Abstract
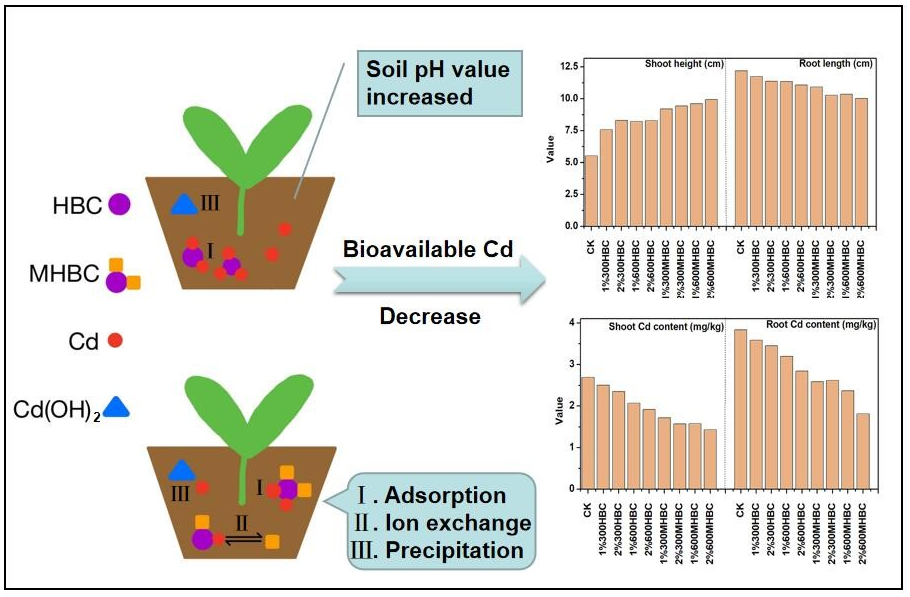
The remediation effects of peanut shell biochar (HBC) and Mg-modified peanut shell biochar (MHBC) prepared under pyrolysis temperatures of 300 °C and 600 °C on Cd2+ polluted brown soil were investigated in a pot experiment. The results showed that the biochar treatment increased soil pH and decreased the bioavailable Cd2+ content in the soil. Compared with the control treatment (CK), the pH value increased by 0.32 to 2.5 upon treatment with 1% and 2% of HBC and MHBC. Bioavailable Cd2+ in the soil decreased by 5.64% to 21.33% with HBC. The MHBC presented better amendment effects than HBC; bioavailable Cd2+ in the soil decreased by 26.2% to 50.1% with the addition of MHBC. The addition of HBC and MHBC increased the shoot height and decreased the root length of the spinach. Moreover, they significantly decreased the accumulation of Cd2+ in the shoots and roots of the spinach. Compared to CK, the Cd2+ content in the shoots decreased by 7.0% to 46.8% upon treatment with 1% and 2% of HBC and MHBC, while the Cd2+ content in the roots decreased by 7.3% to 52.7%. The Cd2+ content in the shoots and roots was more greatly decreased with MHBC than with HBC.
Download PDF
Full Article
Effects of Mg-modified Biochar on the Bioavailability of Cadmium in Soil
Ruifeng Shan,a,b,* Wanting Li,a,b Ya Chen,a,b and Xiaoyin Sun a,b
The remediation effects of peanut shell biochar (HBC) and Mg-modified peanut shell biochar (MHBC) prepared under pyrolysis temperatures of 300 °C and 600 °C on Cd2+ polluted brown soil were investigated in a pot experiment. The results showed that the biochar treatment increased soil pH and decreased the bioavailable Cd2+ content in the soil. Compared with the control treatment (CK), the pH value increased by 0.32 to 2.5 upon treatment with 1% and 2% of HBC and MHBC. Bioavailable Cd2+ in the soil decreased by 5.64% to 21.33% with HBC. The MHBC presented better amendment effects than HBC; bioavailable Cd2+ in the soil decreased by 26.2% to 50.1% with the addition of MHBC. The addition of HBC and MHBC increased the shoot height and decreased the root length of the spinach. Moreover, they significantly decreased the accumulation of Cd2+ in the shoots and roots of the spinach. Compared to CK, the Cd2+ content in the shoots decreased by 7.0% to 46.8% upon treatment with 1% and 2% of HBC and MHBC, while the Cd2+ content in the roots decreased by 7.3% to 52.7%. The Cd2+ content in the shoots and roots was more greatly decreased with MHBC than with HBC.
Keywords: Magnesium-modified biochar; Cadmium; Remediation; Spinach; Bioavailability
Contact information: a: Key Laboratory of Nansihu Lake Wetland Ecological Conservation & Environmental Protection (Shandong Province), College of Geography and Tourism, Qufu Normal University, Rizhao 276826, People’s Republic of China; b: Rizhao Key Laboratory of Territory Spatial Planning and Ecological Construction, Rizhao 276826, People’s Republic of China;
*Corresponding author: ruifengshan@sina.com
GRAPHICAL ABSTRACT
INTRODUCTION
Cadmium (Cd) is widely used in electroplating and metallurgy. The toxicity of Cd causes major environmental problems in soils worldwide. Soils are the largest source and sink of Cd. In China, according to the National Soil Pollution Survey, the standard pollution rate of Cd in 4,095 typical sampling sites was 7% greater than those of other heavy metals (Wang et al. 2015). Research shows that Cd can cause stress to plant growth, resulting in reduced plant biomass even at trace concentrations (Dai et al. 2020). Cadmium(II) induces plant cells to produce excessive reactive oxygen species, generating oxidation products such as hydrogen peroxide and peroxide ions. These species affect the redox balance in the plants and damage the integrity of plant cell membranes to varying degrees (Laspina et al. 2005; Guo et al. 2019; Sidhu et al. 2020). The bioavailability of heavy metals is closely related to the extent of damage to plants and animals. Heavy metals with high bioavailability are more likely to be absorbed by plants. Vegetables, fruits, and crops planted in heavy-metal-contaminated soils or irrigated with sewage containing a high content of heavy metals pose potential threats to human health (Farrag et al. 2012). Therefore, technologies to control the bioavailability of soil Cd should be studied. Present amendment technologies for Cd removal include physical remediation, chemical remediation, and bioremediation. Soil amendments can decrease the bioavailability of heavy metals through adsorption, complexation, and other mechanisms to fix heavy metals and decrease uptake by animals and plants. Soil amendment is an economical, efficient, and environmentally friendly remediation method (Ashrafi et al. 2015; Bashir et al. 2020), but the results of different soil amendments vary greatly (Zhang et al. 2020). At present, the addition of biochar to soil is one of the most promising soil remediation methods.
Biochars are porous carbonaceous materials with high specific surface area, and they are produced by the pyrolysis of organic matter under high temperature (> 300 °C) in anoxic or anaerobic environments (Sun et al. 2017; Cairns et al. 2020; Shan et al. 2020). The addition of biochar can improve soil quality, thereby promoting plant growth. Moreover, biochar has a well-developed pore structure, high specific surface area, and abundant surface functional groups (Sun et al. 2017; Chen et al. 2020). Therefore, biochar is an efficient and low-cost adsorbent that can effectively adsorb inorganic and organic pollutants, thereby decreasing their concentrations and bioavailabilities (Jin et al. 2016; Awad et al. 2020). Ali et al. (2020) used apricot shell and apple tree biochars to transform Cd and Zn from acid-soluble and reducible forms to organic-bound and residue fractions, which are less bioavailable. Consequently, their accumulation in the roots and shoots of mustard were decreased. In recent years, biochar has been widely used for the remediation of soil pollution. However, the remediation efficiency of a single type of pristine biochar for the removal of organic and inorganic pollutants in soil is limited (Fan et al. 2018). Therefore, researchers have combined biochar with other substances such as iron oxides (Micháleková-Richveisová et al. 2017), copper oxide (Li et al. 2020b), calcium carbonate (Wu et al. 2020), and graphene oxide (Shang et al. 2016) to remediate soils contaminated by heavy metals. Moreover, heavy metal fixation by biochar in soils is influenced by the pyrolysis temperature, concentration, and physicochemical properties of the biochar. Wang et al. (2020) observed that the content of Cd accumulated in maize decreased upon the addition of biochar prepared at 300 °C compared to that prepared at 700 °C. In another study, as the concentration of bamboo and rice stalk biochars increased from 0% to 5%, the concentration of CaCl2-extractable Cd in the soil gradually decreased (Yang et al. 2016). Awad et al. (2020) suggested that the contents of heavy metal in the soil and plant issues decreased after adding garden waste biochar and the reduction was higher in 6% of the biochar treatment than 4%. According to the aforementioned studies, the concentration and pyrolysis temperature of the biochar can affect the fraction of heavy metals in the soil, but the mechanisms of the reactions are not clear. Therefore, a new modified biochar should be developed to improve the remediation efficiency of biochar, and its pyrolysis temperature, concentration, mechanisms, and influencing factors should be investigated.
Spinach is one of the most popular vegetables in China. High concentration of Cd can accumulate in edible parts of leafy vegetables, such as spinach and lettuce. The human consumption of such a vegetable which contains a great amount of heavy metal presents a potential risk for human health (Huang et al. 2017). Magnesium ion is an essential element for its growth which can lead to exchange of heavy metal ions in the soil, thereby improving the adsorption efficiency of the biochar. Tao’s team found that the sorption capacity of the Thalia dealbata biochar for Cd in sediment was improved by 24.2 to 25.6% after loading MgCl2 (Tao et al. 2019). Li et al. (2020) suggested that the maximum theoretical saturation adsorption amount of Mg-modified biochar for Cd in wastewater was up to 370 mg/g. These research studies about Mg-modified biochar mainly focused on its sorption behavior in two different environmental matrixes. However, the influence of Mg-modified biochar on the bioavailability of Cd in soil and the effect on plant growth status have not been well understood. Further efforts are necessary to evaluate the effect of the Mg-modified biochar on Cd in soil from the aspect of bioavailability. Therefore, this study attempts to prepare Mg-modified biochar and explore its optimum concentration and pyrolysis temperature to reduce the bioavailability of Cd. Peanut shell biochar (HBC) and Mg-modified peanut shell biochar (MHBC) were prepared via 300 °C and 600 °C pyrolysis and added to soil samples at 1% and 2% concentrations. The growth and Cd content of spinach were used to evaluate the passivation effect of Cd by HBC and MHBC at different concentrations and pyrolysis temperatures.
EXPERIMENTAL
Preparation and Treatment of Soil Samples
Brown surface soil (0 cm to 20 cm) samples were collected from Rizhao, Shandong Province, China. The soil samples were dried, ground, and sieved through 2-mm, 1-mm, and 0.15-mm sieves to prepare for the pot experiment, pH detection, and analysis of total organic matter and heavy metal content, respectively. The basic physicochemical properties of the soil samples are shown in Table 1.
Table 1. Basic Physicochemical Properties of Soil Samples
Preparation and Characterization of Biochar
The pristine biochar was prepared from peanut shells. Peanut shells were first washed three times with deionized water, and they were then dried to a constant weight at 50 °C before being ground into powder. The biochar was produced from the obtained powder by placing it in a porcelain crucible with a cap and pyrolyzing it in a muffle furnace for 2 h at 300 °C (300HBC) and 600 °C (600HBC) under an oxygen-limited N2 atmosphere. The N2 gas was inserted at 10 L/min, and the heating rate was approximately 15 °C/min. The samples were then ground and sieved through a 0.6-mm sieve after the temperature decreased to room temperature.
To prepare the Mg-modified peanut biochar, peanut shells were first soaked in an MgCl2 solution (at a solid–liquid ratio of 1:10 w/v) in an ultrasonic oscillator for 6 h (Tao et al. 2019). They were then cleaned with deionized water, placed in a crucible, and dried at 105 °C before being ground into powder. The pyrolysis process was the same as mentioned above, and the samples were labeled as “300MHBC” and “600MHBC.” The characterization of the biochar is described in Chen et al. (2020).
Experimental Methods
The bioavailability of Cd in the biochar-amended soil was investigated through experiments. The soil treatments were the control treatment (CK), 1%300HBC, 2%300HBC, 1%600HBC, 2%600HBC, 1%300MHBC, 2%300MHBC, 1%600MHBC, and 2%600MHBC. The percentages refer to the mass ratio in the soil (i.e., biochar concentration), 300 and 600 indicate the corresponding pyrolysis temperature (°C), HBC refers to pristine biochar, and MHBC refers to Mg-modified biochar.
A stock solution of 1000 mg/L Cd(NO3)2‧4H2O was added to the soil and mixed evenly. A soil sample with a Cd content of 10 mg/kg was prepared and air dried, followed by homogenization. The polluted soil was added to the plastic pots, and the moisture content of the soil was maintained at 60% of the water holding capacity. After 2 weeks, 1 g of urea was added as base fertilizer and mixed evenly. The pristine biochar and Mg-modified biochar (300HBC, 600HBC, 300MHBC, 600MHBC) were then mixed with the soil at 0%, 1%, and 2% mass fractions. The moisture contents of the soil samples were maintained at 65% of water holding capacity by adding distilled water, and the soil was cultured alternately under moist and dry conditions in a greenhouse for 1 week. All experiments were conducted in triplicate, and the average values were used for the analysis. A total of 27 treatments were established and performed.
Spinach was selected as the indicator of Cd bioavailability in the soil. The spinach seeds were disinfected by soaking in 6% NaClO solution for 20 min. Then, the seeds were removed and rinsed with tap water three times, followed by washing with distilled water. Three seeds were planted in each plastic pot filled with 500 g of soil. The plastic pots were placed in an artificial greenhouse, and their positions were randomly changed every two weeks. The spinach samples were harvested after five weeks of cultivation.
Soil Sampling and Analysis
Soil samples were collected and then air-dried on the 30th day after the spinach was planted in the plastic pots. The soil pH was measured by a pH meter. For that, 5.0 g of soil sample was weighed and sieved (1 mm). The soil sample was placed in a 50-mL centrifuge tube, and 25 mL of deionized water was added to it for a soil:water ratio of 1:5 (w/v). The centrifuge tubes were oscillated at 180 rpm for 30 min, then centrifuged at 4000 rpm for 10 min. Finally, the pH of the supernatant was measured with the pH meter.
Next, soil samples of 5.0 g were screened by a 0.15-mm sieve, then weighed in a 50-mL centrifuge tube. Subsequently, a 0.1 mol/L CaCl2 solution (1:25 soil:water ratio) was added to the tube and oscillated for 2 h at 180 rpm and 25 °C ± 1 °C. The samples were then centrifuged at 4000 rpm for 10 min and filtered through a 0.22-μm membrane. The supernatant was collected to determine the concentration of bioavailable Cd2+.
A soil sample of 0.2 g was moistened with distilled water and placed in a crucible, and 10 mL of HCl was added to it. The sample was heated at 80 °C for evaporation until the solution in the crucible was approximately 3 mL. A mixture solution of HNO3, HF, and HClO4 (5:5:3 v/v/v) was added and heated at 180 °C for 1 h with a lid. Afterwards, the lid was opened to remove silicon and then closed to decompose the black organic carbide when the thick white perchloric acid smoke emerged. Before the lid was opened to remove the white smoke, the black organic matter on the crucible wall was already absent. The crucible was cooled to room temperature, and 1 mL of HNO3 solution was added. The mixture was transferred into a 25-mL colorimetric tube, mixed evenly, and left to stand overnight. The results of atomic absorption testing (see later) determined the total Cd in the soil.
Plant Sampling and Analysis
After 5 weeks of culturing, three spinach samples were randomly selected from each treatment group to measure the plant height and root length with a ruler. The average value was used as the indicator of spinach growth.
The harvested spinach samples were washed with distilled water three times. The shoots and roots of the spinach were separated and dried at 105 °C for 30 min, dried to constant weight at 60 °C, and then ground into powder. A sample of 0.25 g was placed in a crucible, to which 10 mL of HNO3-HClO4 (4:1, v/v) mixture solution was added. The mixture was heated to digest, and the Cd content in the plant tissue was analyzed.
Detection and Statistical Analysis
An atomic absorption spectrophotometer (GFA-7000A, Shimadzu Corporation, Shimadzu, Japan) was used to determine the Cd content in the soil and digested plant tissue solutions. Origin 8.0 software (OriginLab, Northampton, MA, USA) was used for statistical processing of all test data.
RESULTS AND DISCUSSION
Effects of Different Biochar Treatments on Soil pH
Soil pH is the main factor controlling the adsorption, desorption, and precipitation balance of heavy metals. Moreover, it determines the migration and transformation behavior of heavy metals in the soil. Therefore, the soil pH can notably influence the bioavailability of heavy metals in the soil and the extent of the stress caused by them on plant growth. The effects of the different biochar treatments on the soil pH are shown in Fig. 1. The pH of the soil treated with HBC and MHBC increased by 0.32 to 2.50 units compared with CK. This result was consistent with the results of other studies. For example, Wu et al. (2020) reported that the addition of calcium-based magnetic biochar significantly increased the pH of rice rhizosphere and non-rhizosphere soil. The ash produced by the pyrolysis of biomass generates many inorganic salt ions, such as Mg2+, Ca2+, Na+, and K+, which mostly exist as metal oxides and carbonates. Adding them to the soil increases the soil pH. The salt ions of the biochar can also lead to H+ and Al3+ exchange on the surface of soil colloids, thereby increasing the soil pH (Yuan et al. 2010). In addition, when the biochar is alkaline, it can increase the soil pH, to some extent decreasing the content of exchangeable Cd2+ and thereby decreasing the consequent damage to the growth processes of crops (Van Zwieten et al. 2010). The soil pH values for 300HBC, 600HBC, 300MHBC, and 600MHBC at 1% increased by 0.32, 1.43, 0.92, and 2.21 units, respectively, compared with CK. When the biochar concentration was increased to 2%, the soil pH values increased by 1.36, 2.11, 1.89, and 2.50 units, respectively, in comparison with CK. This result suggests that the soil pH increased with increasing concentration of biochar. These results are consistent with the findings of Yang et al. (2016), who reported that the addition of corn stalk biochar significantly increased soil pH, and the improvement caused by 5% corn stalk biochar treatment was greater than with the 1% treatments. This result may be because biochar is an alkaline material, and more alkaline ions carried by the biochar enter the soil with increasing addition of biochar, thereby leading to a greater soil pH. Moreover, the capacities of the HBC and MHBC to increase soil pH increased with increasing pyrolysis temperature. At the same concentration, the biochar produced at higher pyrolysis temperature increased the soil pH to a greater extent. Compared with the HBC, the soil pH increased by 0.39 to 0.78 units with the addition of MHBC, and the soil pH was greatest with the 2%600MHBC treatment. This result demonstrates that the additions of magnesium and biochar had a synergistic effect on soil pH. The Mg2+ loaded on the biochar surface can adsorb negatively charged OH– and CO32-. Consequently, the inorganic magnesium led to a relatively high biochar pH, thereby increasing the soil pH to a greater extent (Lee et al. 2017).
Fig. 1. Effects of different biochar treatments on soil pH
Effects of Different Biochar Treatments on the Bioavailable Cd Content in the Soil
The addition of HBC and MHBC significantly affected the bioavailable Cd2+ content in the soil. As shown in Fig. 2a, in comparison with CK, the content of bioavailable Cd2+ decreased by 5.6%, 6.4%, 26.2%, and 45.0% with 1% concentration of 300HBC, 600HBC, 300MHBC, and 600MHBC, respectively, and the content of bioavailable Cd2+ ranged from 4.35 mg/kg to 5.32 mg/kg. The content of bioavailable Cd2+ in the soil decreased by 21.3%, 19.5%, 40.8%, and 50.1% with 2% 300HBC, 600HBC, 300MHBC, and 600MHBC, respectively, and the bioavailable Cd2+ ranged from 2.16 mg/kg to 3.37 mg/kg. Therefore, the addition of biochar can effectively decrease the content of bioavailable Cd2+ in the soil, which decreased as the concentration of biochar increased from 1% to 2%. This result is consistent with the results of Yang et al. (2016), who reported that the content of CaCl2-extractable Cd2+ in soil decreased as the concentration of biochar increased from 0% to 5%. This result probably occurred because more active adsorption sites were provided for the soil with greater biochar concentrations, which increased the soil pH to a greater extent, thereby improving the fixation capacity of the soil for Cd2+. Moreover, as the biochar concentration increased, the desorption rate of the Cd2+, which was adsorbed by the soil surface, decreased (Abbas et al. 2017). However, Qayyum et al. (2019) demonstrated that the content of bioavailable Cd2+ significantly decreased and was effectively fixed in a soil contaminated by a relatively low Cd2+ level (25 mg/kg) as the concentration of cotton stalk biochar increased from 2% to 5%. However, the results were contrary for soils with relatively greater Cd2+ contamination (50 mg/kg and 100 mg/kg). These results illustrate that the effect of biochar on the fixation of Cd2+ can also be affected by the contamination level of Cd in the soil. Furthermore, for the same concentration, the content of bioavailable Cd2+ in the soil decreased as the pyrolysis temperature increased, and the values were ranked as follows: 300HBC > 600HBC > 300MHBC > 600MHBC. Karimi et al. (2020) reported that a sludge biochar prepared at 600 °C effectively decreased the exchangeable form of Pb2+ and Cd2+ in the soil, and its effect was more significant than that of the biochar prepared at 300 °C. This result further illustrates that the greater pyrolysis temperatures correspond to greater fixation capacity of the biochar for Pb2+ and Cd2+ in the soil. Studies have shown that biochar prepared via low-temperature pyrolysis (< 300 °C) mainly produces aliphatic alkylated oxygen, whereas biochars formed by high-temperature pyrolysis (> 300 °C) contain abundant aromatic structures (Li et al. 2013). Therefore, a greater pyrolysis temperature contributes to the formation of aromatic functional groups on the surface of the biochar, such as O-H, C=C, and C=O. These structures can enhance the adsorption capacity of the biochar by strengthening hydrogen and dipole bonds (Chen et al. 2019). The abundant surface functional groups and aromatic structures of the biochar can also act as electron donors or receptors to adsorb Cd2+ by π-π electron donor–receptor interactions (EDA), thereby decreasing the content of bioavailable Cd2+ in the soil. Compared with the HBC, the MHBC had a stronger inhibitory effect on the bioavailable Cd2+ content in the soil in a short period of time (30 d). The content of bioavailable Cd2+ after the MHBC treatment decreased by 26.2% to 50.1% compared with the CK treatment, which was a greater reduction than that of the HBC treatment (5.6% to 21.3%). This result demonstrates that the Cd2+ fixation capacity of the biochar in the soil was improved by the Mg loading, and the passivation effect of the 2% MHBC prepared at 600 °C was the greatest. This result might be due to the magnesium loaded on the biochar surface after the pyrolysis process in preparing the MHBC. Magnesium hydroxide was precipitated due to the increased soil pH, which promoted the co-precipitation of Mg2+ and Cd2+ in the soil solution (Ngambia et al. 2019; Li et al. 2020), thereby decreasing the content of bioavailable Cd2+ in the soil.
Fig. 2. Effects of different treatments on (a) bioavailable Cd2+ and (b) fraction of bioavailable Cd2+ to total Cd2+
The fraction of bioavailable Cd2+ to total Cd2+ under different biochar treatments are shown in Fig. 2b. The addition of HBC or MHBC decreased the fraction of bioavailable Cd2+ to total Cd2+ in the soil, and as the biochar concentration increased, the fraction of bioavailable Cd2+ tended to decrease. Compared with the CK treatment, the fractions of bioavailable Cd2+ treated with 1%300HBC, 2%300HBC, 1%600HBC, and 2%600HBC decreased by 8.7%, 34.8%, 9.8%, and 34.8%, respectively. By contrast, the fractions of bioavailable Cd2+ in the soil treated with 1%300MHBC, 2%300MHBC, 1%600MHBC, and 2%600MHBC decreased by 44.6%, 55.4%, 59.8%, and 64.1%, respectively. The fractions of bioavailable Cd2+ to total Cd2+ were ranked as 300HBC > 600HBC > 300MHBC > 600MHBC for the same concentration of HBC and MHBC, thus indicating that the fraction of bioavailable Cd2+ in the soil decreased as the pyrolysis temperature increased.
To explore the mechanism of the effect of biochar addition on the content of bioavailable Cd2+ in the soil, the correlation between soil pH and fraction of bioavailable Cd2+ to total Cd2+ was analyzed. As shown in Fig. 3, this correlation was significant (p < 0.01), which further demonstrates that the biochar immobilized Cd2+ in the soil mainly by increasing soil pH and weakening its bioavailability. Huang et al. (2020) reported that the proportion of bioavailable Cd2+ to total Cd2+ was 48.5% in an acidic soil and 5.0% in an alkaline soil. This result is consistent with the results of Ali et al. (2020), which suggested that the addition of biochar derived from apricot shells can decrease the bioavailability of Cd2+ in soil by increasing soil pH and ultimately decreasing crop uptake. Such results can be attributed to two reasons.
First, the addition of HBC and MHBC increased the soil pH, and the consequent greater concentration of OH– in the soil led to the hydrolyzation of Cd2+, which precipitated to Cd(OH)2. The adsorption of Cd2+ was enhanced because the adsorption affinity of the soil for metal hydroxides was greater than that for free metal cations. Consequently, the bioavailability of Cd2+ was decreased (Yuan et al. 2010). Second, in addition to increasing the negative surface charge of clay minerals in the soil, greater soil pH also weakens the competitive adsorption of H+ and Cd2+. Therefore, the adsorption capacity of negatively charged soil colloids for Cd2+ was promoted. Meanwhile, precipitation of compounds such as Cd(OH)2, CdCO3, and Cd3(PO4)2 also occurred, which decreased the bioavailability of Cd2+. However, the fraction of bioavailable Cd2+ to total Cd2+ was not fully consistent with the variation trend of soil pH. For instance, the pH of the soil treated with 1%600HBC was 0.28 units larger than that of the soil treated with 2%300HBC, and the fraction of bioavailable Cd2+ in the former sample was 23% greater, which did not follow the negative correlation (Figs. 1 and 2b). This result indicates that the content of bioavailable Cd2+ in the soil was not completely determined by soil pH, and it may be closely related to the adsorption and desorption processes of HBC and MHBC for Cd2+ in the soil.
The structure of the biochar determines if it can effectively adsorb Cd2+ in the soil through surface adsorption and pore filling, thereby fixating the Cd2+ and decreasing its bioavailability. As shown in scanning electron microscopy (SEM) analysis of HBC and MHBC (Chen et al. 2020), the pristine biochar and Mg-modified biochar had well-developed pore structures and high specific surface area, which exhibited a significant increase after loading MgCl2 on the surface of peanut shell biochar. The pristine biochar presented a relative smooth surface with lesser pores than Mg-modified biochar, which can also explain the adsorption capacity of MHBC for Cd was better than HBC and thus posing a more significant effect on decreasing the bioavailability of Cd in soil. Moreover, according to the SEM analysis, the surface of MHBC was covered with irregular particles, which has been corroborated MgO and Mg(OH)2 crystal particle by Energy Dispersive Spectrometer (EDS) analysis in our previous study (Chen et al. 2020). This indicated that Mg was successfully deposited on the surface of biochar and may improve the adsorption capacity of biochar-soil system for Cd by ion exchange process to some extent. Fourier-transform infrared spectroscopy of HBC and MHBC suggests that their surfaces are rich in functional groups such as -OH corresponded to the peak of 3462 cm-1, -CH3 (2944 cm-1), carbonate (833 cm-1), -C=O (1633 cm-1), and C=C (1450 cm-1), among which acidic oxygen-containing functional groups such as carboxyl can complex with Cd2+ in the soil, thereby decreasing its bioavailability (Chen et al. 2020). In addition, biochars are rich in organic matter, which can also promote the formation of stable complexes between heavy metals (such as Cd2+) and organic matter, thereby decreasing the Cd2+ bioavailability (Zia ur Rehman et al. 2017). In this study, the inherent properties of biochar were changed by MgCl2 modification, and the content of cation ions also increased. These transformation can account for the more significant reduction effect of MHBC on the bioavailability of Cd in soil.
Fig. 3. Correlation between soil pH and fraction of bioavailable Cd2+ to total Cd2+ upon different treatments
Effects of Different Treatments on the Biomass of Spinach
The effects of the HBC and MHBC treatments on the growth of spinach shoots and roots are shown in Fig. 4. The addition of HBC and MHBC increased the shoot height by 37.2% to 80.1% compared with CK. This result can be attributed to the biochar’s alkalinity, which was conducive to the deprotonation of hydroxyl, carboxyl, and other acidic functional groups in the soil. As the negative surface charge of the soil colloids and biochar increased, free heavy metal ions with positive charge were adsorbed to neutralize the surface charge, thus improving the adsorption capacity of the soil and, consequently, its Cd2+ amendment potential. Therefore, the addition of biochar could decrease the bioavailability of Cd2+ in the soil, thereby decreasing the toxic stress on spinach and increasing its yield. The shoot height of the spinach increased with increasing concentrations of HBC and MHBC. Compared with CK, the shoot heights of 1%300HBC, 2%300HBC, 1%600HBC, and 2%600HBC increased by 37.2%, 50.1%, 48.9%, and 49.8%, respectively. Moreover, the shoot heights of 1%300MHBC, 2%300MHBC, 1%600MHBC, and 2%600MHBC increased by 66.5%, 70.8%, 73.8%, and 80.8%, respectively. This result is contrary to the findings of Wu et al. (2020), whose results show that the effect of 1% magnetic calcium-based rice straw biochar on the growth of rice was stronger than that of 2% addition to an As polluted soil. This probably occurred because the negative charges on the surfaces of the soil colloids increased with increasing biochar concentration, which resulted in the repulsion of As anions in the soil. Therefore, the bioavailability of As was enhanced, and the bioavailable As was absorbed more easily by the plant, thereby inhibiting its growth. In contrast, Tao et al. (2019) reported results consistent with the present study. In their study, the Cd2+ stress in the soil was alleviated as the biochar concentration increased from 0% to 5%, which significantly promoted the germination and growth of pakchoi. The differences between these results may be due to the different valence (Cd2+, As anions) which determined the repulsion and adsorption behavior of heavy metal ions then influencing their bioavailability when the negative charges on the surfaces of the soil colloids increased after adding biochar.
Fig. 4. Effects of different biochar treatments on spinach biomass
Fig. 5. Correlations between (a) shoot height and (b) root length of spinach and the fraction of bioavailable Cd2+ to total Cd2+
Therefore, the effect of biochar on plant growth in a heavy-metal-stressed soil depends on the characteristics of the biochar, plant species, and heavy metal properties, among which valence is the most important factor (Chen et al. 2015). The shoot heights of the spinach were ranked as follows: 300HBC < 600HBC < 300MHBC < 600MHBC. This result indicates that the shoot height of the spinach increased with increasing biochar pyrolysis temperature for the same concentration of HBC and MHBC. The shoot height related to the MHBC treatment was 9.20 cm to 9.95 cm, which was significantly greater than those related to the HBC treatment (7.58 cm to 8.29 cm) and CK (5.52 cm). These results indicate that the addition of MHBC was more conducive to the growth of the spinach overground part. This result was consistent with the results of Tao et al. (2019), in which the addition of Mg-modified biochar derived from Thalia dealbata had a more significantly effect than pristine biochar on the germination and growth of pakchoi. A possible reason for these results is that the biochar was rich in basic cations (Mg2+), whose content increased by the modifying process. Abundant Mg2+ can promote the exchange of the Mg2+ loaded on the biochar surface with the Cd2+ in soil, thereby fixing Cd2+ on the biochar surface. In addition, Mg2+ can provide nutrients for the spinach, which promote its growth to some extent. Therefore, the shoot height increased with increasing biochar concentration and pyrolysis temperature, and MHBC had more significant effects on the increase of spinach shoot height. Then the shoot height increased the most in the 2%600MHBC treatment compared with CK. This is consistent with the findings of Lu et al. (2014), who reported that the shoot biomass of Sedum plumbizincicola in 5% bamboo biochar treatment was higher than 1% treatment and CK under Cd stress. On the one hand, this was related to the extractable Cd content which was lower in 5% biochar treatment. The heavy metal Cd has been reported that can decrease spinach biomass (Younis et al. 2016). Then the stress effect on plant growth was lesser in 5% biochar treatment. On the other hand, the total shoot nutrient element N content and the shoot N concentration in 5% biochar treatment were significantly higher than that in 1% and CK. Therefore, different concentration of biochar can affect plant biomass by influencing its nutrient absorption capacity. Moreover, in this study, the pH value of biochar-soil system increased with the pyrolysis temperature of biochar increase. This may decrease the bioavailability of Cd and thus improving the plant biomass.
To further explore the effect of bioavailable Cd2+ on plant growth, the correlation between shoot height and fraction of bioavailable Cd2+ to total Cd2+ was analyzed. As shown in Fig. 5a, the spinach shoot height was negatively correlated with this fraction (p < 0.01). This result may be attributed to the lower content of bioavailable Cd2+, which then caused a lower stress effect on the growth of the spinach. Therefore, the growth inhibition effect of the Cd2+ on the spinach was weak, and the shoots were able to grow relatively tall. In addition, Parra et al. (2017) observed that Ca2+, Zn2+, and Fe2+ were essential elements for plant growth and had similar physical properties to Cd2+, such as charge and ion radius. Other studies have also shown that Cd2+ competes with Ca2+, Zn2+, and Fe2+ for the same transport channel on the root surface when being absorbed into plant tissue (Tian et al. 2016). Therefore, the decreased content of Cd2+ decreased the competition between Cd2+ and essential elements for plant growth, which was conducive to nutrient uptake by the plant.
As shown in Fig. 4, the addition of HBC and MHBC decreased the length of the spinach root. Compared with CK, the root length decreased by 3.7% to 17.8%, and it decreased the most for the 2%600MHBC treatment. As shown in Fig. 4, the root length decreased with increasing biochar concentration. For the 1%300HBC, 2%300HBC, 1%600HBC, and 2%600HBC treatments, the root lengths decreased by 3.7%, 6.8%, 6.8%, and 9.1% compared to the CK, respectively. For the 1%300MHBC, 2%300MHBC, 1%600MHBC, and 2%600 MHBC treatments, the lengths decreased by 10.3%, 15.6%, 15.0%, and 17.8%, respectively. A possible reason for these results is that the 2% HBC and MHBC have more ions, and the increased soil conductivity led to a salt stress, which further inhibited the growth of spinach roots. The lengths of the spinach roots were ranked as follows: 300HBC > 600HBC > 300MHBC > 600MHBC, for the same concentrations of HBC and MHBC. Therefore, the root length decreased with increasing pyrolysis temperature. In addition, compared with HBC, MHBC had a stronger shortening effect on the root length. This result was consistent with the trend of fraction of bioavailable Cd2+ to total Cd2+ upon addition of biochar. As shown in Fig. 5b, there was a highly significant positive correlation between root length and fraction of bioavailable Cd2+ to total Cd2+ (p < 0.01). This probably occurred because the spinach roots were directly exposed to Cd2+ contaminated soil and were more sensitive to a Cd2+ contaminated environment. It was difficult for plant tissues to transport and absorb Cd2+ ions due to the decrease of bioavailable Cd2+ in the soil. Therefore, the spinach shoot contained less Cd2+, which accumulated more in the spinach root, thereby inhibiting its growth.
Effects of Different Biochar Treatments on the Cd Content of Spinach
The Cd2+ contents of the spinach shoots and roots treated with different biochars are shown in Fig. 6. Both treatments decreased the accumulation of Cd2+ in the shoots and roots. Compared with CK, the Cd2+ content upon HBC and MHBC treatment decreased by 7.0% to 46.8% in the shoots and 7.3% to 52.7% in the roots. This result is likely due to the low molecular organic acid being the most active substance in the root exudates, among which oxalic acid was the main component (Muratova et al. 2009). Root exudates decrease soil pH and provide ligands for metal complexation reactions, thereby increasing the concentration of bioavailable Cd2+ in the soil. However, the addition of alkaline biochar neutralized organic acids in the plant root exudates, thereby fixing Cd2+ and decreasing its absorption by the plant. The biochar addition had a relatively mild effect on the decrease of Cd2+ content in the spinach shoots compared with the roots, which suggests that the spinach roots functioned as a barrier to Cd2+ absorption and were the main channel for Cd2+ to be transported to the aboveground parts of the plant.
Fig. 6. Effects of different biochar treatments on the Cd2+ content in spinach
The concentrations of HBC and MHBC had a relatively low effect on the Cd2+ content of spinach shoots. The accumulation of Cd2+ in the spinach shoot decreased as the biochar concentration increased. The Cd2+ content in the shoots decreased by 6% upon treatment with 2%300HBC, compared with that of 1%300HBC, and it decreased by 7.2% with 2%600HBC, compared with that of 1%600HBC. The Cd2+ content upon 2%300MHBC treatment was 8.7% less than that of the 1%300MHBC treatment, and it was 9.5% less for 2%600MHBC compared with that of 1%600MHBC. The accumulation of Cd2+ in the spinach shoot was the lowest (1.43 mg/kg) for the treatment of 2%600MHBC. The accumulation of Cd2+ in the spinach root decreased with increasing biochar concentration. Compared with CK, the greatest reduction occurred for the 2%600MHBC group, at 52.7%. The accumulation of Cd2+ in the spinach shoots and roots decreased with increasing biochar concentration, which suggests that the addition of HBC and MHBC inhibited the absorption of Cd2+, and the increase of biochar concentration improved the inhibition of Cd2+ absorption. In addition, the shoot height increased with increasing biochar concentration, which had a certain dilution effect on the Cd2+ in the spinach shoot. The inhibition effects of biochar on the absorption of Cd2+ in both shoots and roots of the spinach were as follows: 300HBC < 600HBC < 300MHBC < 600MHBC. This result indicates that the inhibition of the Cd2+ adsorption increased with increasing biochar pyrolysis temperature. This result probably occurred because higher pyrolysis temperatures resulted in higher losses of fatty matter, volatile matter, and water, thereby forming a well-developed pore structure on the biochar surface (Zhang et al. 2013), which effectively promotes surface adsorption and pore filling for Cd2+ (Chen et al. 2019). Consequently, the bioavailability and mobility of Cd2+ in the soil decreased, and its absorption was inhibited. Compared with the HBC, the inhibition effect of MHBC on the absorption of Cd2+ was greater. The accumulations of Cd2+ in the shoots and roots of spinach with MHBC decreased by 23.7% to 33.2% and 24.1% to 52.1%, respectively, compared with the HBC at the same pyrolysis temperature and concentration. This result indicates that the magnesium modification had a synergistic effect on pristine biochar in inhibiting the absorption of Cd2+ by the spinach.
As shown in Fig. 6 and Fig. 2b, the variations of the Cd2+ content absorbed by the shoots and roots of the spinach for different treatments were mostly consistent with the fraction of bioavailable Cd2+ to total Cd2+. To further explore the influence of bioavailable Cd2+ in soil on the accumulation of Cd2+ in plants, the correlations between the accumulations of Cd2+ in the shoots (Fig. 7a) and roots (Fig. 7b) of the spinach and the fractions of bioavailable Cd2+ to total Cd2+ were analyzed.
Fig. 7. Correlation between accumulation of Cd2+ in spinach (a) shoots and (b) roots and the fraction of bioavailable Cd2+ to total Cd2+
As shown in Fig. 7, the contents of Cd2+ in both shoots and roots of the spinach were positively correlated with the fraction of bioavailable Cd2+ (p < 0.01). This result indicates that the Cd2+ content in the spinach was mainly affected by the content of bioavailable Cd2+ in the soil. This result is consistent with the results of Huang et al. (2020), in which the Cd2+ absorption by plants was mainly related to bioavailable Cd2+, instead of total Cd2+ content in the soil. The addition of HBC and MHBC decreased the content of bioavailable Cd2+ in the soil, thus decreasing its uptake by plants. The accumulation of Cd2+ in the spinach was greater in the roots than in the shoots, and the average Cd2+ content in the roots was 32.2% greater than that in the shoots. Thus, the distribution of Cd2+ in the spinach presented an obvious root accumulation effect.
CONCLUSIONS
- The addition of peanut shell biochar (HBC) and Mg-modified peanut shell biochar (MHBC) effectively increased the soil pH with increasing biochar concentration and pyrolysis temperature. The effect of MHBC on pH increase was greater than that of HBC.
- Both HBC and MHBC effectively decreased the content of bioavailable Cd2+ in the soil, thereby decreasing the amount of Cd2+ absorbed by the shoots and roots of the spinach. The bioavailable Cd2+ content in the soil and the Cd2+ absorbed by the spinach decreased with increasing biochar concentration and pyrolysis temperature. The MHBC had the greater effect on the decrease of bioavailable Cd2+, indicating that the MHBC can better decrease the bioavailability of heavy metals.
- The addition of the HBC and MHBC significantly increased the shoot height and decreased the root length of the spinach, and the effects were greater as the biochar concentration and pyrolysis temperature increased. The effects of the MHBC on plant height and root length were greater than those of the HBC.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant Nos. 41501542 and 41471389).
REFERENCES CITED
Abbas, T., Rizwan, M., Ali, S., Zia-ur-Rehman, M., Qayyum, M. F., Abbas, F., Hannan, F., Rinklebe, J., and Ok, Y. S. (2017). “Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination,” Ecotoxicology and Environmental Safety 140, 37-47. DOI: 10.1016/j.ecoenv.2017.02.028
Ali, A., Shaheen, S. M., Guo, D., Li, Y., Xiao, R., Wahid, F., Azeem, M., Sohail, K., Zhang, T., Rinklebe, J., et al. (2020). “Apricot shell- and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil,” Environmental Pollution 264. DOI: 10.1016/j.envpol.2020.114773
Ashrafi, M., Mohamad, S., Yusoff, I., and Hamid, F. S. (2015). “Immobilization of Pb, Cd, and Zn in a contaminated soil using eggshell and banana stem amendments: Metal leachability and a sequential extraction study,” Environmental Science and Pollution Research 22, 223-230. DOI: 10.1007/s11356-014-3299-4
Awad, M., Moustafa-Farag, M., Wei, L., Huang, Q., and Liu, Z. Z. (2020). “Effect of garden waste biochar on the bioavailability of heavy metals and growth of Brassica juncea (L.) in a multi-contaminated soil,” Arabian Journal of Geosciences 13, 225-231. DOI: 10.1007/s12517-020-05376-w
Bashir, S., Hussain, Q., Zhu, J., Fu, Q. L., Houben, D., and Hu, H. Q. (2020). “Efficiency of KOH-modified rice straw-derived biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil,” Pedosphere 30, 874-882. DOI: 10.1016/S1002-0160(20)60043-1
Cairns, S., Robertson, I., Sigmund, G., and Street-Perrott, A. (2020). “The removal of lead, copper, zinc and cadmium from aqueous solution by biochar and amended biochars,” Environmental Science and Pollution Research 27, 21702-21715. DOI: 10.1007/s11356-020-08706-3
Chen, T., Zhou, Z. Y., Xu, S., Wang, H. T., and Lu, W. J. (2015). “Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge,” Bioresource Technology 190, 388-394. DOI: 10.1016/j.biortech.2015.04.115
Chen, Y., Jiang, Z., Wu, D., Wang, H., Li, J., Bi, M., and Zhang, Y. (2019). “Development of a novel bio-organic fertilizer for the removal of atrazine in soil,” Journal of Environmental Management 233, 553-560. DOI: 10.1016/j.jenvman.2018.12.086
Chen, Y., Shan, R., and Sun, X. (2020). “Adsorption of cadmium by magnesium-modified biochar at different pyrolysis temperatures,” BioResources 15(1), 767-786. DOI: 10.15376/biores.15.1.767-786
Dai, Y., Liu, R., Zhou, Y., Li, N., Hou, L., Ma, Q., and Gao, B. (2020). “Fire Phoenix facilitates phytoremediation of PAH-Cd co-contaminated soil through promotion of beneficial rhizosphere bacterial communities,” Environment International 136. DOI: 10.1016/j.envint.2019.105421
Fan, Z. X., Zhang, Q., Li, M., Niu, D. Y., Sang, W. J., and Verpoort, F. (2018). “Investigating the sorption behavior of cadmium from aqueous solution by potassium permanganate-modified biochar: Quantify mechanism and evaluate the modification method,” Environmental Science and Pollution Research 25, 8330-8339. DOI: 10.1007/s11356-017-1145-1
Farrag, K., Senesi, N., Soler Rovira, P., and Brunetti, G. (2012). “Effects of selected soil properties on phytoremediation applicability for heavy-metal-contaminated soils in the Apulia region, southern Italy,” Environmental Monitoring and Assessment 184(11), 6593-6606. DOI: 10.1007/s10661-011-2444-5
Guo, B., Liu, C., Liang, Y., Li, N., and Fu, Q. (2019). “Salicylic acid signals plant defence against cadmium toxicity,” International Journal of Molecular Sciences 20(12). DOI: 10.3390/ijms20122960
Huang, R., Dong, M., Mao, P., Zhuang, P., Paz-Ferreiro, J., Li, Y., Li, Y., Hu, X., Netherway, P., and Li, Z. (2020). “Evaluation of phytoremediation potential of five Cd (hyper)accumulators in two Cd contaminated soils,” Science of the Total Environment 721. DOI: 10.1016/j.scitotenv.2020.137581
Huang, Y. Y., He, C. T., Shen, C., Guo, J. J., Mubeen, S., Yuan, J. G., and Yang, Z. Y. (2017). “Toxicity of cadmium and its health risks from leafy vegetable consumption,” Food & Function 8, 1373-1401. DOI: 10.1039/c6fo01580h
Jin, J., Kang, M., Sun, K., Pan, Z., Wu, F., and Xing, B. (2016). “Properties of biochar-amended soils and their sorption of imidacloprid, isoproturon, and atrazine,” Science of The Total Environment 550, 504-513. DOI: 10.1016/j.scitotenv.2016.01.117
Karimi, F., Rahimi, G., Kolahchi, Z., and Nezhad, A. K. J. (2020). “Using industrial sewage sludge-derived biochar to immobilize selected heavy metals in a contaminated calcareous soil,” Waste and Biomass Valorization 11, 2825-2836. DOI: 10.1007/s12649-018-00563-z
Laspina, N. V., Groppa, M. D., Tomaro, M. L., and Benavides, M. P. (2005). “Nitric oxide protects sunflower leaves against Cd-induced oxidative stress,” Plant Science 169(2), 323-330. DOI: 10.1016/j.plantsci.2005.02.007
Lee, Y.-E., Jo, J.-H., Kim, I.-T., and Yoo, Y.-S. (2017). “Chemical characteristics and NaCl component behavior of biochar derived from the salty food waste by water flushing,” Energies 10(10). DOI: 10.3390/en10101555
Li, A. Y., Deng, H., Jiang, Y. H., Ye, C. H., Yu, B. G., Zhou, X. L., and Ma, A. Y. (2020a). “Superefficient removal of heavy metals from wastewater by mg-loaded biochars: Adsorption characteristics and removal mechanisms,” Langmuir: the ACS Journal of Surfaces and Colloids 36, 9160-9174. DOI: 10.1021/acs.langmuir.0c01454
Li, W., Liu, B., Wang, Z., Wang, K., Lan, Y., and Zhou, L. (2020b). “Efficient activation of peroxydisulfate (PDS) by rice straw biochar modified by copper oxide (RSBC-CuO) for the degradation of phenacetin (PNT),” Chemical Engineering Journal 395. DOI: 10.1016/j.cej.2020.125094
Li, X., Shen, Q., Zhang, D., Mei, X., Ran, W., Xu, Y., and Yu, G. (2013). “Functional groups determine biochar properties (pH and EC) as studied by two-dimensional 13C NMR correlation spectroscopy,” PLoS One 8(6). DOI: 10.1371/journal.pone.0065949
Lu, K. P., Yang, X., Shen, J. J., Robinson, B., Huang, H. G., Liu, D., Bolan, N., Pei, J. C., and Wang, H. L. (2014). “Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola,” Agriculture, Ecosystems and Environment 191, 124-132. DOI: 10.1016/j.agee.2014.04.010
Micháleková-Richveisová, B., Frišták, V., Pipíška, M., Ďuriška, L., Moreno-Jimenez, E., and Soja, G. (2017). “Iron-impregnated biochars as effective phosphate sorption materials,” Environmental Science and Pollution Research 24, 463-475. DOI: 10.1007/s11356-016-7820-9
Muratova, A., Golubev, S., Wittenmayer, L., Dmitrieva, T., Bondarenkova, A., Hirche, F., Merbach, W., and Turkovskaya, O. (2009). “Effect of the polycyclic aromatic hydrocarbon phenanthrene on root exudation of Sorghum bicolor (L.) Moench,” Environmental and Experimental Botany 66(3), 514-521. DOI: 10.1016/j.envexpbot.2009.03.001
Ngambia, A., Ifthikar, J., Shahib, L. L., Jawad, A., Shahzad, A., Zhao, M. M., Wang, J., Chen, Z. L., and Chen, Z. Q. (2019). “Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite,” Science of the Total Environment 691, 306-321. DOI: 10.1016/j.scitotenv.2019.07.003
Parra, A., Zornoza, R., Conesa, E., Faz, A., and Gómez-López, M. D. (2017). “Nutritional status and its interaction with soil properties and trace elements in six Mediterranean shrub species grown in reclaimed pyritic tailings,” Ecological Engineering 109, 25-34. DOI: 10.1016/j.ecoleng.2017.08.027
Qayyum, M. F., Rehman, R. A., Liaqat, S., Ikram, M., Ali, S., Rizwan, M., Zia ur Rehman, M., Zafar-ul-Hye, M., and Hussain, Q. (2019). “Cadmium immobilization in the soil and accumulation by spinach (Spinacia oleracea) depend on biochar types under controlled and field conditions,” Arabian Journal of Geosciences 12. DOI: 10.1007/s12517-019-4681-9
Shan, R., Chen, Y., Meng, L., Li, H., Zhao, Z., Gao, M., and Sun X. (2020). “Rapid prediction of atrazine sorption in soil using visible near-infrared spectroscopy,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 224. DOI: 10.1016/j.saa.2019.117455
Shang, M.-r., Liu, Y.-g., Liu, S.-b., Zeng, G.-m., Tan, X.-f., Jiang, L.-h., Huang, X.-x., Ding, Y., Guo, Y.-m., and Wang, S.-f. (2016). “A novel graphene oxide coated biochar composite: Synthesis, characterization and application for Cr(VI) removal,” RSC Advances 6(88), 85202-85212. DOI: 10.1039/C6RA07151A
Sidhu, G. P. S., Bali, A. S., Singh, H. P., Batich, D. R., Kohli, R. K. (2020). “Insights into the tolerance and phytoremediation potential of Coronopus didymus L.(Sm) grown under zinc stress,” Chemosphere 244, 125350. DOI: 10.1016/j.chemosphere.2019.125350
Sun, X., Shan, R., Li, X., Pan, J., Liu, X., Deng, R., and Song, J. (2017). “Characterization of 60 types of Chinese biomass waste and resultant biochars in terms of their candidacy for soil application,” Global Change Biology Bioenergy 9(9), 1423-1435. DOI: 10.1111/gcbb.12435
Tao, Q., Li, B., Li, Q., Han, X., Jiang, Y., Jupa, R., Wang, C., and Li, T. (2019). “Simultaneous remediation of sediments contaminated with sulfamethoxazole and cadmium using magnesium-modified biochar derived from Thalia dealbata,” Science of the Total Environment 659, 1448-1456. DOI: 10.1016/j.scitotenv.2018.12.361
Tian, S., Xie, R., Wang, H., Hu, Y., Ge, J., Liao, X., Gao, X., Brown, P., Lin, X., and Lu, L. (2016). “Calcium deficiency triggers phloem remobilization of cadmium in a hyperaccumulating species,” Plant Physiology 172, 2300-2313. DOI: 10.1104/pp.16.01348
Van Zwieten, L., Kimber, S., Morris, S., Chan, K. Y., Downie, A., Rust, J., Joseph, S., and Cowie, A. (2010). “Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility,” Plant and Soil 327, 235-246. DOI: 10.1007/s11104-009-0050-x
Wang, L., Cui, X., Cheng, H., Chen, F., Wang, J., Zhao, X., Lin, C., and Pu, X. (2015). “A review of soil cadmium contamination in China including a health risk assessment,” Environmental Science and Pollution Research 22, 16441-16452. DOI: 10.1007/s11356-015-5273-1
Wang, Y., Zhang, K., Lu, L., Xiao, X., and Chen, B. (2020). “Novel insights into effects of silicon-rich biochar (Sichar) amendment on cadmium uptake, translocation and accumulation in rice plants,” Environmental Pollution 265. DOI: 10.1016/j.envpol.2020.114772
Wu, J., Li, Z., Wang, L., Liu, X., Tang, C., and Xu, J. (2020). “A novel calcium-based magnetic biochar reduces the accumulation of As in grains of rice (Oryza sativa L.) in As-contaminated paddy soils,” Journal of Hazardous Materials 394. DOI: 10.1016/j.jhazmat.2020.122507
Yang, X., Liu, J., McGrouther, K., Huang, H., Lu, K., Guo, X., He, L., Lin, X., Che, L., Ye, Z., et al. (2016). “Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil,” Environmental Science and Pollution Research 23, 974-984. DOI: 10.1007/s11356-015-4233-0
Younis, U., Malik, S. A., Rizwan, M., Qayyum, M. F., Ok, Y. S., Shah, M. H. R., Rehman, R. A., and Ahmad, N. (2016). “Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes,” Environmental Science and Pollution Research 23, 21385-21394. DOI: 10.1007/s11356-016-7344-3
Yuan, J., and Xu, R. K. (2010). “Effects of rice-hull-based biochar regulating acidity of red soil and yellow brown soil,” Journal of Ecology and Rural Environment 26(5), 472-476. DOI: 10.1080/00949651003724790
Zhang, P., Sun, H., Yu, L., and Sun, T. (2013). “Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars,” Journal of Hazardous Materials 244-245, 217-224. DOI: 10.1016/j.jhazmat.2012.11.046
Zhang, S. Z., Quan, L. T., Zhu, Y. P., Yan, J., He, X. M., Zhang, J., Xu, X. M., Hu, Z. B., Hu, F., Chen, Y. H., Shen, Z. G., and Xia, Y. (2020). “Differential effects of three amendments on the immobilisation of cadmium and lead for Triticum aestivum grown on polluted soil,” Environmental Science and Pollution Research DOI: 10.1007/s11356-020-10079-6
Zia ur Rehman, M., Khalid, H., Akmal, F., Ali, S., Rizwan, M., Qayyum, M. F., Iqbal, M, Khalid, M. U., and Azhar, M. (2017). “Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field,” Environmental Pollution 227, 560-568. DOI: 10.1016/j.envpol.2017.05.003
Article submitted: July 16, 2020; Peer review completed: Aug. 29, 2020; Revised version received and accepted: Sept. 2, 2020; Published: September 4, 2020.
DOI: 10.15376/biores.15.4.8008-8025
