Abstract
The xylan recovered from alkaline black liquor (ABL) of oil palm frond (OPF) has the potential to be converted into value-added products that hold promise, especially for the pharmaceutical industry. This research highlights the maximum xylan relative recovery extracted from ABL by varying the extraction parameters such as the concentration of sodium hydroxide (NaOH) (2 to 10%) (w/v), reaction temperature (60 to 140 °C), and extraction time (30 to 150 min), followed by a 2-stage precipitation method. 6 M of hydrochloric acid (HCl) was used in the first stage to separate the lignin sub-fraction (LF) from the ABL, and 95% ethanol was used in the second stage to obtain xylan sub-fraction (XF). The optimal condition of 6% NaOH (w/v), 100 °C, and 60 min was able to recover 84% of xylan, with low contamination of lignin (7.7%) and glucan (0.3%) in XF, while higher contamination of lignin (19.3%) and glucan (10.9%) with lower amount of xylan (8.8%) were found in LF. The amount of lignin and glucan in LF through the first precipitation was higher than in XF from the second precipitation, leaving remaining fraction of XF mainly composed of xylan. In sum, the developed process reduced the alkali consumption while retaining a high xylan recovery under mild process conditions.
Download PDF
Full Article
Enhancement of High Xylan Recovery from Black Liquor of Alkaline Pretreated Oil Palm Frond and its Physicochemical Properties
Nur Syahirah Ahmad Sobri,a Shuhaida Harun,a,b,* Nor Shahirah Ishak,a Jamaliah Md Jahim,a,b and Abdul Wahab Mohammad a,b
The xylan recovered from alkaline black liquor (ABL) of oil palm frond (OPF) has the potential to be converted into value-added products that hold promise, especially for the pharmaceutical industry. This research highlights the maximum xylan relative recovery extracted from ABL by varying the extraction parameters such as the concentration of sodium hydroxide (NaOH) (2 to 10%) (w/v), reaction temperature (60 to 140 °C), and extraction time (30 to 150 min), followed by a 2-stage precipitation method. 6 M of hydrochloric acid (HCl) was used in the first stage to separate the lignin sub-fraction (LF) from the ABL, and 95% ethanol was used in the second stage to obtain xylan sub-fraction (XF). The optimal condition of 6% NaOH (w/v), 100 C, and 60 min was able to recover 84% of xylan, with low contamination of lignin (7.7%) and glucan (0.3%) in XF, while higher contamination of lignin (19.3%) and glucan (10.9%) with lower amount of xylan (8.8%) were found in LF. The amount of lignin and glucan in LF through the first precipitation was higher than in XF from the second precipitation, leaving remaining fraction of XF mainly composed of xylan. In sum, the developed process reduced the alkali consumption while retaining a high xylan recovery under mild process conditions.
Keywords: Sodium hydroxide; Xylan recovery; Oil palm frond; Alkaline extraction; Hemicellulose; Lignocellulose; Glucan recovery; Acid-insoluble lignin
Contact information: a: Centre for Sustainable Process Technology (CESPRO), Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia; b: Chemical Engineering Programme, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia; *Corresponding author: harun.shuhaida@ukm.edu.my
INTRODUCTION
The utilisation of lignocellulosic biomass (LCB) has been of growing interest for several decades in industrial processes, as it is the most propitious material for renewable energy and bio-based products. LCB is mainly composed of three polymers; cellulose, hemicellulose, and lignin together with small amounts of other components including acetyl groups, minerals, and phenolic substituents. As an equatorial nation, Malaysia can take advantage of the vast availability and systematic replantation program of the palm oil industry as one of the easiest supplies of lignocellulosic biomass. The palm oil industry is a key economic factor that generates a material effect to the agricultural sector, with 2.44 million tonnes of crude palm oil production every year (Sime Darby Plantation Sustainability Annual Report 2014). The abundant lignocellulosic biomasses produced from the oil palm industry are rich with various carbohydrate sugars and other useful chemical compounds (Shuit et al. 2009). In an unorganised development, such as intensive plantation activities, there may be damage to forest systems, changes in the landscapes’ flora and fauna, and extreme land pollution due to the application of pesticides and herbicides. Therefore, waste disposal is huge problem associated with the oil palm industry.
Palm oil trees wastes can be developed into various types of resources, for instance, bioenergy and other value-added products (Abdullah and Sulaiman 2013). Oil palm fronds (OPF) are agricultural wastes of the palm oil industry, and they are structurally lignocelluloses-based that contain cellulose and hemicelluloses (Fazilah et al. 2009). Oil palm fronds have been extensively utilised for the production of bio-based products and as a feedstock in biorefineries for conversion into a wide range of valuable products. Recently, oil palm fronds have been utilised in the manufacturing of activated carbon through pyrolysis (Maulina and Rahmadi 2017) and in the production of oxalic acid through oxidation (Maulina and Anwari 2018). Oil palm fronds are composed of 30.4% hemicellulose (Saleh et al. 2011), which is the second most prolific polysaccharide in nature, representing approximately 20 to 30% of lignocellulosic biomass (Ebringerová et al. 2008). Hemicellulose polymers are complex components found in the cell walls of plants. Hemicellulose forms hydrogen bonds and covalent bonds with cellulose and lignin, respectively. Many notable applications of hemicelluloses by-products have been discovered and industrialised such as xylans.
Xylan is the most abundantly occurring heterogeneous biomolecule present in the hemicelluloses, and it is a promising biomaterial for pharmaceutical as well as food and non-food applications. Xylans have been industrially converted into xylose, xylitol, furfural, and prebiotics (Peng et al. 2009). Xylitol can be manufactured through the hydrolysis of xylan, crystallisation of xylose, and hydrogenation, which has been established in a variety of food products (Ebringerová and Heinze 2000). For instance, xylans in the dietary fibre of cereals aid in decreasing blood cholesterol and reducing postprandial blood glucose and insulin response in humans and animals (Asp et al. 1993).
Different techniques of extracting xylan from different agricultural residues have been studied in order to find novel applications, as there has been a growing interest in the characterisation of xylan. The production of xylan from its source materials will differ as per the method of extraction used in addition to the linkages and hydrogen bonding of cellulose and lignin within it (Samanta et al. 2012). The crucial part in obtaining the xylan is its component in lignocelluloses that are bound tightly, which makes it onerous to separate the xylan from lignins (Sun et al. 2004). This is one of the main reasons why the material properties of xylan have not been industrially exploited. Extraction is needed to alter the structure of the lignocellulose and break down the linkages with other biomolecules within the material.
Various methods have been used to extract xylan from agricultural wastes, and the alkaline extraction method is the most widely used, as it is the most promising method to accomplish complete utilisation of lignocelluloses (Ryberg et al. 2011). A large number of hemicelluloses containing xylan and partial lignin fractions with low molecular weight are degraded and dissolved into the black liquor generated after the extraction process, but many past studies focused on maintaining cellulose content for the production of value-added product. Relatively, less attention has been paid to the black liquor, and it is usually disposed as wastewater, which impacts to the environment.
The isolation of xylan in aqueous media occurs by the hydrolysis of ester linkages between hemicellulose and other components in an alkaline solution (Zulyadi et al. 2016). Alkaline black liquor contains xylan and partial fractions of lignin, which requires two-stage precipitation, starting with the acidification of black liquor, followed by ethanol precipitation to obtain the fractions that contain mainly xylan. The acidification process precipitates 90% of lignin from the black liquor (Zhu and Theliander 2015). Xylan is the precipitate formed through the alkaline solubilisation and an addition of ethanol, since cellulose cannot be dissolved in sodium hydroxide nor potassium hydroxide and it is observed that alcohol would not precipitate lignin (Samanta et al. 2012). Usually, the isolation of hemicellulose is done by using alkaline solution such as sodium hydroxide (NaOH) for softwood and potassium hydroxide (KOH) for hardwood (Peng et al. 2011).
There is limited information regarding the optimum condition of the alkali extraction to raise the recovery of xylan. This study highlights the maximum recovery of xylan extracted from the black liquor of alkaline pretreated OPF by varying the parameters of extraction such as alkaline concentration, temperature, and extraction time. This process was followed by the two-stage precipitation method using 6 M HCl and 95% ethanol to obtain the fractions namely lignin sub-fraction (LF) and xylan sub-fraction (XF), respectively. The maximum xylan percentage relative recovery was investigated, and the chemical composition and physicochemical properties of these fractions were examined by sugar analysis, acid-insoluble lignin (AIL), and Fourier transform infrared (FT-IR).
EXPERIMENTAL
Materials
Preparation of oil palm frond bagasse
The petioles of OPF were collected from the oil palm tree (Elaeis guineensis) plantation at Universiti Kebangsaan Malaysia, Bangi, Selangor, Malaysia on September 2018. The petioles were freshly cut about the average length of 1 to 3 m, and the material was processed on the same day to keep assure of its freshness. The OPF juice was removed by pressing in a sugarcane presser machine model SCM (6.5 hp petrol-driven). The OPF bagasse was dried under sun for 72 h until the moisture content dropped to less than 10%, as measured by a moisture analyser (IR35M, Denver Instrument, New York, NY, USA). After the drying, the OPF was ground to 2 mm particle size using a grinder (Fritsch, Idar-Oberstein, Germany) and sieved through 0.5 mm mesh for the removal of particles in powdered form resulting from the grinding process. The ground samples were sealed in a plastic bag and stored at 4 C. Prior to the optimisation and characterisation studies, the samples were oven-dried at 45 C until the moisture content reached ≤10 %.
Methods
Compositional analysis
The chemical analysis of raw OPF was conducted by following the methods developed by the National Renewable Energy Laboratory (NREL) which include the determination of total extractives, structural carbohydrates, lignin, and ash content (Hames et al. 2008; Sluiter et al. 2008a,b, 2012). In the early stages, the native OPF underwent the water extraction process, followed by ethanol extraction using the accelerated solvent extraction ASE 350 (ASE-Dionex, Sunnyvale, CA, USA) to remove the extractive compounds. Approximately 0.3 g of extractive-free OPF was further subjected to two-step acid hydrolysis process using 3 mL of 72% sulphuric acid at 30 C for 60 min, followed by dilution using 84 mL of deionised water at 121 C for 60 min to quantify the structural carbohydrates content. The methods developed by NREL helped in determining the chemical composition of raw biomass feedstocks for biomass conversion processes.
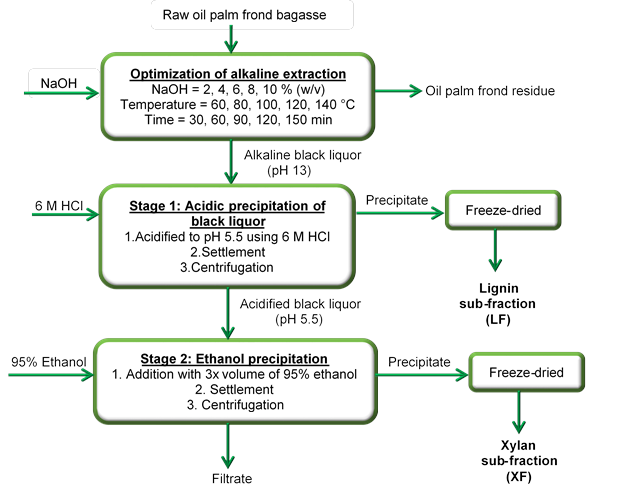
Fig. 1. Scheme for sequential extraction of xylan from OPF
Optimization run of xylan extraction
The scheme for sequential extraction of xylan from OPF was illustrated as can be seen in Fig. 1. Optimization run (OR) of xylan extraction was initially investigated by varying the concentration of NaOH, temperature, and extraction time to optimise the alkaline extraction conditions for maximum xylan relative recovery. Various concentrations of NaOH solution (2, 4, 6, 8, and 10%) (w/v), temperatures (60, 80, 100, 120, and 140 C), and extraction time (30, 60, 90, 120, and 150 min) were evaluated upon soaking the OPF with the ratio of 1:10 (solid to liquid). The black liquor was collected after the extraction process and was further subjected to acidification by 6 M hydrochloric acid (HCl) until it reached pH 5.5. The acidified black liquor was brought for centrifugation at 10000 rpm for 15 min. The precipitate formed, namely the lignin sub-fraction (LF), was filtered and freeze-dried using Martin Christ Alpha 1-4 LSC freeze-dryer until constant weight was achieved. The supernatant from the precipitation of LF was added with 3 volumes of 95% (v/v) ethanol until it became saturated for about 10 min. The mixture was brought for centrifugation again for 15 min at 10000 rpm. The precipitate formed, the xylan sub-fraction (XF), was freeze-dried after filtration until the weight became constant. Xylan obtained in the form of pellet (LF and XF) was used as xylan source and stored at room temperature for further analyses and characterisations while the supernatant was kept for sugar analysis. The following formula (Eqs. 1, 2, and 3) were used to calculate the relative recovery of xylan, glucan, and lignin, respectively, after the extraction,
where:
Verification run of xylan extraction under optimized condition
The optimization run (OR) was performed by using one-factor-at-a-time (OFAT) method, and the concentration of NaOH, temperature and time were varied to obtain the maximum xylan relative recovery. In order to ensure the accuracy of the data obtained from OR, triplicates of each verification run (VR1, VR2, and VR3) were further carried out under the optimal condition obtained from OR with the ratio of 1:10 (solid to liquid). The relative recovery values of xylan, glucan and lignin obtained from VR were calculated based on the Eqs. 1, 2, and 3, respectively.
Determination of acid-insoluble lignin content
The lignin content in OPF bagasse, LF, and XF was measured by acid-insoluble lignin (AIL), which was determined after a two-step hydrolysis process with 72% and 4% sulphuric acid, as previously described (Sluiter et al. 2012). Approximately 3 mL of 72% H2SO4 was added to 0.3 g of each fraction, and the mixtures were incubated at 30 °C for 60 min. Subsequently, 84 mL of deionised water was added to the mixtures, which were autoclaved at 121 °C (1.03 × 105 Pa) for 60 min. The filter was pre-weighed to be used for filtering off the lignin. The filtered hydrolysate was analysed for sugar content, while the residues were placed in a desiccator before being weighed. The AIL content was obtained from the percentage of weight loss.
Sugar composition and phenolic acid of extracted xylan
Hydrolysis of LF and XF fractions using sulphuric acid (H2SO4) was performed to determine their sugar composition and phenolic acid. The sugar composition was quantified using high performance liquid chromatography (HPLC) (UltiMate 3000 LC system, Dionex, Sunnyvale, CA, USA) detected by a refractive index (RI) detector (RefractoMax 520, ERC, Buchholz, Germany) set at 40 °C by using Rezex ROA-Organic Acid H+ column (300 mm × 7.8 mm; Phenomenex, Torrance, CA, USA), with a guard column (50 mm × 7.8 mm). The column temperature was maintained at 60 °C, and 0.005 N H2SO4 was isocratically eluted at a flow rate of 0.6 mL/min with the sample injection volume set at 20 µL. Concentrations of ferulic acid and coumaric acid were also analysed by using Agilent 1100 HPLC system (California, USA) detected by ultraviolet-diode array detector (UV-DAD) set at 220 nm by using Gemini C-18 column (Phenomenex, Torrance, CA, USA). The column temperature was set at 40 °C, and 20 mM sulphuric acid/ acetonitrile (1:10) was isocratically eluted at a flow rate of 0.8 mL/min with the sample injection volume set at 20 µL. Each of the mobile phase was initially vacuum filtered and degassed.
Fourier transform infrared spectroscopy (FTIR) analysis
The infrared spectra of OPF bagasse, extracted fractions (LF and XF), and commercial beechwood xylan were carried out using a Nicolet 6700 FTIR spectrophotometer (Thermo Fisher Scientific, MA, USA) with the attenuated total reflectance (ATR) method in the frequency range of 4000 to 400 cm⁻1 with resolution of 4 cm⁻1 to analyse the changes in functional groups in the xylan fractions.
RESULTS AND DISCUSSION
Compositional Analysis of Oil Palm Frond (OPF) Bagasse
The potential of an agricultural residual as a feedstock for biorefinery processes was initially predicted based on its chemical composition to attain a dependable and comparable compositional analysis of OPF, as it allows the biomass’ economical potential in biochemical industries to serve as a feedstock (Tan et al. 2016). The composition of OPF obtained in this study and by other researchers is shown in Table 1.
Table 1. Comparative View of Compositional Analysis of Raw OPF with Other Researchers
In this study, glucan was the major structural carbohydrate in OPF bagasse, at 34.7 ± 4.2%, followed by xylan, 19.4 ± 3.8%. Hence, the total structural carbohydrates content in OPF bagasse was 54.1 ± 7.9%. The results were similar with other studies in which glucan and xylan are the major structural carbohydrates present in OPF bagasse (Fauzi et al. 2016; Luthfi et al. 2016; Tan et al. 2016; Manaf et al. 2018). However, the total structural carbohydrates in this study was slightly lower than in others (Table 1). The variations in the source of OPF, maturity of the plant, and the conditions of weather might contribute to these differences. Because OPF was rich in xylan, the extraction of xylan from OPF was continued for further analyses. The total lignin content was 20.7 ± 0.5%. Lignin is also one of the major building blocks in lignocellulosic biomass; therefore, it is important to determine the total lignin percentage in the raw sample to enhance the effectiveness of the overall process. The content of total structural carbohydrates especially xylan proves that the OPF has the potential to be used as a feedstock in biorefineries for the production of value-added products.
Optimization Run (OR) of Xylan Extraction
During alkaline extraction, black liquor was generated after treating the ground OPF bagasse (Fig. 2a) with NaOH (Fig. 2b). The pH remained relatively constant but slightly decreased due to consumption of OH⁻ as hemicellulose and lignin were solubilised. An intense colour change took place from black (Fig. 3a) to yellowish orange (Fig. 3b) during the acidification of black liquor with 6 M HCl as the pH was lowered from 13.0 to 5.5. During the precipitation using ethanol to extract xylan sub-fraction (XF), two layers were formed (Fig. 3c). The extract was centrifuged, and the precipitated xylan was freeze-dried for 16 h before being weighed and powdered (Fig. 4). The recovery of xylan from OPF was calculated using Eq. 1, and it was observed based on different concentrations, temperatures and extraction time. Henceforth, xylan extraction was carried out in bulk for subsequent analysis after obtaining the best levels of alkali and its condition. Xylan recovery for XF, which was after the precipitation with ethanol, resulted in high recovery of xylan as compared to LF.
Fig. 2. (a) Untreated OPF bagasse after grinding process, extraction of OPF bagasse soaked in NaOH (b) before extraction, and (c) after extraction
In these alkaline processes, xylan and lignin components were solubilised into the aqueous alkaline black liquor by the peeling reaction and nucleophilic substitution. The pH of the black liquor remained relatively constant but slightly decreased due to consumption of OH⁻ as xylan and lignin were solubilised. In order to obtain pure xylan precipitate, the lignin in the black liquor must be precipitated out via acidification process by lowering the pH of black liquor. The xylan precipitate (XF) was then obtained via the ethanol precipitation from the acidified black liquor. The precipitation of lignin from black liquor by acidification occurs because of the protonation of ionised phenolic groups on lignin molecules. The protonation of phenolic groups reduces the electrostatic repulsive forces between lignin molecules, which then become less hydrophilic, leading to precipitation; lignin sub-fraction (LF) and the remaining liquid was poured into ethanol to precipitate out the xylan sub-fraction (XF) (Zhu and Theliander 2015). The two stages of precipitation defined the different molecular weight of hemicellulose, in which hemicellulose with higher molecular weight was usually obtained from the first precipitation using acid, while the hemicellulose with lower molecular weight was obtained from the second stage of precipitation using ethanol (Nacos et al. 2006).
Fig. 3. (a) Alkaline black liquor obtained after extraction process, (b) changes in colour during pH change, (c) formation of 2 layers after ethanol precipitation, (d) precipitated xylan formed by centrifugation
Fig. 4. Xylan fractions obtained after (a) acidic precipitation; LF and (b) ethanol precipitation; XF
Effect of concentration of NaOH on the xylan relative recovery
To investigate the effect of NaOH concentration on xylan relative recovery, different NaOH concentrations (2, 4, 6, 8, and 10%) (w/v) were used at a fixed time (60 min) and temperature (100 C). Ester linkages formed in the lignocellulosic matrix of biomass was initially hydrolysed by alkaline before being extracted into alkaline medium. These are the two processes involved to extract the xylan from lignocellulosic biomass (Doner and Hicks 1997; Peng et al. 2010). Hemicellulose polysaccharides were isolated in substantial amounts under alkaline extraction, which showed that it was the most efficient method. After the extraction took place, a moiety of hemicellulose material was brought into solution due to the swelling of cellulose, hemicelluloses, and celluloses by hydroxyl ions (Sun et al. 2005). Based on Table 2, the recovery of xylan for both LF and XF were increased from 10.6 ± 0.3% to 22.6 ± 0.3% and 23.3 ± 0.1% to 64.7 ± 0.7%, respectively, at 2% (w/v) to 6% (w/v). However, as the concentration was getting higher, the xylan relative recovery for LF and XF started to decrease from 18.9 ± 0.3% to 17.8 ± 0.1% and 53.9 ± 0.5% to 47.1 ± 0.4%, respectively, at 8% (w/v) to 10% (w/v). The relative recovery of all xylan fractions decreased at higher concentrations, indicating that appreciable amounts of xylans were leached out (Nasir and Saleh 2016). This also indicates that mild conditions were the best for xylan extraction. The highest xylan recovered of 22.6 ± 0.3% for LF and 64.7 ± 0.7% for XF were obtained at 6% NaOH (w/v); hence, it was chosen for further analysis.
Effect of temperature on the xylan relative recovery
The effects of different temperatures (60, 80, 100, 120, and 140 C) were studied at fixed parameters of 6% NaOH (w/v) and 60 min. According to Table 2, the xylan relative recovery for LF and XF increased from 17.4 ± 0.4% to 25.4 ± 0.4% and 39.0 ± 0.3% to 66.8 ± 0.2%, respectively, at 60C to 100 C, but it decreased from 22.5 ± 0.4% to 20.2 ± 0.3% for LF and 62.4 ± 0.9% to 46.9 ± 0.5% for XF at 120 C to 140 C. Warm alkaline solutions enhance hemicellulose degradation, but in cold alkaline solutions, hemicellulose has a finite solubility of (Nasir and Saleh 2016). The highest xylan relative recovery obtained for LF and XF was 25.4 ± 0.4% and 66.8 ± 0.2%, respectively, at 100 C. Thus, high temperature is needed in extracting the xylan to promote penetrating the lignified cell wall (Mosier et al. 2005). However, at higher temperatures, the decreased xylan recovery may be due to the degradation of hemicellulose by end-wise peeling reactions (Höije et al. 2005). High temperature caused the cleavage of linkages between the glycosyl units, which led to minor degradation (Bian et al. 2012). Therefore, a reaction temperature of 100 C was chosen as optimum temperature for further analysis.
Effect of extraction time on the xylan relative recovery
The effects of different extraction times (30, 60, 90, 120, and 150 min) on xylan relative recovery during alkaline extraction were examined after an optimum concentration and temperature were obtained at 6% NaOH (w/v) and 100 C, respectively. As can be observed from Table 2, the xylan recovery for LF and XF were increased from 7.4 ± 0.5% to 8.8 ± 0.4% and 54.1 ± 0.2% to 84.0 ± 0.1%, respectively, at an extraction time of 30 to 60 min. However, the xylan recovery dropped from 8.3 ± 0.7% to 7.7 ± 0.6% for LF and 62.6 ± 0.3% to 43.8 ± 0.3% for XF at the longer extraction time of 90 to 150 min.
Table 2. Optimization Run (OR) of Xylan Extraction for Percentage Relative Recovery of Xylan, Glucan, and Lignin in Alkaline Black Liquor, Lignin, and Xylan Fractions Under Varying Operating Conditions
The results indicated that increasing the extraction time may escalate the recovery of xylan, as previously noted (Saleh et al. 2012; Nasir and Saleh 2016). Hydrolytic cleavage may degrade polysaccharides and induce changes in the polysaccharide molecule structure at elevated time (Azhar et al. 2015). However, the change in polysaccharide molecule structure was induced due to extended time of extraction (Cai et al. 2008). In sum, the optimal conditions for xylan extraction from OPF were a NaOH concentration at 6% (w/v) and temperature of 100 C for 60 min with a dry OPF-to-NaOH ratio of 1:10 (g/L).
Fig. 5. Comparative view of xylan, glucan and lignin yield in raw OPF, lignin sub-fraction (LF) and xylan sub-fraction (XF) from OR obtained under optimal condition of 6 % NaOH (w/v), 100 (C) and 60 min.
The xylan relative recovery of each condition performed was higher in XF as compared to LF. However, the results for glucan and lignin relative recovery were found to be higher in LF rather than XF. Based on the results obtained under optimal condition of 6% NaOH (w/v), 100 C and 60 min, greater lignin recovery of 19.3 ± 0.1% was observed compared to 10.9 ± 0.1% of glucan in LF. The trend was also similar in XF where lignin (7.7 ± 0.2%) was higher than glucan (0.3 ± 0.3%). The relative recovery of glucan was very low, which indicated that significant amount of glucan was unhydrolyzed in the black liquor, as it was retained in the alkaline pretreated OPF. Also, the amount of lignin in LF after acidification process was higher than XF, leaving remaining fraction of XF mainly composed of xylan. According to Fig. 5, the result of xylan yield calculated from Eq. 4 showed an increase from 1.7 ± 0.1% to 16.3 ± 0.1% in LF and XF, respectively. These values corresponded to increase in the relative recovery of xylan from 8.8 ± 0.4% to 84.0 ± 0.1% in LF and XF, respectively. This could explain that the optimal condition obtained in this study provides high xylan recovery of 84 ± 0.1% with low contamination of lignin (7.7 ± 0.2%) and glucan (0.3 ± 0.3%).
Verification Run (VR) of Xylan Extraction Under Optimized Condition
The main objective of this study was to evaluate the factors influencing the maximum amount of xylan that could be recovered from the black liquor. An optimization run (OR) of xylan extraction was performed by using a one-factor-at-a-time (OFAT) method, and the results showed that 8.8 ± 0.4% and 84 ± 0.1% of xylan was successfully recovered in LF and XF, respectively. Three variables, namely NaOH concentration, temperature, and time, were the main factors affecting the xylan recovery. The verification was run under the optimal conditions of 6% NaOH (w/v), 100 C and 60 min, and the results are given in Table 3. The average results of xylan recovered from verification run (VR1, VR2 and VR3) in LF and XF were 8.5 ± 0.3% and 83.3 ± 0.6%, respectively, which is very close to the results obtained from OR. Hence, the consistency of the results showed that the optimal condition was able to provide maximum recovery of xylan from black liquor, preserving the xylan polymeric form.
Table 3. Comparative View of Optimization Run (OR) and Verification Run (VR) of Xylan Extraction based on Percentage Relative Recovery under Optimized Condition
Composition of Lignin Sub-Fraction (LF) and Xylan Sub-Fraction (XF)
Sugar composition of LF and XF
The sugar composition of extracted xylan varies, depending on the isolation method used and the type of raw materials used during the process (Peng et al. 2009). Various different sugar substituents such as glucose, arabinose, mannose, or acidic groups such as acetic and uronic acid attached to the xylose backbone describes the heterogeneity of xylan. According to Table 4, xylose was the predominant monosaccharide in XF, which contained of 18.5 ± 0.1% and 1.9 ± 0.1% of xylose in LF. Apart from xylose, glucose was found at a slight amount of 0.1 ± 0.1% in XF and 4.2 ± 0.2% in LF. This result is in accordance with other research findings in which xylose was predominantly found with a moderate amount of glucose in hemicelluloses of Indian wheat (Revanappa et al. 2007). Other monomers such as arabinose were not found in either fraction of xylan, which may be due to the decomposition of arabinose. During the solubilisation and hydrolisation of hemicelluloses, acetyl groups in hemicellulose are liberated into acetic acid (Manaf et al. 2017). Taken together, the results showed that xylose is the major unit in the xylan fractions, with glucose and acetic acid as substituents attached to the backbone of xylose.
Acid-insoluble lignin and phenolic acid content
To identify the purity of the xylan samples, the samples were subjected to acid hydrolysis to determine the lignin content based on the measurement of acid-insoluble lignin (AIL). It is the residue left from the acid hydrolysis of the samples due to the degradation of polysaccharides to monosaccharides (Sun et al. 2005). Polysaccharides in the plant cell walls contain various types of linkages that are tightly linked with lignin. Some examples are the hydroxyl group located at the -position of lignin side chain with the alcoholic hydroxyl of sugar residue, which is known as an ether linkage (Freudenberg 1965) or the cinnamic unit in lignin with the alcoholic –OH of polysaccharides, which is known as an ester linkage (Lam et al. 1992).
Fig. 6. Comparative view of acid-insoluble lignin content in raw OPF, lignin sub-fraction (LF), and xylan sub-fraction (XF) from OR obtained at optimal condition: 6% NaOH (w/v), 100 (C), 60 min.
Table 4. Sugar Composition, Phenolic Acid, and Acid-Insoluble Lignin Content in LF and XF
The AIL content of LF showed a noticeable decrement compared with the untreated OPF bagasse through the alkaline extraction process (Fig. 6), which showed that NaOH removed much of the hemicelluloses from the OPF sample. After the sub-fractionation via a graded precipitation with 95% ethanol, the AIL content in XF was decreased, which is also in accordance with other reports (Zhao et al. 2009). The phenolic acids attached to the lignin might have an effect on the properties of the materials used. Based on Table 4, only trace amounts of phenolic acids were found in the xylan fractions. Therefore, approximately 84 ± 0.1% of xylan was recovered from its original content in raw OPF bagasse, containing about 7.2 ± 0.2% of AIL in XF under the optimised conditions.
Fourier Transform Infrared (FT-IR) Analysis
An analytical technique to determine the conformational properties, as well as physicochemical characteristics of carbohydrates was implemented by using FT-IR spectroscopy as it is a rapid, sensitive, and low-cost technique (Kačuráková et al. 1999; Peng et al. 2009). It identifies the functional groups in the sample that match up to its signature molecule. The purity of the sample was studied from the FT-IR absorbance patterns (Gonçalves and Ruzene 2001). Hence, the present research investigated the FT-IR spectra of raw OPF bagasse, extracted xylan, and commercial beechwood xylan. From Fig. 7, the anomeric region ranged from 950 to 655 cm⁻1, and a small band at 901 cm⁻1 was assigned to the C–1 group frequency or ring frequency, which is the -glycosidic linkages between the sugar units in all xylan fractions. This could be the residues of xylose, in which the -form bonds linked the macromolecule backbone. Small peaks observed at 700 to 655 cm⁻1 are -anomers characteristic in the side chains (Sun and Sun 2002). A sharp band at 1040 to 1038 cm⁻1 in all xylan fractions spectra appeared from typical of xylans in the isolated hemicelluloses. It is also assigned to C–C and C–O stretching and the glycosidic (C–O–C) contributions in xylan (Sun and Tomkinson 2002).
Fig. 7. FT-IR spectra of (a) untreated raw OPF bagasse, (b) lignin sub-fraction; LF, (c) xylan sub-fraction; XF and (d) commercial beechwood xylan
Fig. 8. FT-IR spectra of lignin sub-fractions (LF) from OR, VR1, VR2, and VR3 under optimal condition of 6% NaOH (w/v), 100 C and 60 min.
Fig. 9. FT-IR spectra of xylan sub-fractions (XF) from OR, VR1, VR2, and VR3 under optimal condition of 6% NaOH (w/v), 100 C and 60 min.
The typical characteristics of arabinoxylans are observed between bands at 1170 and 1000 cm⁻1. The absorption bands at 1635, 1608, 1602, and 1593 cm⁻1 in all xylan spectra corresponded to the bending mode of water that has been absorbed, as the hemicelluloses shows high affinity toward water (Peng et al. 2012). The IR spectra of raw OPF (Fig. 7a) and LF (Fig. 7b) are almost similar especially in the region 1506 to 1247 cm-1 , which was found to be reduced in the IR spectra of XF (Fig. 7c) and beechwood xylan (Fig. 7d). The syringyl and guaiacyl propane units, which are characteristic of lignin, are observed at 1374 and 1247 cm-1, respectively in both raw OPF and LF. The peak at 1247 cm-1 is assigned to the linkages of C–O in guiacyl aromatic methoxy groups (Fan et al. 2011). Also, the guaiacyl lignin was reported to restrict the swelling of fiber and accessibility of enzyme than syringyl lignin (Ramos et al. 1992). Hence, the disappearance of these peaks in XF indicates the destruction in lignin structure during alkaline extraction.
The band observed at 1506 cm⁻1 in both raw OPF (Fig. 7a) and LF (Fig. 7b) was attributable to the lignin that was associated with the xylan fractions. However, the peak disappeared in XF (Fig. 7c) and beechwood xylan (Fig. 7d), indicating the absence of or a negligible amount of lignin in the xylan fractions. Bands at 1417, 1320, and 1040 cm−1 indicated hemicellulose (Stewart et al. 1995; Sun et al. 1999). The appearance of a strong band at 3375 cm⁻1 was due to the hydrogen-bonded in hydroxyl groups, while the band at 2937 to 2908 cm⁻1 was assigned to the CH3 group, which is the symmetric C–H vibration in cellulose and hemicelluloses (Fang et al. 2002). The absence of signal at 1732 cm⁻1 in Fig. 7(b-d) suggested that the application of sodium hydroxide had cleaved the ester bonds completely (Samanta et al. 2012), such as the acetyl and uronic ester groups from the hemicelluloses of OPF. Evidence of such cleavage also was shown in an earlier investigation (Peng et al. 2009), in which the ester linkages were able to be cleaved completely by using concentration of sodium hydroxide at 3%, which is particularly useful for the depolymerization or conversion to monomeric components. The IR spectra of LF and XF obtained from OR, VR1, VR2, and VR3 are shown in Figs. 8 and 9, respectively, to ensure the consistency of the sample. From the observation, the LF and XF were similar and consistent in each run performed under optimal condition of 6% NaOH (w/v), 100 C and 60 min. In sum, the lignin was clearly reduced in XF during alkaline extraction, and both XF and commercial beechwood xylan contained an identical set of transmission bands that differed only in intensity, indicating a similar structure of these xylans.
Mass Balance for Alkaline Extraction and Precipitation of Xylan from Oil Palm Frond
Mass balance is a vital application that can help in analysing the overall process flow by monitoring the input and output of the material from the process. Based on the compositional analysis of untreated OPF bagasse and xylan analysis after each process, an overall mass balance of xylan recovery from the alkaline extraction was developed based on tracking the amount of xylan yield. Figure 10 depicts the recovery of xylan in the untreated and treated OPF bagasse, as well as the extracted hemicellulose, which are expressed as the percentage equivalent to the component in the untreated OPF bagasse. The mass balance was performed on the OPF extracted at the conditions of 6% NaOH (w/v) with 1:10 solid to liquid ratio at temperature of 100 C and extraction time of 60 min. The black liquor obtained corresponding to 31.7 g of the original material was further filtered, neutralised, and precipitated using 95% ethanol to obtain the hemicellulose fraction. The alkaline extraction generated a pretreated OPF that was enriched in glucan (87.6%) and lignin (70.5%) based on their original content in raw OPF, while most of the xylan (93.8%) was hydrolysed into black liquor. This study focused more on the xylan recovery in black liquor toward utilization of OPF-derived hemicellulose for the production of value-added products along with bringing the palm industry to achieve zero waste strategies. The analysis showed that the xylan sub-fraction (XF) in the final process was successfully recovered, with up to 84% of xylan from the original content of xylan in raw OPF. In sum, the developed process was able to reduce the alkali consumption while retaining a high xylan recovery in mild process conditions.
Fig. 10. Overall mass balance of xylan, glucan and lignin yield from alkaline extraction of hemicellulose from OPF. Lignin includes summation of acid-insoluble lignin and acid-soluble lignin.
CONCLUSIONS
- The extraction of xylan was achieved under mild conditions of extraction using 6% NaOH (w/v), temperature of 100 C, and extraction time at 60 min, achieving up to 8.8% and 84% recovery of xylan in the lignin subfraction (LF) and the xylan subfraction (XF), respectively.
- Xylan content in XF was higher than LF, showing that the amount of lignin could affect the percentage recovery of xylan from oil palm fronds.
- Alkaline extraction of oil palm fronds by sodium hydroxide followed by ethanol precipitation led to the maximum recovery of xylan.
- Oil palm fronds are a low-cost feedstock for bioconversion, and alkaline extraction using sodium hydroxide produced valuable, pure, xylan-rich hemicellulose.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Higher Education, Malaysia through the grant provided under the Fundamental Research Grant Scheme (FRGS/1/2016/TK02/UKM/02/4) and the Long-term Research Grant Scheme (LRGS/2013/UKM-UKM/PT/01) on a project entitled “Enhancement of High Xylan Recovery from Black Liquor of Alkaline Pretreated Oil Palm Frond and its Physicochemical Properties”.
REFERENCES CITED
Abdullah, N., and Sulaiman, F. (2013). “The oil palm wastes in Malaysia,” in: Biomass Now – Sustainable Growth and Use, InTech Open, London, pp. 75-100. DOI: 10.5772/55302
Asp, N.-G., Björck, I., and Nyman, M. (1993). “Physiological effects of cereal dietary fibre,” Carbohydrate Polymers 21(2–3), 183-187. DOI: 10.1016/0144-8617(93)90016-W
Azhar, S., Henriksson, G., Theliander, H., and Lindström, M. E. (2015). “Extraction of hemicelluloses from fiberized spruce wood,” Carbohydrate Polymers 117, 19-24. DOI: 10.1016/j.carbpol.2014.09.050
Bian, J., Peng, F., Peng, X. P., Xu, F., Sun, R. C., and Kennedy, J. F. (2012). “Isolation of hemicelluloses from sugarcane bagasse at different temperatures: Structure and properties,” Carbohydrate Polymers 88(2), 638-645. DOI: 10.1016/j.carbpol.2012.01.010
Cai, W., Gu, X., and Tang, J. (2008). “Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta,” Carbohydrate Polymers 71, 403-410. DOI: 10.1016/j.carbpol.2007.06.008
Doner, L. W., and Hicks, K. B. (1997). “Isolation of hemicellulose from corn fiber by alkaline hydrogen peroxide extraction,” Cereal Chemistry 74(2), 176-181. DOI: 10.1094/CCHEM.1997.74.2.176
Ebringerová, A., and Heinze, T. (2000). “Xylan and xylan derivatives – biopolymers with valuable properties, 1. Naturally occurring xylans structures, isolation procedures and properties,” Macromolecular Rapid Communications 21(9), 542-556. DOI: 10.1002/1521-3927(20000601)21:9<542::AID-MARC542>3.3.CO;2-Z
Ebringerová, A., Hromádková, Z., Hříbalová, V., Xu, C., Holmbom, B., Sundberg, A., and Willför, S. (2008). “Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities,” International Journal of Biological Macromolecules, 42(1), 1-5. DOI: 10.1016/j.ijbiomac.2007.08.001
Fan, S., Zakaria, S., Chia, C., Jamaluddin, F., Nabihah, S., Liew, T., and Pua, F. (2011). “Bioresource technology comparative studies of products obtained from solvolysis liquefaction of oil palm empty fruit bunch fibres using different solvents,” Bioresource Technology 102(3), 3521-3526. DOI: 10.1016/j.biortech.2010.11.046
Fang, J. M., Fowler, P., Tomkinson, J., and Hill, C. A. S. (2002). “Preparation and characterisation of methylated hemicelluloses from wheat straw,” Carbohydrate Polymers 47, 285-293.
Fauzi, N. A., Harun, S., and Jahim, J. (2016). “Physiochemical changes and mass balance of raw and alkaline pretreated oil palm frond: Pressed versus non-pressed sample,” International Journal of Applied Engineering Research 11(19), 9886-9893.
Fazilah, A., Azemi, M. N. M., Karim, A. A., and Norakma, M. N. (2009). “Physicochemical properties of hydrothermally treated hemicellulose from oil palm frond,” Journal of Agricultural and Food Chemistry 57(4), 1527-1531. DOI: 10.1021/jf8028013
Freudenberg, K. (1965). “Lignin: Its constitution and formation from p-hydroxycinnamyl alcohols,” Science 148(3670), 595-600.
Gonçalves, A. R., and Ruzene, D. S. (2001). “Bleachability and characterization by Fourier transform infrared principal component analysis of acetosolv pulps obtained from sugarcane bagasse,” Applied Biochemistry and Biotechnology 91–93(1), 63-70. DOI: 10.1385/ABAB:91-93:1-9:63
Hames, B., Ruiz, R., Scarlata, C., Sluiter, A., Sluiter, J., and Templeton, D. (2008). Preparation of Samples for Compositional Analysis: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42620), National Renewable Energy Laboratory (NREL), Golden, CO, USA.
Höije, A., Gröndahl, M., Tømmeraas, K., and Gatenholm, P. (2005). “Isolation and characterization of physicochemical and material properties of arabinoxylans from barley husks,” Carbohydrate Polymers 61(3), 266-275. DOI: 10.1016/j.carbpol.2005.02.009
Jin, A. X., Ren, J. L., Peng, F., Xu, F., Zhou, G. Y., Sun, R. C., and Kennedy, J. F. (2009). “Comparative characterization of degraded and non-degradative hemicelluloses from barley straw and maize stems: Composition, structure, and thermal properties,” Carbohydrate Polymers 78(3), 609-619. DOI: 10.1016/j.carbpol.2009.05.024
Kačuráková, M., Ebringerová, A., Hromádková, Z., Wellner, N., Wilson, R. H., and Belton, P. (1999). “Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies,” Food Hydrocolloids 13(1), 35-41. DOI: 10.1016/S0268-005X(98)00067-8
Lam, T. B. T., Iiyama, K., and Stone, B. A. (1992). “Changes in phenolic acids from internode walls of wheat,” Phytochemistry 31(8), 2655-2658.
Luthfi, A. A. I., Jahim, J. M., Harun, S., Tan, J. P., and Mohammad, A. W. (2016). “Biorefinery approach towards greener succinic acid production from oil palm frond bagasse,” Process Biochemistry 51(10), 1527-1537. DOI: 10.1016/j.procbio.2016.08.011
Manaf, S. F. A., Jahim, J. M., Harun, S., and Luthfi, A. A. I. (2018). “Fractionation of oil palm fronds ( OPF ) hemicellulose using dilute nitric acid for fermentative production of xylitol,” Industrial Crops & Products 115, 6-15. DOI: 10.1016/j.indcrop.2018.01.067
Manaf, S. F. A., Luthfi, A. A. I., Jahim, J. M., and Harun, S. (2017). “Interaction effects of pH and inhibitors in oil palm frond (OPF) hemicelullosic hydrolysate on xylitol production: A statistical study,” Journal of Physical Science 28, 241-255.
Maulina, S., and Anwari, F. (2018). “Utilization of oil palm fronds in producing activated carbon using Na2CO3 as an activator,” Materials Science and Engineering 309, 012087. DOI: 10.1088/1757-899X/309/1/012087
Maulina, S., and Rahmadi, I. (2017). “The utilization of oil palm fronds in producing oxalic acid through oxidation,” AIP Conference Proceedings 1879(1), 050002. DOI: 10.1063/1.5000472
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pretreatment of lignocellulosic biomass,” Bioresource Technology 96, 673-686. DOI: 10.1016/j.biortech.2004.06.025
Nacos, M. K., Katapodis, P., Pappas, C., Daferera, D., Tarantilis, P. A., Christakopoulos, P., and Polissiou, M. (2006). “Kenaf xylan – A source of biologically active acidic oligosaccharides,” Carbohydrate Polymers 66(1), 126-134. DOI: 10.1016/j.carbpol.2006.02.032
Nasir, M. A. M., and Saleh, S. H. (2016). “Characterization of hemicelluloses from oil palm empty fruit bunches obtained by alkaline extraction and ethanol precipitation,” Malaysian Journal of Analytical Sciences 20(4), 849-855. DOI: 10.17576/mjas-2016-2004-19
Peng, F., Bian, J., Ren, J., Peng, P., Xu, F., and Sun, R. (2012). “Fractionation and characterization of alkali-extracted hemicelluloses from peashrub,” Biomass and Bioenergy 39, 20-30. DOI: 10.1016/j.biombioe.2010.08.034
Peng, F., Ren, J. L., Xu, F., Bian, J., Peng, P., and Sun, R. C. (2009). “Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse,” Journal of Agricultural and Food Chemistry 57(14), 6305-6317. DOI: 10.1021/jf900986b
Peng, F., Ren, J. L., Xu, F., Bian, J., Peng, P., and Sun, R. C. (2010). “Fractional study of alkali-soluble hemicelluloses obtained by graded ethanol precipitation from sugar cane bagasse,” Journal of Agricultural and Food Chemistry 58(3), 1768-1776. DOI: 10.1021/jf9033255
Peng, P., Peng, F., Bian, J., Xu, F., Sun, R. C., and Kennedy, J. F. (2011). “Isolation and structural characterization of hemicelluloses from the bamboo species Phyllostachys incarnata Wen,” Carbohydrate Polymers 86(2), 883-890. DOI: 10.1016/j.carbpol.2011.05.038
Ramos, L. P., Breuil, C., and Saddler, J.N. (1992). “Comparison of steam pretreatment of eucalyptus, aspen, and spruce wood chips and their enzymatic hydrolysis,” Appl. Biochem. Biotech. 34(5), 37-48.
Revanappa, S. B., Bhagwat, S. G., and Salimath, P. V. (2007). “Studies on pentosans in Indian wheat (Triticum aestivum) varieties in relation to chapati making quality,” Food Chemistry104, 896-902. DOI: 10.1016/j.foodchem.2006.12.024
Saleh, S.-H., Azemi, M. N. M., and Ahmad, R. (2011). “Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeis guineensis Jacq.) frond fibres for xylose and xylooligosaccharides production,” Bioresource Technology 102(2), 1234-1239. DOI: 10.1016/j.biortech.2010.08.017
Saleh, S. H., and Aziz, A. A. (2016). “Polymer characterization of cellulose and hemicellulose,” Polymer Science: Research Advances, Practical Applications and Educational Aspects, 404-411.
Saleh, S. H., Ridzuan, N., Hanapi, N. S. M., and Karim, S. K. A. (2012). “Effects of temperature , time and pressure on the hemicelluloses yield extracted using subcritical water extraction,” Procedia Engineering 42(August), 562-565. DOI: 10.1016/j.proeng.2012.07.448
Samanta, A. K., Jayapal, N., Kolte, A. P., Senani, S., Sridhar, M., Suresh, K. P., and Sampath, K. T. (2012). “Enzymatic production of xylooligosaccharides from alkali solubilized xylan of natural grass (Sehima nervosum),” Bioresource Technology 112, 199-205. DOI: 10.1016/j.biortech.2012.02.036
Shuit, S. H., Tan, K. T., Lee, K. T., and Kamaruddin, A. H. (2009). “Oil palm biomass as a sustainable energy source: A Malaysian case study,” Energy 34(9), 1225-1235.
Sime Darby Plantation Sustainability Report (2014). Retrieved from http://www.simedarbyplantation.com/sites/default/files/sustainability/Sime_Darby_Plantation_Sustainability_Report_2014.pdf
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008a). Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42622), National Renewable Energy Laboratory (NREL), Golden, CO, USA.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2012). Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42618), National Renewable Energy Laboratory (NREL), Golden, CO, USA..
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008b). Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42619), National Renewable Energy Laboratory (NREL), Golden, CO, USA.
Stewart, D., Mcdougall, G. J., and Baty, A. (1995). “Fourier-transform infrared microspectroscopy of anatomically different cells of flax (Linum usitatissimum) stems during development,” Journal of Agricultural and Food Chemistry 43(7), 1853-1858. DOI: 10.1021/jf00055a019
Sun, J. X., Sun, X. F., Sun, R. C., and Su, Y. Q. (2004). “Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses,” Carbohydrate Polymers 56(2), 195-204. DOI: 10.1016/j.carbpol.2004.02.002
Sun, R. C., Fang, J. M., Mott, L., and Bolton, J. (1999). “Fractional isolation and characterization of polysaccharides from oil palm trunk and empty fruit bunch fibres,” 53, 253-260.
Sun, R. C., and Sun, X. F. (2002). “Fractional and structural characterization of hemicelluloses isolated by alkali and alkaline peroxide from barley straw,” Carbohydrate Polymers 49(4), 415-423. DOI: 10.1016/S0144-8617(01)00349-6
Sun, R. C., and Tomkinson, J. (2002). “Characterization of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw,” Carbohydrate Polymers 50(3), 263-271. DOI: 10.1016/S0144-8617(02)00037-1
Sun, X. F., Sun, R., Fowler, P., and Baird, M. S. (2005). “Extraction and characterization of original lignin and hemicelluloses from wheat straw,” Journal of Agricultural and Food Chemistry 53(4), 860-870. DOI: 10.1021/jf040456q
Tan, J. P., Jahim, J. M., Harun, S., Wu, T. Y., and Mumtaz, T. (2016). “Utilization of oil palm fronds as a sustainable carbon source in biorefineries,” International Journal of Hydrogen Energy 41(8), 4896-4906. DOI: 10.1016/j.ijhydene.2015.08.034
Xu, F., Sun, R. C., Sun, X. F., Geng, Z. C., Xiao, B., and Sun, J. X. (2004). “Analysis and characterization of acetylated sugarcane bagasse hemicelluloses,” International Journal of Polymer Analysis and Characterization 9(4), 229-244. DOI: 10.1080/10236660490920228
Zhao, X., Cheng, K., and Liu, D. (2009). “Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis,” Applied Microbiology and Biotechnology 82, 815-827. DOI: 10.1007/s00253-009-1883-1
Zhu, W., and Theliander, H. (2015). “Precipitation of lignin from softwood black liquor: An investigation of the equilibrium and molecular properties of lignin,” BioResources 1027(1), 1696-1714. DOI: 10.1063/1.4861115
Zulyadi, N. H., Saleh, S. H., and Sarijo, S. H. (2016). “Fractionation of hemicellulose from rice straw by alkaline extraction and ethanol precipitation,” Malaysian Journal of Analytical Science 20(2), 329-334. DOI: 10.17576/mjas-2016-2002-15
Article submitted: February 22, 2019; Peer review completed: April 8, 2019; Revised version received: May 13, 2019; Accepted: May 16, 2019; Published: May 23, 2019.
DOI: 10.15376/biores.14.3.5400-5421
