenolic hydroxyl group contents and also decreased the methoxyl group content. The molecular weight of EOL varied depending on the extraction condition. The FT-IR and NMR spectra revealed that the main structure did not significantly change. From the spectra, it is clear that EOL extracted from bamboo can be classified as an HGS (H–p-hydroxyphenyl, G–guaiacyl, and S–syringyl, respectively) type. Clear β-O-4, β-β, and β-5’ linkages were observed.
Download PDF
Full Article
Effects of Extraction Conditions on the Characteristics of Ethanol Organosolv Lignin from Bamboo (Phyllostachys pubescens Mazel)
Liangliang Fan,a,b Rongsheng Ruan,a,b Yuhuan Liu,a,b,* Yunpu Wang,b,* and Chunming Tu a,b
The structure and properties of ethanol organosolv lignin (EOL) extracted from bamboo under various conditions were characterized. EOL yield increased at high temperatures of 160 to 200 °C and a reaction time of 1 to 3 h. The nitrogen content in lignin was low, with a maximum of 0.62%. The carbon content increased with increasing temperature and prolonged time, whereas oxygen content showed an inverse trend. EOL extracted from bamboo showed high purity levels (more than 95.5% Klason lignin) with low impurity contents (carbohydrate and ash). The severity of the process increased the carboxylic acid and phenolic hydroxyl group contents and also decreased the methoxyl group content. The molecular weight of EOL varied depending on the extraction condition. The FT-IR and NMR spectra revealed that the main structure did not significantly change. From the spectra, it is clear that EOL extracted from bamboo can be classified as an HGS (H–p-hydroxyphenyl, G–guaiacyl, and S–syringyl, respectively) type. Clear β-O-4, β-β, and β-5’ linkages were observed.
Keywords: Lignin; Organosolv; Bamboo; Extraction conditions; Structure; Properties
Contact information: a: Nanchang University, State Key Laboratory of Food Science and Technology, Nanchang 330047, China; b: Nanchang University, Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang 330047, China;
* Corresponding authors: liuyuhuan@ncu.edu.cn; wangyunpu@ncu.edu.cn
INTRODUCTION
Bamboo (Phyllostachys pubescens Mazel), a perennial woody grass that belongs to the Gramineae family and Bambuseae subfamily, is extensively distributed worldwide. China is the largest bamboo-producing country, wherein 300 species in 44 genera occupy 3% of the global forests (Scurlock et al.2000). As a fast-growing and highly productive plant, bamboo is widely used in various industries, including pulp and papermaking, food, textile, furniture, and household goods. Nevertheless, the utilization rate of crude bamboo only ranges from 35% to 40% (Li and Kobayashi 2004), and the remaining crude bamboo is buried or burned. This causes a significant waste of resources and poses environmental hazards.
Lignin, celluloses, and hemicelluloses serve as structural components of vascular tissues of high-order land plants (Chen and Sarkanen 2010). Lignin, the second-most abundant terrestrial organic polymer on earth, is a polyphenolic amorphous material containing three typical phenylpropane structural units (H, G, and S units, respectively) (Davin and Lewis 2005).
H (R1 = H, R2 = H), G (R1 = OMe, R2 = H), and S units (R1 = OMe, R2 = OMe) (Fig. 1) are connected through ether linkages (about 2/3) and carbon–carbon linkages (about 1/3). Ether linkages include β-O-aryl ether (β-O-4) and 4-O-5 (biphenyl ether). Carbon–carbon linkages contain 1, 2-diaryl propane (β-1), resinol (5-5), biphenyl (5-5′), and phenylcoumaran (β-5/α-O-4) (Ralph et al. 1998; del Río et al. 2008; Martínez et al. 2008).
Lignin is utilized in various industries because of its polyphenolic structure; for example, lignin is used as a raw material to produce aromatic chemicals such as vanillin and phenols (Silva et al. 2009; Yoshikawa et al. 2013). Lignin is also applied to manufacture dispersants, surfactants, flocculants, and activated carbons (Holtzapple 2003). Lignin can be obtained from the black liquor produced through the alkaline kraft process in wood pulping (Calvo-Flores and Dobado 2010; Zhao et al. 2010). More than 90% of this lignin is simply used as a boiler combustible for energy generation (Lora and Glasser 2002).
Fig. 1. Typical C9 structure of lignin
*H unit (R1=R2=H); G unit (R1=OCH3, R2=H); S unit (R1=R2=OCH)
Because the utilization of lignin from black liquor is limited, sulfur-free lignin extracted through various organosolv processes with organic solvents (especially ethanol) has gained much interest (Yáñez-S et al. 2013). Among the reagents for organosolv pretreatments, ethanol is the most promising because of its economic and environmental friendliness. It can also be recycled and reused (Pan et al.2006). Lignin extraction through the use of ethanol-based organosolv process has been widely used in various types of biomass, such as flax (Linum usitatissimum L.) (Buranov et al. 2010), wheat straw (Monteil-Rivera et al. 2013), fiber hemp (Zomers et al. 1995), Buddleja davidii (Hallac et al. 2010), Eucalyptus globulus wood (Yáñez-S et al. 2014), and Eucommia ulmoides Oliver (Zhu et al. 2015). Compared with native lignin, lignin extracted through ethanol-based organosolv processes can be transformed into low-molecular weight fragments, which can be used to prepare high-value chemicals and fuels.
In this work, we isolated and extracted bamboo lignin with 50% aqueous ethanol. The effects of extraction conditions on the properties and structure of ethanol organosolv lignin (EOL) from bamboo have not been previously reported. Ethanol organosolv pretreatments were performed on bamboo culms under a wide range of extraction temperature (160 to 200 °C) and time (1 to 4 h). The effects of extraction conditions on the properties and structure of isolated lignin and the elucidated lignin breakdown mechanism in the organosolv process were then investigated.
EXPERIMENTAL
Materials
Bamboo culms were obtained from Nanchang, Jiangxi province, China. Dried raw materials were cut into small pieces (1 to 3 cm), ground, and sieved to obtain an 80- to 100-mesh fraction. The fraction was extracted with methylbenzene/ethanol (2:1, v/v) in a Soxhlet apparatus for 6 h before being air-dried. The dewaxed bamboo contained cellulose (44.2% ± 0.8%), hemicelluloses (19.0% ± 0.9%), lignin (28.3% ± 0.4%), and ash (1.8% ± 0.1%). This composition was determined using the NREL method (Sluiter et al. 2008a, b).
Methods
Extraction conditions for EOL preparation
Bamboo powder was ethanol-organosolv-pretreated under various conditions in a GSH-2-Type stainless steel autoclave (Weihai Chemical Machinery Co. Ltd., Shandong, China). This was done at a working temperature of 350 °C and a volume of 2 L. Briefly, 150 g of bamboo powder (dry basis) and 50% ethanol aqueous solution (8:1, v/w) were carefully loaded into the autoclave initially heated to 30°C. The autoclave was set at desired temperatures (160 °C, 180 °C, and 200 °C) with a 10 °C/min heating rate and kept within a ± 2 °C range. After keeping 1 to 4 h of the desired temperature, the reactor was cooled with 4 oC cooling water. The pH of the cooled organosolv liquor (COL) was determined. After the pH was determined, the reaction mixture was vacuum filtered and the remaining solid fraction was washed with 50% ethanol aqueous solution until the liquid became almost colorless. This was then followed by 1 L of hot water. The filtrate and washing solution were combined and concentrated under reduced pressure. After adding 10 volumes of water into the concentrated solution, lignin was obtained through filtration and washed thoroughly with water. The sample was then dried in an oven at 50 °C for 24 h.
The autoclave pressure and the severity factor of the process are shown in Table 1, and the severity factor was calculated using the H-factor parameter (Yáñez-S et al. 2014), whose formula is shown as follows,
(1)
where T is reaction temperature (K), and t is reaction time (h).
Table 1. Autoclave Pressure and Severity Factor under Various Extraction Conditions
Purification of lignin
The obtained lignin was purified according to the method proposed by Pan and Sano (1999). Briefly, the lignin was dissolved in dioxane/ethanol (8:2, v/v), and the precipitate was removed by centrifugation. The supernatant was added dropwise to 10 volumes of diethyl ether/petroleum ether (1:1, v/v) with stirring. The precipitate was centrifuged off, then washed twice with diethyl ether/petroleum ether (1:1, v/v).
The purified lignin was dried and weighed to determine EOL recovery yield,
(2)
where KL is the Klason lignin content of untreated bamboo powder (g Klason lignin per 100 g of wood on dry basis).
Elemental analysis
Carbon, hydrogen, and nitrogen contents of the purified samples were determined using an elemental analyzer (Vario EL III, Elementar, Germany). The oxygen content was calculated based on the difference from the total sample.
Determination of Klason lignin content
Klason lignin content was determined using the NREL LAB method (Sluiter et al. 2008b). EOL samples were treated with 72% H2SO4 for 1 h in a water bath at 30 °C. The samples were then diluted to 4% and autoclaved at 121 °C for 1 h. The hydrolysis solution was cooled to room temperature and then filtered. Solid residue was washed with distilled water until neutral and then dried. Klason lignin content was calculated using the following equation:
(3)
Sugar analysis
Aliquots (3 mL) of 72% H2SO4 were added to 0.3 g of lignin in pressure tubes. The tubes were placed in a water bath at 30 °C for 1 h and stirred continuously to completely dissolve the lignin sample. The solution was diluted to 4% by adding water, and the tubes were autoclaved at 121 °C for 1 h (Sluiter et al. 2008b). After hydrolysis, the samples were filtered through a 0.22-μm syringe filter and then injected into an HPLC system (Agilent 1260, USA). Calibration was performed with standard solutions of L-arabinose, D-glucose, D-xylose, D-mannose, D-galactose, glucuronic acid, and galacturonic acid.
Ash analysis
Ash content was analyzed according to NREL standard procedure (Sluiter et al. 2008a).
Characterization of functional groups
The contents of various functional groups were determined to predict the properties of EOL. The methoxyl group was determined through 1H NMR (Mousavioun and Doherty 2010). The carboxylic acid and phenolic hydroxyl groups were determined through the titration method (Mousavioun and Doherty 2010).
Molecular weight determination
Lignin samples were prepared in an eluent (0.1 M NaOH) at 0.2 mg/mL prior to analysis. The eluent was then filtered through a 0.45-μm syringe filter. A differential refractive index detector was used, and the column was operated at ambient temperature and eluted with 0.1 M NaOH at a flow rate of 1.0 mL/min. Dextran standards with molecular weights of 1,200, 10,000, 40,000, 70,000, and 500,000 g/mol were used to prepare a standard calibration curve. The weight-average molecular weight (Mw) and number-average molecular weight (Mn) of lignin were calculated using the equation of the standard curve.
FT-IR spectroscopy
The FT-IR spectra of EOL were determined using an FT-IR spectrophotometer (Bruker Tensor 27, Germany) with KBr pellets containing 1% finely ground samples. Each spectrum recorded 32 scans ranging from 4,000 to 400 cm−1 with a 2 cm−1 resolution in transmission mode.
NMR spectroscopy
The solution-state NMR spectra of the samples were determined on a Bruker AVIII 400 MHz spectrometer (Germany) at 25 °C. The 1H NMR spectra were recorded at 100 MHz using 15 mg of lignin fractions in 1 mL of d-DMSO. After 30,000 scans, the 13C NMR spectra were obtained at 400 MHz using 80 mg of the sample in 1 mL of d-DMSO.
Statistical analysis
Each experiment was conducted in triplicate, and the data were expressed as means ± one standard deviation (SD). In case of variation analysis, significance was p < 0.05 level of Duncan test using analytical software SPSS statistics (Version 16.0, SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
COL pH and EOL Recovery Yield
The ethanol organosolv process, known as the Alcell process, is based on the autocatalytic effect (Pye and Lora 1991). Plant materials can produce organic acids including acetic and uronic acids, which can function as catalysts for delignification in the cooking process. The organosolv process conditions affected COL pH and lignin yield. An increase in temperature caused a decrease in COL pH and an increase in lignin recovery yield (Fig. 2a). This was due to enhanced hemicellulose hydrolysis, which results in a higher production of acetic acid from the acetyl side groups of hemicellulose as well as an increase in delignification (Wildschut et al. 2013). These findings indicate that higher temperature increases the autocatalytic effect in the ethanol organosolv process. With a prolonged reaction time of less than 3 h at 180 °C, the delignification and lignin recovery both increased (Fig. 2b). Many studies have obtained similar results (Wildschut et al. 2013; Huijgen et al. 2014; Yáñez-S et al. 2014). However, the reaction time had a negligible effect on the pH of COL, which indicated that the autocatalytic effect on the organosolv process was only slightly influenced by reaction time.
Fig. 2. (a) COL pH and EOL recovery yield under different temperature (for 1 h); (b) COL pH and EOL recovery yield under different time (at 180 °C)
*Based on starting lignin in dry bamboo powder.
Different letters on the same column indicate significant differences (p < 0.05).
Nevertheless, in the present study, the recovery of lignin after 4 h of reaction was lower than that after 3 h. McDonough (1992) reported that all organosolv processes are dependent on a chemical breakdown of lignin prior to dissolution. Moreover, the condensation reaction under severe conditions may result in lower lignin solubility in the liquor (Yáñez-S et al. 2014). In the organosolv process of reaction time ranging from 3 to 4 h, the effect of condensation reaction on the solubility of lignin was significant and subsequently caused a decrease in the recovery of lignin.
Elemental Analysis
Table 2 shows the elemental composition of the isolated EOL of bamboo. The displayed data show that the carbon content is between 60.21% and 61.18% and that the oxygen content is between 32.36% and 33.35%.
Table 2. Elemental Composition of EOL from Bamboo under Various Extraction Conditions
In Table 2, the nitrogen content ranges from 0.48% to 0.62%. This is probably due to the presence of a small amount of nitrogenous compounds in EOL. Most of these nitrogenous compounds were dissolved in the aqueous ethanol liquid and easily removed during the process of lignin extraction (Mousavioun and Doherty 2010). Higher temperature and prolonged time decreased nitrogen content because increasing the severity of the organosolv process promoted the isolation of nitrogenous compounds from the lignin. By comparing the elemental composition of EOL, it was found that the carbon content increased, while the oxygen content decreased slightly with increasing temperature and prolonged reaction time, suggesting that EOLs undergo a condensation reaction (Yáñez-S et al. 2014). Acetic acid released the protonated hydroxyl group in the Cα of lignin side chains as a resonance-stabilized benzyl carbocation, which can readily form a band with an electron-rich carbon atom of another lignin unit (Hallac et al. 2010; Yáñez-S et al. 2014), thereby increasing carbon content while decreasing oxygen content.
EOL Composition
The Klason lignin content of EOL extracted from bamboo was higher than 95.5% (Table 3), which indicates the high purity of the extracted lignin. The carbohydrates present in EOL mainly originated from polysaccharides that covalently bonded to lignin in the bamboo sample. Several studies have confirmed the existence of covalent bonds between lignin and carbohydrates (Lawoko et al. 2006; Ghaffar and Fan 2013; Oinonen et al. 2015). The low ash content in EOL was primarily derived from the element silicon, but minor amounts of sodium, iron, and potassium in lignin samples also contributed (Mousavioun and Doherty 2010).
Table 3. Composition of EOL Extracted from Bamboo under Various Conditions
Table 3 shows that EOL1 presented higher Klason lignin content than EOL2 and EOL3, but lower xylose content than EOL2 and EOL3. This is probably because the higher temperature in the organosolv process increased hemicellulose hydrolysis, resulting in higher concentrations of sugars in the organosolv liquor (Huijgen et al. 2014). Besides, the high temperatures under acidic conditions may promote the dehydration of hemicellulose sugars to form furfural compounds, thus inducing the formation of a lignin–furfural condensation product (Huijgen et al. 2014). As a result, EOL2 and EOL3showed higher xylose and lower Klason lignin content than EOL1. However, with prolonged reaction time, the total carbohydrate content decreased. This finding could be due to enhanced carbohydrate hydrolysis caused by severe conditions. Compared with that in the raw material (bamboo), the ash content in the EOL samples decreased by more than 1.0%. Furthermore, it seems that ash content decreased under severe extraction conditions, especially when the reaction time was more than 2 h. This finding could be attributed to more metal salt being released from bamboo at severe extraction conditions and that it is easily removed in the organosolv process. Overall, less carbohydrate and ash remained in lignin, which resulted in the high purity of lignin.
Functional Groups
The contents of carboxylic acid, phenolic hydroxyl, and methoxyl functional groups are shown in Figs. 3 and 4. The contents of carboxylic acid and phenolic hydroxyl groups increased gradually with increasing temperature and prolonged reaction time (Fig. 3). Gosselink et al. (2004) and Tejado et al.(2007) found that the phenolic hydroxyl group content is related to the organosolv process. Also, pulping can increase the carboxylic acid content of lignin when compared with organosolv pulping (Mousavioun and Doherty 2010). These findings imply that the existence of acetic acid in the extraction process promotes oxidation reactions (Erdocia et al. 2014), and the severity of the organosolv process enhances the release of acetic acid, thus promoting oxidation reactions and increasing the production of carboxylic acid and phenolic hydroxyl groups. The content of the methoxyl group slightly decreased with an increase in temperature and prolonged reaction time (2 to 4 h) (Fig. 4). This is because the acid produced by the autocatalytic effect in the ethanol organosolv process facilitated the loss of the methoxyl groups (Li et al. 2012).
Fig. 3. (a) Carboxylic acid and hydroxyl group contents under different temperature (for 1 h); (b) Carboxylic acid and hydroxyl group contents under different time (at 180 °C).
Different letters on the same column indicate significant differences (p < 0.05).
Fig. 4. (a) Methoxyl group contents under different temperature (for 1 h); (b) Methoxyl group contents under different time (at 180 °C).
Different letters on the same column indicate significant differences (p < 0.05).
Molecular Weight Distribution
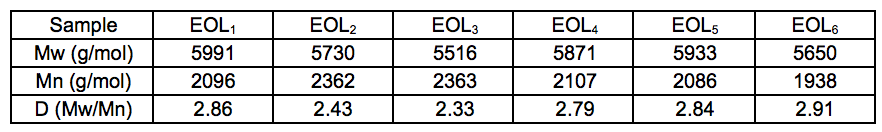
The weight-average (Mw) molecular weight, number-average (Mn) molecular weight, and polydispersity (Mw/Mn) of EOL were determined to estimate the effects of extraction conditions on the polymer structures (Table 4). A comparison of the Mw of EOL extracted under various temperatures demonstrated that high reaction temperatures could decrease the molecular weight of EOL. The higher hydrogen ion concentration generated from higher temperatures may enhance the cleavage of ether linkages. McDonough (1992) reported that higher concentrations of hydronium ion in liquor (low pH values) facilitate the breakdown of α and β-ether bonds in lignin, resulting in the formation of molecular fragments with low molecular weights, also, the homogeneity of the resulting lignin increased, resulting in an decrease in the average polydispersity and an increase in the Mn molecular weight. With prolonged reaction time, the EOL molecular weight increased from the 5730 g/mol of EOL2 to the 5933 g/mol of EOL5, which indicated the occurrence of lignin repolymerization reactions. On the other hand, the increase in the polydispersity index was attributed to the simultaneous competition between degradation (depolymerization) and condensation (repolymerization), which causes an increase in the heterogeneity of the resulting lignin (Hallac et al. 2010). The increased polydispersity was indicated by a broader distribution of the molecular weights, resulting in lower Mnmolecular weight. The abnormal decrease in the molecular weight of EOL6 may have been due to the condensation reaction, which decreased lignin solubility. These high-molecular weight polymers cannot be isolated from the residue. The lower yield of lignin at EOL6 (Fig. 2) also confirmed these findings.
Table 4. Molecular Weight of EOL Extracted from Bamboo under Various Conditions
FT-IR Spectroscopy
FT-IR spectroscopy can be used to study the physicochemical properties and structure of lignin (El Hage et al. 2009; Sun et al. 2012; Wen et al. 2013). The FT-IR spectra of all EOL obtained under various extraction conditions were characterized in the 400 to 4000 cm−1 range (Figs. 5 and 6). Minimal differences were observed among the six EOL samples, which indicated that the structure of lignin extracted from bamboo did not undergo major modifications under varied extraction conditions.
The spectrum of lignin extracted at 160 °C for 1 h (lignin EOL1) is shown in Fig. 5. A wide absorption band appears at 3417 cm−1, which is assigned to the O-H stretching absorption of phenolic hydroxyl and aliphatic hydroxyl groups. The bands at 2937 and 2843 cm−1 are assigned to the C-H stretching vibrations in the methyl and methylene groups, respectively. The band at 1660 cm−1 is due to carbonyl stretching of conjugated p-substituted aryl ketones (Wen et al. 2013). The bands at 1598, 1511, and 1417 cm−1 are attributed to aromatic skeleton vibrations, which imply that they are the primary structure of lignin. The band at 1459 cm−1 is assigned to C-H in-plane deformation (such as methyl, methylene, and methoxyl groups) (Jahan et al. 2007). The bands at 1268, 1031, and 912 cm−1originated from aromatic ring breathing vibration, aromaticin-plane bending, and out-of-plane C–H bending of guaiacyl (G) units, respectively; those of syringyl (S) units were observed at 1328, 1223, and 1121 cm−1, respectively (Li et al. 2012). The band at 832 cm−1 is due to C–H out-of-plane deformation in positions 2 and 6 of S and all positions of p-hydroxyphenyl (H) units (Li et al. 2012).
Fig. 5. FT-IR spectra of EOL extracted from bamboo at various temperatures
Only minimal differences were observed in EOL under all conditions. As shown in Figs. 5 and 6, the signal at 1660 cm−1 disappeared with increasing temperature (lignin extracted at 200 °C) and prolonged reaction time (more than 2 h). Additionally, the signal at about 1700 cm−1 became clearer, which indicated the presence of carbonyl stretching in non-conjugated ketones. This phenomenon contributes to the increase in carboxylic acid contents (Fig. 3) under varied conditions.
Fig. 6. FT-IR spectra of EOL extracted from bamboo at various reaction times
NMR Spectroscopy
1H and 13C NMR spectroscopy were used for further study of the structural properties of EOL extracted from bamboo. It was found that the NMR spectra of the six EOL samples were the same. Figures 7 and 8 showed the 1H and 13C NMR spectra of EOL1, respectively.
Fig. 7. 1H NMR spectra of EOL1
Fig. 8. 13C NMR spectra of EOL1
From the 1H NMR spectra of EOL1 (Fig. 7), the signals that occurred between 6.0 ppm and 8.0 ppm possibly originated from aromatic protons in S units, G units, H units, p-coumarate acid, and ferulate acid (Hussin et al. 2013). The signals from 7.49 to 7.52 ppm represented the aromatic protons of p-coumarate acid and ferulate acid (Seca et al. 2000), whereas the signals at 6.28 to 6.83 ppm were due to the aromatic protons of the S and G units. Three weak signals between 4.0 and 5.5 ppm are shown in the spectra: the signal at 5.33 ppm originated from Hα in benzyl aryl ethers. The signals at 4.88 and 4.02 ppm correspond to Hβ and Hγ of the β-O-4 structure, respectively (Xu et al. 2005). The methoxyl protons produced a sharp signal at 3.62 to 3.85 ppm. The signal at approximately 3.45 ppm was due to the protons of the water in DMSO, and the intense signal at 2.50 ppm is attributed to the protons in DMSO. The signals at 0.7 and 1.5 ppm were assigned to the protons in aliphatic groups.
The 13C spectrum of EOL1 is shown in Fig. 8, and the corresponding resonance is assigned according to the values reported in the literature (Nimz et al. 1981; Xu et al. 2005; Sun et al. 2012). For p-coumaric acid, signals are assigned at 168.44 (C-γ), 160.06 (C-4), 144.66 (C-α), and 115.82 ppm (C-β). The small signal at 179.31 ppm indicates the presence of p-coumaric acid, which is connected to lignin via ester bonds. Etherified ferulic acid is identified by signals at 168.44 (C-γ) and 144.66 ppm (C-α). G units provide signals at 152.62 (C-4, etherified), 148.39 (C-3, etherified), 136.88 (C-1, etherified), 132.24 (C-1, nonetherified), 124.03 (C-6, nonetherified), 120.18 (C-6, etherified), and 111.10 ppm (C-2). S units are related to the signals at 152.62 (C-3/C-5, etherified), 148.39 (C-3/C-5, nonetherified), 136.88 (C-4, nonetherified), and 104.10 ppm (C-2/C-6, etherified). The signals caused by the C-4 of etherified G units and the C-4 of nonetherified S units imply that most of the phenolic hydroxyl group in EOL originates from S units. The signals at 104.10 ppm confirm the existence of the p-hydroxyphenyl (H) unit. The typical structure of β-O-4 in lignin is shown at the signals 85.84 (C-β), 71.56 (C-α), and 60.56 ppm (C-γ). The signal at 70.91 ppm is attributed to the C-γ of β-β units. The signals at 65.11 and 54.15 ppm originate from the C-γ and C-β of β-5′ units, respectively. The intense signals at 56.04 and 56.43 ppm are indicative of OCH3 in S and G units, respectively. The signals at 14.41 to 34.13 ppm are related to γ-methyl, α-, and β-methylene groups in the n-propyl side chains.
CONCLUSIONS
- Cooled organosolv lignin (COL) pH decreased with increasing temperature and prolonged time, indicating higher autocatalytic effects and more organic acid released in the organosolv process, which resulted in an increase in lignin recovery. In addition, the acid produced in the organosolv process facilitated the decrease of methoxyl groups and promoted oxidation reactions, which led to the increase of carboxylic acid and phenolic hydroxyl group.
- The organic acid in the organosolv process promoted the formation of the carbon cation, which resulted in condensation reaction. Hence, the carbon content increased, while the oxygen content decreased slightly with increasing temperature and prolonged reaction time. However, the simultaneous competition between degradation (depolymerization) and condensation (repolymerization) making the lignin molecular weight varied.
- FT-IR and NMR spectra indicated that the extraction conditions did not significantly change the core structure of lignin, and the major lignin side chains were β-O-4, β-β, and β-5’ linkages. Also, the enzymatic hydrolysis lignin (EOL) can be classified as an HGS type.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (21466022 and 21266022) and the International Cooperation Project of MOST. P. R. CHINA (2014DFA61040).
REFERENCES CITED
Buranov, A. U., Ross, K. A., and Mazza, G. (2010). “Isolation and characterization of lignins extracted from flax shives using pressurized aqueous ethanol,” Bioresour. Technol. 101(19), 7446-7455. DOI: 10.1016/j.biortech.2010.04.086
Calvo-Flores, F. G., and Dobado, J. A. (2010). “Lignin as renewable raw material,” ChemSusChem3(11), 1227-1235. DOI: 10.1002/cssc.201000157
Chen, Y. -r., and Sarkanen, S. (2010). “Macromolecular replication during lignin biosynthesis,” Phytochemistry 71(4), 453-462. DOI: 10.1016/j.phytochem.2009.11.012
Davin, L. B., and Lewis, N. G. (2005). “Lignin primary structures and dirigent sites,” Curr. Opin. Biotechnol. 16(4), 407-415. DOI: 10.1016/j.copbio.2005.06.011
del Río, J. C., Rencoret, J., Marques, G., Gutiérrez, A., Ibarra, D., Santos, J. I., Jiménez-Barbero, J. S., Zhang, L., and Martínez, A. n. T. (2008). “Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants,” J. Agric. Food Chem. 56(20), 9525-9534. DOI: 10.1021/jf800806h
El Hage, R., Brosse, N., Chrusciel, L., Sanchez, C., Sannigrahi, P., and Ragauskas, A. (2009). “Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus,” Polym. Degrad. Stab. 94(10), 1632-1638. DOI: 10.1016/j.polymdegradstab.2009.07.007
Erdocia, X., Prado, R., Corcuera, M. Á., and Labidi, J. (2014). “Effect of different organosolv treatments on the structure and properties of olive tree pruning lignin,” J. Ind. Eng. Chem. 20(3), 1103-1108. DOI: 10.1016/j.jiec.2013.06.048
Ghaffar, S. H., and Fan, M. (2013). “Structural analysis for lignin characteristics in biomass straw,” Biomass Bioenergy 57(0), 264-279. DOI: 10.1016/j.biombioe.2013.07.015
Gosselink, R. J. A., Abächerli, A., Semke, H., Malherbe, R., Käuper, P., Nadif, A., and van Dam, J. E. G. (2004). “Analytical protocols for characterisation of sulphur-free lignin,” Ind. Crop. Prod. 19(3), 271-281. DOI: 10.1016/j.indcrop.2003.10.008
Hallac, B. B., Pu, Y., and Ragauskas, A. J. (2010). “Chemical transformations of Buddleja davidiilignin during ethanol organosolv pretreatment,” Energy Fuels 24(4), 2723-2732. DOI: 10.1021/ef901556u
Holtzapple, M. T. (2003). “Lignin,” in: Encyclopedia of Food Sciences and Nutrition (Second Edition), B. Caballero (ed.), Academic Press, Oxford, pp. 3535-3542.
Huijgen, W. J. J., Telysheva, G., Arshanitsa, A., Gosselink, R. J. A., and de Wild, P. J. (2014). “Characteristics of wheat straw lignins from ethanol-based organosolv treatment,” Ind. Crop. Prod. 59, 85-95. DOI: 10.1016/j.indcrop.2014.05.003
Hussin, M. H., Rahim, A. A., Ibrahim, M. N. M., and Brosse, N. (2013). “Physicochemical characterization of alkaline and ethanol organosolv lignins from oil palm (Elaeis guineensis) fronds as phenol substitutes for green material applications,” Ind. Crop. Prod. 49, 23-32. DOI: 10.1016/j.indcrop.2013.04.030
Jahan, M. S., Chowdhury, D. N., Islam, M. K., and Moeiz, S. I. (2007). “Characterization of lignin isolated from some nonwood available in Bangladesh,” Bioresour. Technol. 98(2), 465-469. DOI: 10.1016/j.biortech.2006.01.005
Lawoko, M., Henriksson, G., and Gellerstedt, G. (2006). “Characterisation of lignin-carbohydrate complexes (LCCs) of spruce wood (Picea abies L.) isolated with two methods,” Holzforschung 60(2), 156-161. DOI: 10.1515/HF.2006.025
Li, Z. -h., and Kobayashi, M. (2004). “Plantation future of bamboo in China,” J. Forest. Res. 15(3), 233-242. DOI:10.1007/BF02911032
Li, M. -F., Sun, S. -N., Xu, F., and Sun, R. -C. (2012). “Formic acid based organosolv pulping of bamboo (Phyllostachys acuta): Comparative characterization of the dissolved lignins with milled wood lignin,” Chem. Eng. J. 179, 80-89. DOI: 10.1016/j.cej.2011.10.060
Lora, J. H., and Glasser, W. G. (2002). “Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials,” J. Polym. Environ. 10(1-2), 39-48. DOI: 10.1023/A:1021070006895
Martínez, Á. T., Rencoret, J., Marques, G., Gutiérrez, A., Ibarra, D., Jiménez-Barbero, J., and José, C. (2008). “Monolignol acylation and lignin structure in some nonwoody plants: a 2D NMR study,” Phytochemistry 69(16), 2831-2843. DOI: 10.1016/j.phytochem.2008.09.005
McDonough, T. J. (1992). “The chemistry of organosolv delignification,” TAPPI J. 76(8), 186-193.
Monteil-Rivera, F., Phuong, M., Ye, M., Halasz, A., and Hawari, J. (2013). “Isolation and characterization of herbaceous lignins for applications in biomaterials,” Ind. Crop. Prod. 41, 356-364. DOI: 10.1016/j.indcrop.2012.04.049
Mousavioun, P., and Doherty, W. O. S. (2010). “Chemical and thermal properties of fractionated bagasse soda lignin,” Ind. Crop. Prod. 31(1), 52-58. DOI: 10.1016/j.indcrop.2009.09.001
Nimz, H., Robert, D., Faix, O., and Nemr, M. (1981). “Carbon-13 NMR spectra of lignins, 8. Structural differences between lignins of hardwoods, softwoods, grasses and compression wood,” Holzforschung 35(1), 16-26. DOI: 10.1515/hfsg.1981.35.1.16
Oinonen, P., Zhang, L., Lawoko, M., and Henriksson, G. (2015). “On the formation of lignin polysaccharide networks in Norway spruce,” Phytochemistry 111, 177-184. DOI: 10.1016/j.phytochem.2014.10.027
Pan, X.-J., and Sano, Y. (1999). “Atmospheric acetic acid pulping of rice straw IV: Physico-chemical characterization of acetic acid lignins from rice straw and woods. Part 1. Physical characteristics,” Holzforschung 53(5), 511-518. DOI: 10.1515/HF.1999.084
Pan, X., Gilkes, N., Kadla, J., Pye, K., Saka, S., Gregg, D., Ehara, K., Xie, D., Lam, D., and Saddler, J. (2006). “Bioconversion of hybrid poplar to ethanol and co‐products using an organosolv fractionation process: Optimization of process yields,” Biotechnol. Bioeng. 94(5), 851-861. DOI: 10.1002/bit.20905
Pye, E. K., and Lora, J. (1991). “The AlcellTM process: A proven alternative to kraft pulping,” TAPPI J. 74(3), 113-118.
Ralph, J., Peng, J., and Lua, F. (1998). “Isochroman structures in lignin: A new β-1 pathway,” Tetrahedron Lett. 39(28), 4963-4964. DOI: 10.1016/S0040-4039(98)00968-X
Scurlock, J. M. O., Dayton, D. C., and Hames, B. (2000). “Bamboo: An overlooked biomass resource?” Biomass Bioenergy 19(4), 229-244. DOI: 10.1016/S0961-9534(00)00038-6
Seca, A. M., Cavaleiro, J. A., Domingues, F. M., Silvestre, A. J., Evtuguin, D., and Neto, C. P. (2000). “Structural characterization of the lignin from the nodes and internodes of Arundo donax reed,” J. Agric. Food Chem. 48(3), 817-824. DOI: 10.1021/jf9910988
Silva, E. A. B. d., Zabkova, M., Araújo, J. D., Cateto, C. A., Barreiro, M. F., Belgacem, M. N., and Rodrigues, A. E. (2009). “An integrated process to produce vanillin and lignin-based polyurethanes from kraft lignin,” Chem. Eng. Res. Des. 87(9), 1276-1292. DOI: 10.1016/j.cherd.2009.05.008
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008a). “Determination of ash in biomass,” NREL Report No. TP-510-42622, National Renewable Energy Laboratory, Golden, CO.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2008b). “Determination of structural carbohydrates and lignin in biomass,” NREL Report No. TP-510-42622, National Renewable Energy Laboratory, Golden, CO.
Sun, S. -N., Li, M. -F., Yuan, T. -Q., Xu, F., and Sun, R. -C. (2012). “Sequential extractions and structural characterization of lignin with ethanol and alkali from bamboo (Neosinocalamus affinis),” Ind. Crop. Prod. 37(1), 51-60. DOI: 10.1016/j.indcrop.2011.11.033
Tejado, A., Pena, C., Labidi, J., Echeverria, J., and Mondragon, I. (2007). “Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis,” Bioresour. Technol. 98(8), 1655-1663. DOI: 10.1016/j.biortech.2006.05.042
Wen, J.-L., Xue, B.-L., Xu, F., Sun, R.-C., and Pinkert, A. (2013). “Unmasking the structural features and property of lignin from bamboo,” Ind. Crop. Prod. 42, 332-343. DOI: 10.1016/j.indcrop.2012.05.041
Wildschut, J., Smit, A. T., Reith, J. H., and Huijgen, W. J. (2013). “Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose,” Bioresour. Technol. 135, 58-66. DOI: 10.1016/j.biortech.2012.10.050
Xu, F., Sun, R. -C., Sun, J. -X., Liu, C. -F., He, B. -H., and Fan, J.-S. (2005). “Determination of cell wall ferulic and p-coumaric acids in sugarcane bagasse,” Anal. Chim. Acta 552(1), 207-217. DOI: 10.1016/j.aca.2005.07.037
Yáñez-S, M., Rojas, J., Castro, J., Ragauskas, A., Baeza, J., and Freer, J. (2013). “Fuel ethanol production from Eucalyptus globulus wood by autocatalized organosolv pretreatment ethanol–water and SSF,” J. Chem. Technol. Biot. 88(1), 39-48. DOI: 10.1002/jctb.3895
Yáñez-S, M., Matsuhiro, B., Nuñez, C., Pan, S., Hubbell, C. A., Sannigrahi, P., and Ragauskas, A. J. (2014). “Physicochemical characterization of ethanol organosolv lignin (EOL) from Eucalyptus globulus: Effect of extraction conditions on the molecular structure,” Polym. Degrad. Stab. 110, 184-194. DOI: 10.1016/j.polymdegradstab.2014.08.026
Yoshikawa, T., Yagi, T., Shinohara, S., Fukunaga, T., Nakasaka, Y., Tago, T., and Masuda, T. (2013). “Production of phenols from lignin via depolymerization and catalytic cracking,” Fuel Process. Technol. 108, 69-75. DOI: 10.1016/j.fuproc.2012.05.003
Zhao, X. -Y., Cao, J. -P., Morishita, K., Ozaki, J. -I., and Takarada, T. (2010). “Electric double-layer capacitors from activated carbon derived from black liquor,” Energy Fuels 24(3), 1889-1893. DOI: 10.1021/ef901299c
Zhu, M.-Q., Wen, J.-L., Su, Y.-Q., Wei, Q., and Sun, R.-C. (2015). “Effect of structural changes of lignin during the autohydrolysis and organosolv pretreatment on Eucommia ulmoides Oliver for an effective enzymatic hydrolysis,” Bioresour Technol 185, 378-385. DOI: 10.1016/j.biortech.2015.02.061
Zomers, F. H., Gosselink, R. J., Van Dam, J. E., and Tjeerdsma, B. F. (1995). “Organosolv pulping and test paper characterization of fiber hemp,” TAPPI J. 78(5), 149-155.
Article submitted: May 20, 2015; Peer review completed: August 24, 2015; Revised version received and accepted: October 6, 2015; Published: October 14, 2015.
DOI: 10.15376/biores.10.4.7998-8013
