Abstract
Kenaf (Hibiscus cannabinus L.) is a relatively new industrial crop which has been identified as an alternative source of fiber in the papermaking industry in Malaysia. In this study, experimental unbleached kenaf kraft paper samples were used as a substrate to produce water-resistant paper by employing a special coating. In the preparation of the coating formulation, commercially precipitated calcium carbonate (PCC) was used as the filler, in addition to 0 to 0.32 w/w g of hydrophobic stearic acid (SA). Polymer latex (PL) was added at 0.4 to 0.16 w/w g into the coating compound to control the surface roughness of the samples. The paper morphology was examined by employing a scanning electron microscope (SEM). Hydrophobic kenaf kraft paper prepared in this study had water contact angle (θ) greater than 90°. Hydrophobic paper made with formulation PL4c resulted in the highest value contact angle of 147°. The process of surface coating by dipping increased the water contact angle and this treated paper achieved a high hydrophobic level. For mechanical properties, the coated kenaf paper showed decreasing tensile strength as the addition of stearic acid increased.
Download PDF
Full Article
Effects of Physical Treatments on the Hydrophobicity of Kenaf Whole Stem Paper Surface Using Stearic Acid
Hazwani Husna Abdullah,a Ainun Zuriyati Mohamed Asa’ari,a,* Nurul Izzati Mohd Zawawi,a Luqman Chuah Abdullah,a and Sarani Zakaria b
Kenaf (Hibiscus cannabinus L.) is a relatively new industrial crop which has been identified as an alternative source of fiber in the papermaking industry in Malaysia. In this study, experimental unbleached kenaf kraft paper samples were used as a substrate to produce water-resistant paper by employing a special coating. In the preparation of the coating formulation, commercially precipitated calcium carbonate (PCC) was used as the filler, in addition to 0 to 0.32 w/w g of hydrophobic stearic acid (SA). Polymer latex (PL) was added at 0.4 to 0.16 w/w g into the coating compound to control the surface roughness of the samples. The paper morphology was examined by employing a scanning electron microscope (SEM). Hydrophobic kenaf kraft paper prepared in this study had water contact angle (θ) greater than 90°. Hydrophobic paper made with formulation PL4c resulted in the highest value contact angle of 147°. The process of surface coating by dipping increased the water contact angle and this treated paper achieved a high hydrophobic level. For mechanical properties, the coated kenaf paper showed decreasing tensile strength as the addition of stearic acid increased.
Keywords: Coating; Kenaf kraft paper; Water contact angle; Hydrophobic; CaCO3
Contact information: a: Laboratory of Biopolymer and Derivatives, Institute of Tropical Forestry and Forest Products (INTROP), Universiti Putra Malaysia, 43400 UPM Serdang, Selangor; b: School of Applied Physics, Faculty of Science & Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor; *Corresponding author: ainun.introp@gmail.com
INTRODUCTION
Kenaf has received much attention as an alternative source of fiber from a pool of different agricultural materials. Since there was a shortage of fiber from the natural forest and increasing world demand for paper-based product, kenaf has becoming a potential fiber source. In addition, kenaf has comparable properties among the other established non-wood fibers such as bagasse, wheat, rice straw, and bamboo (Ashori et al. 2006).
The stem of kenaf is characterized by a thin outer layer of bast and a wood-like inner core. The stem has almost 35-40% of its material composed of slender and long fibers similar in length to those of southern pine and other softwood fibers. The woody core of the stem consists of 60-65% of short fibers, similar to most hardwood fibers (Shmulsky and Jones 2011). In a fiber morphology study on nine varieties of kenaf planted at the Universiti Putra Malaysia, the longest fiber length of kenaf bast was found to be approximately 2.96 mm (TK variety), while the shortest bast fiber length was 2.20 mm (V19 variety) (H`ng et al. 2009). For the core, two varieties of kenaf, V36 and NS, yielded the longest value of 0.89 mm and the shortest value of 0.69 mm, respectively (H`ng et al. 2009).
Kenaf has excellent physical and mechanical properties, having long bast fibers and low lignin content. All these properties have promoted kenaf as a new fiber source for papermaking in the pulp and paper industry (Kaldor 1992). Magnetic paper was successfully prepared from kenaf pulp using several methods, such as lumen loading, in situ synthesis, and chemical co-precipitation (Zakaria et al. 2005; Chia et al. 2008). In the same study, the authors found that increasing the loading of nano-sized ferrite particle increased the magnetic properties of the paper. Research studies on the application of kenaf fiber into various types of products have been carried out many years ago (Kalaycroglu and Nemli 2006; Nishino et al. 2003; Gita et al.2002). At present, some studies are still ongoing, while some of the products have been successfully commercialized. In Indonesia, Toyota has produced door trim from kenaf and polypropylene, while Mazda has used a bioplastic material from kenaf fiber to manufacture car interiors (Kalia et al. 2011). Paper products from kenaf fiber are used for specialty papers, tea bags, newsprint, non-sticking paper, bank paper, and other specialty papers (Asunción 2003).
One of the most important properties of paper that have received much attention by the industries and researchers nowadays is the resistance of the product to moisture or water. Paper exhibits hydrophilic properties. It absorbs water from the environment either when exposed to high humidity or when in contact with food with high moisture content. Absorption of water by the paper decreases its physical and mechanical properties (Rhim 2004). Generally, material surface is classified as hydrophilic if the contact angle is less than 90°. When the contact angle higher than 90°, the surface is called hydrophobic, and when the contact angle is greater than 150°, the surface is called superhydrophobic (Kumar 2010).
Physical surface treatment, such as coatings is one of the methods used to produce paper with better resistance towards moisture or water penetration. Various studies have been conducted by researchers using various materials in coating formulating of paper (Wang et al. 2011; Butkinaree et al. 2008; Rhim et al. 2006). Butkinaree et al. (2008) gelatinized starch matrix with 1-3% stearic acid at 75 oC to produce biodegradable paperboard for food packaging. Rhim et al. (2006) studied the effect of biopolymers, such as alginate and soy protein, on the mechanical and water resistance properties of corrugated paperboard. Wang et al. (2011) improved the water repellency of the base paper by using two silane-based sol-gel coatings. A thin coating layer formed on the surface of the paper clearly changed the surface properties and improved its hydrophobicity. Precipitated calcium carbonate (PCC) incorporated with fatty acid is an example of a well-known coating formulation, which provides hydrophobic properties to the base material (Rothon 2003).
Wang et al. (2010), developed hydrophobic calcium carbonate particles by treating them with dodecanoic acid. The authors found that 40 °C and 4:1000 molar ratio of dodecanoic acid to Ca2+ was the optimum condition to synthesize calcite nano-particles via the carbonation method. The optimum condition yielded calcium carbonate with a higher water contact angle. Hu and Deng (2010) modified PCC using oleic acid. Increasing the oleic acid content in the PCC gave the maximum value for the advancing water contact angle. The optimum content of oleic acid has been found to be 2.1 wt5%, resulting in a 164° advancing water contact angle. In the other study conducted by Jiang et al. (2006), they found that thermoplastic polyurethane (TPU) reinforced with nano-particle calcium carbonate (NPCC) coated with stearic acid demonstrated an increase of tensile strength with the addition of NPCC, but the poor dispersion of NPCC may limit the mechanical improvement of the TPU-based composite.
Several studies have been conducted regarding the development of paper-based products from kenaf fiber, but most of them only focused on the elementary process in papermaking, such as pulping, bleaching, and beating (Ashori et al. 2008; Villar et al. 2008; Mehta et al. 2003). However, there has been little work on the application of coating to kenaf paper. Studies have been carried out on the coating of filter paper, linerboard, and paperboard made from hardwood fiber (Ford et al. 2009; Butkinaree et al. 2008; Kiuberis et al. 2005; Rhim et al. 2004). The main purpose of this present study is to study the effect of physical treatment on the surface hydrophobicity of whole stem kenaf paper. The physical treatment was conducted by applying a coating mixture onto the paper’s surface. The tensile strength of the experimentally manufactured coated paper was also investigated to better understand the impact of the product on paper strength.
EXPERIMENTAL
This study has taken into consideration the method reported earlier by Hu et al. (2009). However, in line with the objectives of the research, several modifications were made, which included the type of base paper, the parameters of stearic acid (SA), polymer latex (PL) used, the drying method, and the duration of paper drying.
Materials
Kenaf variety V36 stalks were collected from the plantation site of the National Kenaf and Tobacco Board (NKTB), Bachok, Kelantan, Peninsular Malaysia. Precipitated calcium carbonate (PCC), namely Albacar® HO, was supplied by Solvay Chemical International, France. The PCC was used as the inorganic filler component in the coating formulation. Acronal NX 4787 X, polymer latex emulsion, was supplied by BASF Indonesia. The polymer was added into the coating formulation as binder. Stearic acid and potassium stearate were purchased from Peter Greven Asia Sdn. Bhd.
Methods
Preparation of base paper
Base paper for coating purpose was prepared using kenaf kraft pulp. Prior to cooking, the green kenaf stalks were chipped to 2-3 cm in length and about 0.5 cm in thickness using Mini-Chipper machine. The kenaf chips were dried to 10-15% moisture content in order to avoid any fungal attack. The cooking was carried out in a cooking digester using kraft pulping method with 17% alkali active and 25% sulphidity. About 300 g (oven-dried) whole stem kenaf was placed into the digester vessel. The ratio of kenaf chips to cooking liquor was 1:7. The cooking temperature was 170 °C for 2 h, in addition to a 1 h heat-up to cooking temperature. Once cooked, the unbleached kenaf pulp was washed, screened, and centrifuged. The unbleached pulp was then beaten at 500 revolutions using PFI Mill. The unbleached handsheets were prepared in accordance to TAPPI Standard method T 205. These papers were conditioned at temperature of 23±1 °C and relative humidity of 50±2% for 24 h in accordance to TAPPI Standard method T402 sp-08.
Preparation of Coating Formulation
In preparing coating formulations, 40 g of PCC and 90 mL of distilled water were added into every mixture. The percentages of SA and PL were measured based on the dry weight of the PCC. In this study, an amount of 0, 0.4, and 0.8% of SA was used. The suspension containing SA and PCC was stirred for 30 min at 75 °C. After cooling down to room temperature, the PL was added into the suspension. This suspension was stirred for 30 min with agitation. The percentage levels of PL chosen were 1, 2, 3, and 4%.
Preparation of Coated Papers
The coating suspension was applied onto the surface of the base kenaf kraft paper using a roller coater. The coated paper was air-dried for 60 min before immersing into a container of potassium stearate (PS) suspension of 0.0015 mol/L for 3 min. The immersion process in the PS suspension was called treatment stage. The treatment was expected to improve the hydrophobicity of the coated paper. After treating the paper with PS, the coated paper was rinsed with distilled water at 75 °C. This stage was carried out mainly to remove excess stearate salt presence at the paper surface. The coated paper was air-dried for another 60 min prior to paper testing.
Characterization of Coated Paper
The coated paper was characterized by its physical, mechanical, and morphological properties. The physical properties also encompassed water contact angle (WCA) measurement and paper roughness. The WCA was measured using FECA Contact-Angle Meter, while paper smoothness was carried out in accordance with TAPPI Standard Method T 535 um-91. The mechanical property included tensile strength, which was measured using a Buchel-Van Der Korput Horizontol tensile tester. The test was in accordance with TAPPI standard method T 949 om-01. The paper morphology focused on the distribution of PCC particles on the paper surface. A SEM Carl Zeiss Model 1450VP with variable pressure was used for this purpose.
RESULTS AND DISCUSSION
Effect of Dipping Treatment on the Water Contact Angle Value
The untreated samples exhibited hydrophobic surfaces with WCA > 90° as shown in Table 1. However, the value increased after treating the coated papers by dipping them into PS solution. Dipping is a type of physical treatment approach used for coating (Hu et al. 2009). The dipping treatment allowed the stearate salt to adsorb onto the paper fibres and also onto the polymer thin layer. These adsorptions resulted in a paper surface that was more hydrophobic (Hu et al. 2009). Thus, it was proven that dipping is an important treatment in enhancing and increasing paper hydrophobicity. Potassium stearate is an organic salt made from a long-chain of fatty acid. As shown in Fig. 1, PS consists of two distinct regions; carboxyl head, which is hydrophilic, and hydrocarbon tail, which is hydrophobic (Yves 2007). The hydrophilic head of the stearate salt attaches to the surface of kenaf fibres and the PCC particles, while leaving the hydrophobic tail oriented towards the air. Therefore, the response has improved the hydrophobic behavior of the coated paper. In other words, by conducting the dipping treatment, the stearate salt adsorbed onto the kenaf fibres and covered the spaces between the PCC particles. Excessive PS beyond the saturated adsorption could also decrease the WCA value. As shown in Table 1, the highest WCA value is achieved by 4c either before or after dipping treatment followed by 2c; however, the coating layer of these samples were observed to be easily peeled from the base paper. This might not be a good result for manufacturing purposes. Due to the good hydrophobic properties, the whole paper samples were treated using the dipping method.
Table 1. Effect of Dipping Treatment on the Contact Angle of Coated Paper
Fig. 1. Chemical structure of potassium stearate (PS) consists of carboxyl (hydrophilic head) and hydrocarbon (hydrophobic tail)
Effect of Stearic Acid Addition on the Hydrophobicity of Kenaf-Coated Paper
In this section, the samples are grouped based upon the different amount of SA. Hence, the effect on the addition of stearic acid (SA) towards the paper’s hydrophobicity was studied. All samples prepared in the experiment are categorized as hydrophobic paper due to its >90° WCA value, as shown in Fig. 2. The same increment trend can be seen in the four series of samples. The degree of WCA slightly increased with the increasing amount of SA which can be seen in 2a, 2b, and 2c. The same trends of WCA results are also found for the series of samples 4, 6, and 8. This result proves that SA offers significant benefits in the processing and properties of coated paper such as reducing high surface energy and decreasing agglomeration of particles like PCC (Osman et al. 2004).
After increasing a certain dosage of SA, the efficiency of WCA is observed stagnant as in samples 6c and 8c. It is believed that the formulation contains 0.4% SA at 3 and 4% of PL and is sufficient enough to achieve such water repellent property.
The highest WCA is 147°, performed by sample 4c in which the formulation contained 40 g PCC, 0.8% SA, and 2% PL. The image of water droplet on the paper surface is shown in Fig. 3. The droplet is observed having minimum contact with the paper surface, thus providing the least wetting effect. It tends to form a round shape rather than spread out over the area of the surface. As reported by Mittal (2009), the water droplet exhibited more internal cohesion than adhesion to the surface, and thus easily rolled off the hydrophobic paper surface. It tends to limit the contact area between the droplet and the surface of base paper.
Fig. 2. Effect of SA on the measurement of WCA for the coated and treated paper
Fig. 3. Image of a water droplet that formed WCA 147° on the coated and treated paper surface
Grisham and Garrett (2005) depicted the chemical structure of SA, which encompasses a hydrophilic head and hydrophobic long tail, as shown in Fig. 4. In the coating suspension, the SA is expected to absorb onto the PCC surfaces, as illustrated in Fig. 5. Hu et al. (2009) elucidated the hydrophilic carboxyl group, which refers to the head of SA, will be bonded to the surface of the PCC to form monolayers of stearate salt molecules. Meanwhile, the hydrophobic hydrocarbon group, which refers to the long tail of SA, will be oriented perpendicular to the surface (Hu et al. 2009). Shi et al. (2010, 2011) reported that the surface of PCC was positively charged, and it can be attracted to the fatty acid which is negatively charged. The attachment of SA to PCC can be further described from a study performed by Osman et al. (2004). The authors modified the calcium carbonate particles by using SA in the interaction between the filler with polydimethylsiloxane (PDMS). An ultra-thin layer of hydrophobic alkyl chains is detected which is formed and chemically bonded to the filler surface.
Fig. 4. Chemical structure of stearic acid (SA) which consists of a hydrophilic head and hydrophobic tail (adapted from Grisham and Garrett 2005)
Fig. 5. A schematic diagram of hydrophobic principle as explained by Hu et al. (2009)
Effect of Polymers Latex on Coated Paper
This section reports on the hydrophobicity performance of paper towards the amount of PL added. Therefore, the samples are grouped based on the percentage of SA addition in the coating formulation. In order to further understand the efficiencies of the PLs, these samples are compared among 0, 0.4, and 0.8% of SA addition. The application of coating formulation onto all samples composed of PCC, SA, and PL apparently showed good resistance to water (Fig. 6). This statement is based on the result that the WCA was greater than 90°. However, the WCA value slightly decreased after the addition of PL at 4%. This can be seen from samples 8a, 8b, and 8c. Meanwhile, six out of the twelve samples had excellent WCA values (>140°), labeled as 2c, 4a, 4b, 4c, 6b, and 6c. These samples showed outstanding hydrophobicity properties. In fact, sample 4c closely reached superhydrophobic value (i.e. ≥150°). This might be due to the high surface roughness, which enhanced the coating process (Table 2). Higher surface roughness can be obtained from the presence of PCC, which spread onto the paper surfaces with the aid of PL. The highest contact angle is achieved by sample 4c, while 6a showed the lowest angle. As for sample 4c, the filler particle adhered together with the presence of polymer latex. The addition of PL on samples 6c and 8c possess better adhesion between the filler particles. However, PL has created smoother surface on the coated paper which resulted in lower contact angle. The presence of a minimum amount of PL produced a rougher surface; this result is comparable to that reported by Alava and Niskanen (2006), in which the authors found that the presence of latex in the coating formulation kept the coating particles together.
Samples with 0% SA, which are namely as 2a, 4a, 6a, and 8a, passed the standard values requirement for hydrophobic paper. This means that the presence of PL is the prime factor in obtaining hydrophobic properties for the papers. The PCC is known as hydrophilic filler. However, the combination of PCC with PL furnishes hydrophobic behavior to the coated paper. The contact angle of the coated paper might rise to >90° even without or with low percentages of SA in the coating formulation. Holix (2006) reported that the incorporation of unsaturated carboxylic acid during the carboxylation process of the latex make these polymers highly hydrophobic compared to other coating components. Zhu et al. (2008) found that polystyrene-butyl acrylate (PSBA) showed hydrophobic properties on nanocomposites, which was probably due to the formation of hydrophobic cross-linking on the PSBA structure. Wang et al.(2007) also reported the changes in wettability of a colloidal crystal film from hydrophilic to super-hydrophobic by introducing different ratios of styrene-n-butyl acrylate (nBA/ST) at different temperatures.
Fig. 6. Effect of PL on the measurement of WCA for the coated and treated paper
Effect of Stearic Acid and Polymer Latex on Paper Roughness and Tensile Strength
Roughness test was conducted in order to measure surface roughness of coated paper. In other words, the regularities acquired from paper surface were determined. Higher value of roughness indicates a rougher paper surface. Zachary and Bushon (2006) reported that the larger solid-liquid interface area and net energy of rough surface are responsible for the increase of contact angle for the hydrophobic surface. Wenzel (1949) revealed that a base substrate obtaining WCA, < 90 ° may be produced from a smoother surface. Due to the smoother surface, the water droplet is reluctant to form a spherical shape. It then tends to wet the paper surface. Meanwhile, the WCA < 90 ° may refer to a rougher surface. This statement is similar to Rios et al. (2007) who also showed that a higher degree of roughness gave a higher water contact angle. In the case of producing water-resistant paper, the paper surface roughness is proven as one of the dominant factors that contribute to hydrophobicity.
The decreasing surface roughness may be due to the action of PL in inducing more aggregates of PCC to attach on the fibres and also to fill in the voids between the fibres. The enormous amount of PCC that attached on and between the fibres may result in lowering the paper surface roughness. This phenomenon can be seen from the roughness value of 6c and 8c in Table 2.
Table 2. Roughness and Tensile Index of Coated and Treated Paper
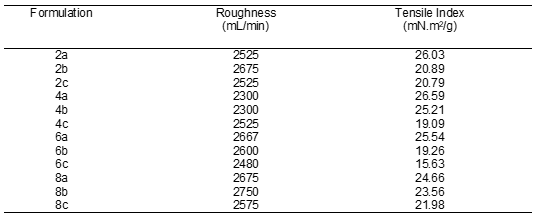
Figure 7 illustrates the amplified micrograph images of the distribution of PCC particles on the kenaf base paper. Figure 7 (a-d) represents samples 2c, 4c, 6c, and 8c. The parameter that differs among these samples is the content of PL added during stock preparation. The filler particles in 2c and 4c did not uniformly cover the paper surface as compared to samples 6c and 8c. The nonuniformity of PCC distribution is an advantage to the water resistance properties of 2c and 4c. Therefore, the paper roughness was higher than sample 6c’s as stated in Table 2. According to Liu et al (2006), in order to attain higher hydrophobicity of the paper surface, researchers usually create a rougher surface followed by modification with material of lower surface energy.
Fig. 7. The distribution of PCC particles on the coated and treated paper surfaces at 1, 2, 3, and 4% PL and 0.8% SA
Tensile strength is an important mechanical property of paper. Referring to Table 2, the increasing content of SA led to a decrease in tensile strength. In Fig. 8, the micrograph observed via SEM clearly exhibits two distinct layers of coating and the kenaf base paper. The reduction in tensile strength for the coated and treated papers is probably due to the dimensional changes of the kenaf base paper and the coating formulation layers. During the coating process, the presence of moisture or water in the coating formulation was attracted to the kenaf base paper. Thus, this resulted in the water absorption which has disrupted the inter-fiber bonds of the base paper. Krotcha and Han (2001) found that the penetration of water or solvent in paperboard decreased the tensile strength due to the swelling of the fibres. They also found that the decrease in the interaction force between the cellulose fibres of the coated paper resulted from the interference of the coating material, thereby reducing the tensile strength of the sample. Husband et al. (2010) reported that the presence of moisture could disrupt the interface between the binder and pigment. In their study, they used kaolin and PCC as the chosen pigments which are dispersed with sodium poly(acrylate). However, the weakening condition is temporary due to the wetness and is related to the rate of absorption of water by the coating materials. Osman et al. (2004) observed that excessive amounts of stearic acid in LDPE-CaCO3 composite reduce its tensile strength. The reduction might be due to the presence of free stearic acid molecule in the polymer matrix as a heterogeneous phase.
Fig. 8. Cross section of coated and treated paper with addition of SA and PL
CONCLUSIONS
Based on the results from this study, the coated and treated kenaf papers with a simple formulation and modified technique was successfully produced. Dipping the coated paper into potassium stearate (PS) solution resulted in paper with a higher resistance to water penetration. The coated and treated paper showed the highest WCA value (147°), which was almost superhydrophobic. Therefore, the optimum parameter in order to obtain such paper was by adding 0.8% SA and 2% PL.
ACKNOWLEDGEMENTS
This study was financed by the Economic Planning Unit (EPU), Prime Minister’s Department Malaysia. The Institute of Tropical Forestry and Forest Products (INTROP) and the Faculty of Forestry, Universiti Putra Malaysia are gratefully acknowledged for providing the facilities.
REFERENCES CITED
Alava, M., and Niskanen, K. (2006). “The physics of paper,” Rep. Prog. Phys. 69(3), 669-772.
Ashori, A., Jalaluddin, H., Raverty, W. D., and Mohd, N. M. Y. (2006). “Chemical and morphological characteristic of Malaysian cultivated kenaf (Hibiscus cannabinus) Fiber,” Polym. Plast. Technol. Eng. 45(1), 131-134.
Ashori, A., Raverty, W. D., Vanderhoek, N., and Word, J. V. (2008). “Surface topography of kenaf (Hibiscus cannabinus) sized paper,” Bioresource Technol. 99(2), 404-410.
Asunción, J. (2003). The Complete Book of Papermaking, Sterling Publishing Co., Inc. (Lark Books).
Butkinaree, S., Jinkran, T., and Yoksan, R. (2008). “Effect of biodegradable coating on barrier properties of paperboard food packaging,” J. Metals Mater. Min. 18(2), 219-222.
Chia, C. H., Sarani, Z., Ngiuyen, K. L. and Abdullah, M. (2008). “Utilisation of unbleached kenaf fibers for the preparation of magnetic paper,” Ind. Crop Prod. 28(3), 333-339.
Ford, E., Popil, R. E., and Kumar, S. (2009). “Breathable water-resistant linerboard coatings by electrospraying application,” BioRes. 4(2), 714-729.
Gita, N. R., Sellers, T., Tao., W., and Crook, L. G. (2002). “Kenaf nonwovens as substrates for laminations,” Ind. Crop Prod. 23(1), 1-8.
Grisham, C. M., and Garrett, R. (2005). Biochemistry, Third Ed., Thomson Brooke/Cole, Belmont, CA.
H’ng, P. S., Khor, B. N., Tadashi, N., Aini, A. S. N., and Paridah, M. T. (2009). “Anatomical structure and fiber morphology of new kenaf varieties,” Asian Journal of Scientific Research2(3), 161-166.
Holix, H. (2006). Handbook of Paper and Board, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
Hu, Z., and Deng, Y. (2010). “Superhydrophobic surface fabricated from fatty acid –modified precipitated calcium carbonate,” Ind. Eng. Chem. Res. 49(12), 5625-5630.
Hu, Z., Zen, X., Gong, J., and Deng, Y. (2009). “Water resistance improvement of paper by superhydrophobic modification with microsized CaCO3 and fatty acid coating,” Colloids Surf., A 351(1-3), 65-70.
Jiang, L., Lam, Y. C., Tam, K. C., Li, D. T., and Zhang, J. (2006). “The influence of fatty acid coating on the rheological and mechanical properties of thermoplastic polyurethane (TPU)/nano-sized precipitated calcium carbonate (NPCC) composites,” Polym. Bull. 57(4), 575-586.
Kalaycroglu, H., and Nemli, G. (2006). “Producing composite particleboard from kenaf (Hibiscus cannabinus L.) stalks,” Ind. Crop Prod. 24(2), 177-180.
Kaldor, A. F. (1992). “Kenaf, an alternative fiber for the pulp and paper industries in developing and developed countries,” Tappi J. 75(10), 141-145.
Kalia, S., Kaita, S. B., and Kaur, I. (2011). Cellulose Fibers: Bio and Nano-polymer composites, Springer-Verlag Berlin, Heidelberg, Germany.
Kiuberis, J., Tautkus, S., Kazlauskas, R., Pakutinskiene, I., and Karieva, A. (2005). “Protective coating for paper: New development and analytical characterization,” J. Cult. Herit. 6(3), 245-251.
Kumar, C. (2010). Nanostructured Thin Film and Surfaces. Nanomaterials for the Life Sciences, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
Mehta, D. C., Roy, D. N., and Whiting, P. (2003). “Bleaching study of kenaf mechanical pulps,” TAPPI J. 2(8), 29-32.
Nishino, T., Hiro, K., Kotera, M., Nakamae, K., and Inagaki, H. (2003). “Kenaf reinforced biodegradable composite,” Compos. Sci. Technol. 63(9), 1281-1286.
Osman, M. A., Atallah, A., and Suter, U. W. (2004). “Influence of excessive filler coating on the tensile properties of LDPE-calcium carbonate composite,” Polymer 45(4), 1173-1183.
Rhim, J. W. (2004). “Physical and chemical properties of water resistant sodium-alginate films,” Lebensm. Wiss. Technol. 37(3), 232-330.
Rhim, J. W., Lee, J. H., and Hong, S. I. (2006). “Water resistance and mechanical properties of biopolymer (alginate and soy protein) coated paperboards,” Lebensm. Wiss. Technol. 39(7), 806-813.
Rios, P. F., Dodiuk, H., Kenig, S., McCarthy, S., and Dotan, A. (2007). “Transparent ultra-hydrophobic surfaces,” J. Adhesion Sci. Technol. 21(5-6), 399-408.
Rothon, R. N. (2003). Particulate-Filled Polymer Composites, 2nd Edition, Smithers Rapra Publishing, Shawbury, United Kingdom.
Shi, X., Bertóti, I., Pukánszky, B., Rosa, R., and Lazzeri, A. (2011). “Structure and surface coverage of water-based stearate coating on calcium carbonate nanoparticles,” J. Colloid Interface Sci. 362(1), 67-73.
Shi, X., Rosa, R., and Lazzeri, A. (2010). “On the coating of precipitated calcium carbonate with stearic acid in aqueous medium,” Langmuir 26(11), 8474-8482.
Shmulsky, R., and Jones, P. D. (2011). Forest Product and Wood Sources; An Introduction, Sixth Ed., Wiley-Blackwell, United Kingdom.
Villar, J. C., Revilla, E., Gomez, N., Carbajo, J. M., and Simón, J. L. (2008). “Improving the use of kenaf for kraft pulping by using mixture of bast and core fibers,” Ind. Crops Prod.,29(2-3), 301-307.
Wang, C., Piao, C., Zhai, X., Hickman, F. N., and Li, J. (2010). “Synthesis and characterization of hydrophobic calcium carbonate particles via a dodecanoic acid inducing process,” Powder Technol. 198(1), 131-134.
Wang, J., Wen, Y., Hu, J., Song, Y., and Jiang, L. (2007). “Fine control of the wettability transition temperature of colloidal crystal film: From superhydrophilic to superhydrophobic,” Adv. Funct. Mater. 17(2), 219-225.
Wang, S., Jämsä, S., Mahlberg, R., Nikkola, J., Mannila, J., Ritschkoff, A. C., and Peltonen, J. (2011). “Surface characteristic and water absorption of sol-gel coated impregnated paper pressed onto plywood,” Adv. Mater. Res. Vols. 291-294(2011), 159-162.
Yves, M. (2007). The Hydrogen Bond and the Water Molecule: The Physics and Chemistry of Water, Aqueous and Bio-media, Elsevier, Oxford, United Kingdom.
Zakaria, S., Ong, B. H., Ahmad, S. H., Abdullah, M., and Yamauchi, T. (2005). “Preparation of lumen-loaded kenaf pulp with magnetite (Fe3O4),” Mater. Chem. Phys. 89(2-3), 216-220.
Zhu, A., Cai, A., Yu, Z., and Zhou, W. (2008). “Film characterization of poly(styrene-butylacrylate-acrylic acid)-silica nanocomposite,” J. Colloid Interface Sci 322(1), 51-58.
Article submitted: March 7, 2013; Peer review completed: May 27, 2013; Revised version accepted: June 11, 2013; Published: June 13, 2013.
