Abstract
NaY zeolite-containing ceramic papers were prepared by a papermaking technique with a dual polyelectrolyte retention system that implied the use of cationic and anionic polymers. To improve their mechanical properties, we found that some borate compounds could be successfully used as ceramic binders. Three types of sodium and/or calcium borates were tested as binders: colemanite, nobleite, and anhydrous ulexite. The improvement in the mechanical properties depends both on the borate used and on the calcination temperature. By XRD it was determined that the faujasite structure collapsed after calcination at 700°C, which limited the final calcination temperature of zeolitic ceramic papers. Different amounts of NaY zeolite were added to ceramic papers and, as observed by SEM, faujasite particles were well distributed throughout the ceramic paper structure. Ceramic papers containing 1.2 wt.% zeolite after calcination at 650°C resulted in structured catalysts that were easy-to-handle, and which can be adapted to different conformations.
Download PDF
Full Article
Enhancing Mechanical Properties of Ceramic Papers Loaded with Zeolites using Borate Compounds as Binders
Juan P. Cecchini,a Ramiro M. Serra,a María A. Ulla,a Miguel A. Zanuttini,b and Viviana G. Milt a,*
NaY zeolite-containing ceramic papers were prepared by a papermaking technique with a dual polyelectrolyte retention system that implied the use of cationic and anionic polymers. To improve their mechanical properties, we found that some borate compounds could be successfully used as ceramic binders. Three types of sodium and/or calcium borates were tested as binders: colemanite, nobleite, and anhydrous ulexite. The improvement in the mechanical properties depends both on the borate used and on the calcination temperature. By XRD it was determined that the faujasite structure collapsed after calcination at 700°C, which limited the final calcination temperature of zeolitic ceramic papers. Different amounts of NaY zeolite were added to ceramic papers and, as observed by SEM, faujasite particles were well distributed throughout the ceramic paper structure. Ceramic papers containing 1.2 wt.% zeolite after calcination at 650°C resulted in structured catalysts that were easy-to-handle, and which can be adapted to different conformations.
Keywords: Ceramic papers; Zeolitic fibrous catalysts; Flexible structured catalysts; Borate binders
Contact information: a: Instituto de Investigaciones en Catálisis y Petroquímica (INCAPE, FIQ-UNL-CONICET), Santiago del Estero 2829, 3000 Santa Fe, Argentina; b Instituto de Tecnología Celulósica, FIQ, UNL, Santiago del Estero 2564, Santa Fe, S3000 AOJ, Argentina;
* Corresponding author: vmilt@fiq.unl.edu.ar
INTRODUCTION
Ceramic fiber materials, in the form of wool, blankets, or papers, are mainly used for thermal insulation. Depending on the working temperature, different kinds of fibers can be employed as mineral fibers, refractory ceramic fibers (RCF), or high temperature glass fibers.
A ceramic paper is a flexible but consolidated structure that has the advantage of accommodating different geometries. The commercial production, in this case, includes the addition of high-temperature resistant adhesives to achieve the necessary handling strength. These organic adhesives must burn out cleanly, producing the desired features of high flexibility, homogeneous pore size distribution, and good thermal properties.
A ceramic paper can also be the structural base of a catalytic bed. For this application, the ideal ceramic paper should be easy-to-handle in practical use and should allow for rolling and folding without breaking. The catalyst can be deposited on a finished ceramic paper, or catalytic particles can be immobilized in the fibrous network if they are added during the paper preparation. This type of structured catalyst has been applied for different reactions, such as methanol steam reforming (Koga et al. 2006; Fukahori et al. 2006a; Fukahori et al. 2006b; Koga et al. 2008), reduction of NOx in exhaust gases (Koga et al. 2009; Ishihara et al. 2010), and soot removal (Banús et al. 2010). Moreover, paper-like composites containing photoactive TiO2 powders have been applied for VOC decomposition (Fukahori et al. 2007). Besides, the immobilization of molecular sieve porous materials (zeolite) as active particles that form adsorbent structures, has also been analyzed (Cecchini et al. 2011).
For all these catalytic applications, the use of a ceramic binder instead of an organic adhesive is necessary. Also, the addition of cellulosic fibers in low proportion favors the fiber mat consolidation, not only when the mat is wet, but also when the mat is dry. Then, after calcination, the paper should have permanent mechanical properties as well as a stable porous structure suitable for gas flow-through applications. The kind and the amount of ceramic binder should be selected in order to obtain a strong but flexible structure. The binder should fulfill three characteristics: (i) Be insoluble in water if the preparation is carried out in aqueous medium, (ii) Be stable and not reactive to fibers or to the active particles added in a degree that can affect the mechanical integrity of fibers or chemical properties of the active particles during paper manufacturing, and (iii) Develop the bonding action by thermal treatments that do not affect the activity of the catalyst particles.
In a previous study we used a zeolite-containing ceramic paper for toluene removal (Cecchini et al. 2011). Although adsorption properties of these structures were good, the resulting papers were not easy to handle. Moreover, they broke when manipulated. This led us to further study on how to enhance the mechanical properties of ceramic papers. In our experiments we found that some borate compounds can be successfully used as ceramic binders for paper-structured catalytic beds. Focusing on the importance of mechanical properties of ceramic papers and considering the scant information reported, this paper analyzes the effect of calcination temperature on tensile strength and stiffness of ceramic paper prepared with three borate binders. Alumina-silicate fibers were used, and a papermaking procedure, including a dual polyelectrolyte system, was applied in the paper formation. After that, for a selected preparation condition, the effects of the addition of different amounts of NaY zeolite on the mechanical properties of the ceramic papers were evaluated. TGA, SDTA, XRD, and SEM were used to determine the retention of particle material during the formation of the paper and to characterize the composites.
EXPERIMENTAL
Raw Materials
Fibers
Refractory ceramic fibers (RCF, 50 wt.% SiO2, 48 wt.% Al2O3, and 2 wt.% impurities) were obtained from a ceramic insulation from CARBO. The fibrous mat was dispersed in tap water, and the fibers were separated from low slenderness particles by an elutriation process. The yield in fibers was around 50%, with an average fiber length of 660 µm and an average diameter of 6 µm. Cellulosic fibers, from a dry commercial bleached softwood Kraft pulp, were re-wetted at least 24h and dispersed by a standard disintegrator before use.
Borate compounds
Three kinds of borate compounds were studied as binders: (i) commercial anhydrous ulexite (B5O9NaCa) provided by BORAX, (ii) commercial colemanite (B6O11Ca2. 5H2O) provided by BORAX, and (iii) nobleite (B6O10Ca. 4H2O). The latter was synthesized according to Erds et al. (1961), using Ca(OH)2 and H3BO3 as starting materials, preparing a stoichiometric solution, softly stirring during 30 h at 48°C, and further treating for 10 days at 68°C. In all cases, only binder particles that passed an 80-mesh sieve were used for paper manufacturing. It should be pointed out that the commercial available anhydrous ulexite had been calcined at 900°C, which is not the case of the other two borate compounds here explored.
Zeolite
NaY zeolite (Si/Al = 2.8) was prepared by hydrothermal synthesis. The gel, whose molar composition was: H2O:SiO2:Na2O:Al2O3 = 80:1:0.38:0.025 (Si/Al = 6), was prepared starting from NaOH, H2O, Na2Al2O4, and colloidal SiO2 (Ludox, 40 wt.% of SiO2). After annealing at room temperature for 24h under stirring, the gel was put into an autoclave that was hermetically closed and put in an oven at 100°C for 24 h. Then, zeolite crystals were filtered, washed with abundant distilled water, and dried at 100°C in an oven overnight.
Ceramic Paper Preparation
A papermaking technique was used. For the sheet formation stage, a dual polyelectrolyte retention system (cationic and anionic polymer) was employed in order to reduce the loss of fine material (borate compound and zeolite) as much as possible. The cationic polymer was polyvinyl amine (PVAm) (Luredur PR 8095) from BASF, molecular weight 4.105 g∙mol-1and charge density 4.5 meq∙g-1, and the anionic polymer was A-PAM from AQUATEC, molecular weight 104 to 105 g∙mol-1 and charge density 2.7 meq∙g-1.
Under gentle agitation, 5.0 g of ceramic fiber, 0.75 g of cellulose fiber, 1.56 g of anhydrous ulexite, and 33 mL of PVAm solution (1 g∙L-1) were incorporated to 500 mL of NaCl solution (0.01N). After 3 minutes of slight stirring, 20 mL of A-PAM polymer solution (0.4 g∙L-1) was added. From this suspension, a handsheet was formed by the SCAN standard method (SCAN-C 26:76 and SCAN-M 5:76) but instead using tap water (180 mS) and applying double the standard pressing pressure. The wet sheet was dried under an atmosphere where the temperature and the relative humidity were controlled (23°C and 50% RH) during 24 h and finally calcined in air for 2 h at four different temperatures (600, 650, 700, or 750ºC); resulting samples were denominated as PCerU600, PCerU650, PCerU700, and PCerU750. Similarly, ceramic papers were prepared using either colemanite or nobleite as binders, and they were referred to as papers prepared with ulexite but using the letter C or N instead of U to denote colemanite or nobleite. Ceramic papers thus prepared resulted in highly porous structures with a resistance to passage of air lower than 0.5 s Gurley∙100 mL-1. To measure these low values, Gurley equipment (TAPPI T 460 om-02) was used, but the flow section was reduced to half of the original. Although these values are outside the range accepted by standards, they clearly indicate a high porosity. Besides, paper density resulted in values ranging between 0.21 and 0.32 g∙cm-3.
Following the same procedure as that used for the preparation of PCerU650, zeolite papers were prepared by adding NaY zeolite (1.0, 2.0, and 4.0 g) at the beginning of the preparation procedure. Firstly, zeolite was milled, and the fraction of particles that passed through the 80-mesh sieve was dispersed in water of 180 mS during five minutes under ultrasound. Papers containing zeolite were calcined at 650°C during 2 h, and they were denoted as PCerZ(X)U650, X being the zeolitic wt.%. Figure 1 schematizes the preparation procedure. An estimation of the retention of inorganic materials was done considering the weight of components loaded into the paper manufacturing machine and the weight of the calcined paper. For the ceramic paper without zeolite (PCerU650) retention was 91%, whereas for the paper with 1.2 wt.% zeolite (PCerZ(1.2)U650) it was 81%.
Fig. 1. Scheme of the ceramic papers preparation
Characterization
Mechanical characterization
Tensile strength and stiffness of ceramic papers were determined using an INSTRON 3344 universal tester equipped with a 10 N load cell. The standard procedure TAPPI T494 – 01 om was followed.
Scanning electron microscopy (SEM)
The morphology of the ceramic papers was studied using a SEM Jeol JSM-35C instrument, which was operated at 20 kV acceleration voltage. Samples were glued to the sample holder with Ag painting and then coated with a thin layer of Au in order to improve the images.
XRD characterization
Crystalline phases were determined with a Shimadzu XD-D1 instrument with monochromator using Cu K radiation at a scan rate of 1 deg∙min-1, from 2 = 5 to 50°. In the case of powders, they were compressed in the sample holder, whereas for ceramic papers, pieces of about 2 cm x 2 cm, were supported in a special sample holder designed for the XRD analysis. The software package of the equipment was used for the phase identification from the X-ray diffractograms.
TGA-SDTA
The thermal analysis of the ceramic papers was studied in a Mettler Toledo TGA/SDTA 851 instrument. The weight changes and the differential thermal process of the catalytic ceramic papers (10 mg of samples) were analyzed from 25 to 900ºC with a heating rate of 10ºC∙min-1 in air flow (80 mL∙min-1).
Textural properties
Nitrogen adsorption–desorption isotherms were obtained at −196°C on a Quantachrome Autosorb 1C instrument. Previously, samples were outgassed at 350°C for 2h under vacuum. The Brunauer–Emmett–Teller (BET) equation was used for calculating the specific surface area of the materials from nitrogen adsorption isotherms.
RESULTS AND DISCUSSION
Effect of the Calcination Temperature on the Zeolitic Structure
Figure 2 shows the XRD patterns of the synthesized NaY zeolite powder calcinated for two hours at different temperatures (600, 650, 700, and 750ºC). After calcination at 600ºC, the zeolite presented diffraction peaks that did not differ from those corresponding to the fresh faujasite (not shown), and the crystallinity was partially reduced after calcination at 650ºC. Nevertheless, after the calcination at higher temperature (750ºC), the intensity of the diffraction peaks was drastically decreased, and the presence of an amorphous halo in the 2 region from 15° to 40° could be clearly seen. The calcination at 750ºC caused the collapse of structure.
Fig. 2. XRD patterns of NaY zeolite calcined at different temperatures
Borate Thermal Analysis
Figure 3 shows the thermal analysis (TGA and SDTA) of the three borate compounds considered: (i) commercial anhydrous ulexite, (ii) commercial colemanite, and (iii) laboratory synthesized nobleite. As reported, borate compounds suffer thermal processes of three types: dehydration, crystallization, and fusion.
Fig. 3. (a) Thermal behavior (TGA) of borates used, where w/w° is the relative weight, i.e., the weight at any temperature T divided by the initial weight (w°). (b) Exo-endothermic evolutions when heating borates under air stream (SDTA profiles). T-TREF is the difference between the real temperature and the reference value.
Figure 3 shows the different behaviors exhibited when heating the borate compounds. In the case of ulexite, no weight loss was observed up to 900°C since the borate compound used was the corresponding anhydride. However, colemanite presented weight losses at two temperature zones, the first one between 400 and 550ºC, which corresponds to constitutive water, and the latter observed at higher temperatures (650 to 750ºC), which could be attributed to carbonate impurities (Gazulla et al. 2005). Finally, it could be noted that for nobleite, the loss of water occurred at temperatures below 450°C.
In agreement with TGA experiments, SDTA profiles (Fig. 3b) showed endothermic peaks that correspond to dehydration processes, which occur at lower temperatures for nobleite than for colemanite. Another process that can be appreciated is that of recrystallization, characterized by the exothermic peak observed at ca. 750°C for the three types of borates used. Nevertheless, none of the three borates under study melts up to 900°C.
Mechanical Tests
Figure 4 shows a typical curve obtained in the INSTRON apparatus, where Tensile Load is plotted against Elongation. Two parameters were obtained from these plots: the Breaking Load (BL, N) and the Elastic Module (EM, MPa).
Fig. 4. Mechanical behavior: typical curve obtained for the ceramic papers (as example the curve obtained for the PCerU650 sample is shown as example). The tensile index (TI) of ceramic papers was determined from breaking load value, indicated by the square, and the specific elastic modulus (EM) was calculated from the slope of the elastic portion of the curve
The Tensile Strength is expressed here as a Tensile Index (TI) (Equation 1),
(1)
where G is the Grammage (weight per surface unit) and W is the paper strip width (m).
The elastic modulus was obtained from the linear portion of the curve at the beginning of the test, as indicated in Fig. 4 and using Equation 2,
(2)
where F is tensile load, S is paper section calculated as the probe width multiplied by the paper thickness, ΔL is the elongation, and L is the distance between the testing grips. It should be stressed that a high tensile strength and high elasticity (low elastic module) are the desired characteristics of a ceramic paper.
It is interesting to point out that during these tests for ceramic papers, failure does not occur as a “clean rupture,” i.e. tensile load exist beyond the elongation corresponding to maximum load, which indicates that, instead of individual fiber break, many fibers are pulled out during the rupture.
Figure 5 shows the effect of calcination temperature (600 to 750ºC) on the average values (of at least five samples) of tensile strength for the three borates added as ceramic binders, where the error bars indicate the standard deviations from the averages. Some tensile strength values show variability, which can be attributed to the intrinsic error of the method. As observed, papers prepared with colemanite and nobleite and calcinated at higher temperatures exhibited the highest tensile strength values. For the paper prepared using nobleite (PCerN), a maximum strength was observed after calcination at 700ºC. Nevertheless, for the low calcination temperature (650ºC), the strength of both (PCerN and PCerC) was lower than that corresponding to the paper prepared with ulexite (PCerU).
As Fig. 3 shows, although none of the borates used melted during the calcination step, individual borate particles began to sinter as calcination temperature increased, reinforcing the ceramic fiber network through the joining of ceramic fibers in several points, thus enhancing paper mechanical strength properties.
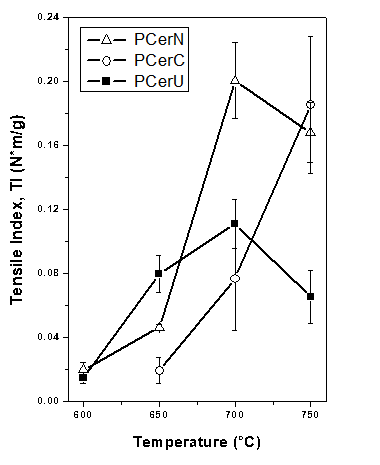
Fig. 5. Tensile Index of ceramic papers
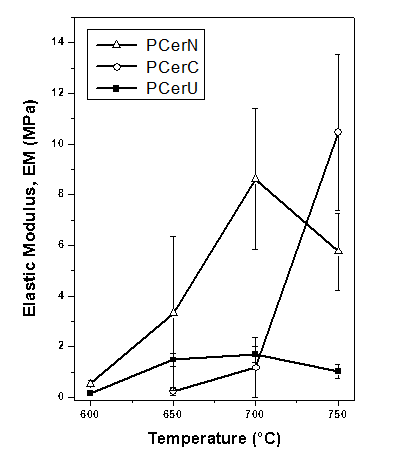
Fig. 6. Elastic modulus (EM) of different ceramic calcined at different temperatures papers calcined at different temperatures
Figure 6 shows the elastic modulus as a function of the calcination temperature. Nobleite- and colemanite-prepared papers exhibited the highest elastic modulus values (higher rigidity) especially after high calcination temperatures (700 and 750ºC). On the contrary, papers prepared with ulexite (PCerU) presented low rigidity with similar values over the whole temperature range.
Zeolite Quantification
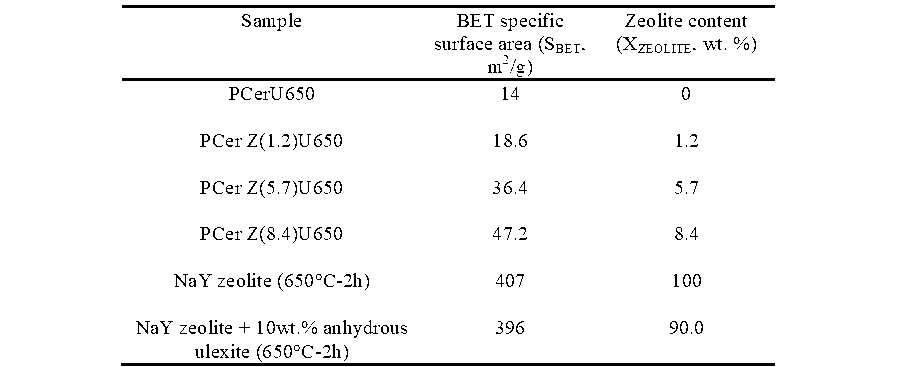
The zeolite content in the zeolitic ceramic papers was estimated from BET specific surface area values (shown in Table 1) and considering Equation 3, as suggested by Vu et al. (2002),
SBET,PCerZ(x)U650 = SBET,ZEOLITE * XZEOLITE + SBET,PCerU650 * (1-XZEOLITE) (3)
where SBET,PCerU650 corresponds to the specific surface area of the ceramic fiber, which constitutes the main compound of the calcined ceramic paper without zeolite and XZEOLITE is the weight fraction of zeolite.
As expected, the increase in the amount of zeolite NaY added during paper manufacturing was reflected in a higher content of faujasite in the zeolitic ceramic paper (Table 1), despite the intrinsic error inherent in the BET test.
In order to check that the addition of borate did not block faujasite micropores and hence did not affect the zeolite surface area, a mechanic mixture of NaY zeolite and anhydrous ulexite (10 wt.%) was prepared. The specific surface area obtained for this
mixture after calcination at 650°C for 2h was the same as that for the calcined faujasite powder. This means that the results of the zeolitic content quantification in the ceramic papers are reliable.
Table 1. Zeolite Quantification from BET
Effect of Zeolite Addition on Mechanical Properties
For the preparation of a ceramic paper containing zeolite, the calcination temperature should be limited to the lowest possible value in order to preserve its intrinsic properties (crystallinity, pore volume, etc.). As discussed above, the 650ºC value can be considered an acceptable calcination temperature if the NaY zeolite is considered. For this temperature, Figs. 5 and 6 show that good tensile index and elastic modulus values were obtained for PCerU. For this reason, the addition of NaY zeolite was analyzed for a paper formulation based on ulexite and calcined at 650ºC.
Figure 7 comparatively shows the tensile strength of ceramic papers with different contents of Y zeolite. As it can be expected, paper strength was affected by the presence of zeolite. The TI value was reduced by ca. 64 % when 5.7 wt.% of zeolite was incorporated into the ceramic paper. Nevertheless, the addition of 1.2 wt.% of zeolite (PCerZ(1.2)U650) did not affect paper strength. Figure 8 shows the effect of the addition of zeolite on the Elastic Module, and the effect was similar to that shown in the tensile strength, but more pronounced in the case of the ceramic paper containing 5.7 wt.% of NaY zeolite.
Balancing the paper strength and the zeolite loading, paper PCerZ(1.2)U650 resulted in paper that was easy to handle and permitted rolling and folding without breaking, which emphasizes its potential practical use.
Thermal Behavior of Ceramic Papers
The thermograms of the ceramic papers containing zeolite showed two weight loss processes (Fig. 9): The first was caused by the removal of water and the other was due to the destruction of the cellulose.
Fig. 7. Effect of the addition of Y zeolite on the tensile index of ceramic papers
Fig. 8. Elastic modulus (EM) of zeolite ceramic papers
Fig. 9. Thermal behavior of zeolitic ceramic papers
Fig. 10. SDTA profiles of PCerU
The DTA analysis (Fig. 10) is only shown for PCerU650 (similar profiles were obtained for the other papers, not shown) and has two exothermic peaks: one at 341°C related to the carbonization and thermal oxidation of the cellulose and a second peak at 469°C attributed to the oxidation of coal.
The retention of inorganic materials was estimated from the ash content of the papers after treatment of the samples above 900°C and it was referred to their dry weights. Table 1 shows values of retention of inorganic solids (theoretical and practical values) and they were much closer to similar published values (Ichiura et al. 2001). The destruction of the zeolitic matrix at temperatures higher than 750°C, detected while determining textural properties, was not evidenced in the TGA curves (Fig. 9).
Ceramic Paper Morphology
Figure 11 shows the SEM micrographs of the ceramic papers containing the three studied borate compounds and calcined at the two extreme calcination temperatures analyzed (600 and 750°C).
In the case of the ceramic papers prepared using colemanite as binder (PCerC), the calcination at 600°C caused the colemanite to be deposited on the ceramic fibers without sintering (Fig. 11a). The calcination at 750°C (Fig. 11b) did not affect the morphology observed after calcination at 600°C. This could be associated with the fast loss of water observed in the TGA experiments for the colemanite, which makes colemanite crystal matrix grow quickly due to uneven stresses. Correspondingly, the SEM pictures show colemanite particles with holes, caused by the fast dehydration process. In addition, these tensions produced fractures and fragmentation of the borate crystals. The porosity of the structure also increased with the calcination temperature (Celik and Suner 1995).
The addition of nobleite (PCerN600, Fig. 11c) produced effects on the ceramic paper similar to those produced by colemanite after calcination at 600°C. That is, nobleite particles were deposited on ceramic fibers without any sintering effect. After calcination at 750°C (Fig. 11d), individual particles began to sinter but without notably enhancing the paper quality, as previously observed in the mechanical tests. This could be attributed to the fact that temperatures higher than 1050°C are needed to melt calcium borates. Probably, calcinations at higher temperatures could result in better mechanical properties.
On the other hand, papers prepared with ulexite after calcination at 600°C (Fig. 11e) show that ulexite particles could bind ceramic fibers, and that this effect was pronounced after calcination at 750°C (Fig. 11f). The benefit effect of ulexite was clearly shown, where groups of ceramic fibers appeared to be joined by ulexite particles. As previously reported (Flores and Valdez 2007), after calcination at temperatures higher than 600°C, ulexite particles begin to agglomerate weakly, maintaining their individuality and shape. Increasing temperatures make this union stronger, ulexite taking the appearance of a sinter and thus enhancing the mechanical properties of ceramic papers.
Fig. 13. Morphology of ceramic papers prepared using ulexite as binder with different Y zeolite content. a) PCerU650, b) PCerZ(1.2)U650, c) PCerZ(5.7)U650, and d) PCerZ(8.4)U650
Fig. 14. Effect of borate compounds addition on paper flexibility. a) Ceramic paper prepared without the addition of binder, b) and c) Ceramic paper prepared with the addition of borates (PCerU650), and d) “Trees” cut from the ceramic with (left) and without (right) borates
Figures 12 and 13 show how ulexite progressively bound the ceramic fibers for bare ceramic papers and for those containing Y-zeolite, respectively, as the calcination temperature increased. As it can be observed, faujasite particles deposited not only on ceramic fibers but also on ulexite. As the mechanical characterization indicated, the addition of more than 1.2 wt.% of zeolite was detrimental to paper handling. Probably, higher amounts of zeolite hindered the binder-ceramic fibers contact and thus caused a negative effect on the mechanical properties of the zeolitic ceramic papers.
To compare the easy manipulation of papers when borate compounds were added with those prepared without the binder addition, Fig. 14 shows how the sheets could be easily bent and cut. Note the smooth edges of the tree cut from the ceramic paper prepared with binder in comparison with that cut from the ceramic paper without borates.
CONCLUSIONS
- The proposed methodology for the preparation of fibrous structured catalysts has proved to be adequate and has allowed us to prepare ceramic papers with different contents of NaY zeolite. The zeolitic ceramic papers obtained here were easy to handle, such that the sheets could be bent and cut without cracking.
- The calcium borate compounds tested here were found to be suitable as ceramic binders. The mechanical properties of ceramic papers varied significantly depending on the calcium borate used as well as on the calcination temperature. Although none of the borates used melted under the calcination conditions, individual particles began to sinter as calcination temperature increased, thus joining ceramic fibers and giving ceramic papers the necessary mechanical strength properties.
- Ceramic papers prepared using nobleite and colemanite as binders exhibited the highest paper strength values after calcination at 700 to 750°C. However, although better binding properties could be expected when increasing the calcination temperature, this value is limited to the maximum temperature that NaY zeolite resists without any damage to the faujasite structure. In this way, if calcination temperature is limited to 650°C, the anhydrous ulexite provided the better strength and elasticity.
- As expected, the incorporation of zeolite adversely affected the mechanical properties of ceramic papers. However, the addition of 1.2 wt.% of NaY zeolite preserved mechanical properties.
ACKNOWLEDGMENTS
The authors wish to acknowledge the financial support received from ANPCyT, CONICET, and UNL. Thanks are also given to Román Suarez and Rubén Tarcaya from BORAX S.A. for the borates, and to Elsa Grimaldi for the English language editing.
REFERENCES CITED
Banús, E., Ulla, M., Galván, M., Zanuttini, M., Milt, V., and Miró, E., (2010). “Catalytic ceramic paper for the combustion of diesel soot,” Catal. Comm. 12(1), 46-49.
Cecchini, J., Serra, R., Barrientos, C., Ulla, M., Galván, M., and Milt, V. (2011). “Ceramic papers containing Y zeolite for toluene removal,” Micropor. Mesopor. Mater. 145, 51-58.
Celik, M., and Suner, F. (1995). “A thermodynamic analysis of the decrepitation process,” Thermochim. Acta 245, 167-174.
Erds, R., McAllister, J., and Vlisidis, A. (1961). “Nobleite, another new hydrous calcium borate from the Death Valley region, California,” Amer. Mineralogist 14, 560-570.
Flores, H., and Valdez, S., (2007). “Thermal requirements to obtain calcined and frits of ulexite,” Thermochim. Acta 452, 49-52.
Fukahori, S., Koga, H., Kitaoka, T., Tomoda, A., Suzuki R., and Wariishi, H. (2006a). “Hydrogen production from methanol using a SiC fiber-containing paper composite impregnated with Cu/ZnO catalyst,” Appl. Catal. A: General 310, 138-144.
Fukahori, S., Kitaoka, T., Tomoda, A., Suzuki, R., and Wariishi, H. (2006b). “Methanol steam reforming over paper-like composites of Cu/ZnO catalyst and ceramic fiber,” Appl. Catal. A: General 300, 155-161.
Fukahori, S., Iguchi, Y., Ichiura, H., Kitaoka, T., Tanaka, H., and Wariishi, H. (2007). “Effect of void structure of photocatalyst paper on VOC decomposition,” Chemosphere 66, 2136-2141.
Gazulla, M., Gómez, M., Orduña, M., and Silva, G. (2005). “Chemical, mineralogical and thermal characterisation of natural and synthetic borates,” Bol. Soc. Esp. Ceram. 44 (1), 21-31.
Ichiura, H., Kubota, Y., Wu, Z., and Tanaka, H. (2001). “Preparation of zeolite sheets using a papermaking technique. Part I: Dual polymer system for high retention of stock components,” J. Mater. Sci. 36, 913-917.
Ishihara, H., Koga, H., Kitaoka, T., Wariishi, H., Tomoda, A., and Suzuki, R. (2010). “Paper-structured catalyst for catalytic NOx removal from combustion exhaust gas,” Chem. Eng. Sci. 65, 208-213.
Koga, H., Fukahori, S., Kiyaoka, T., Tomoda, A., Suzuki, R., Wariishi, H. (2006). “Autothermal reforming of methanol using paper-like Cu/ZnO catalyst composites prepared by a papermaking technique,” Appl. Catal. A: General 309, 263-269.
Koga, H., Fukahori, S., Kitaoka, T., Nakamura, M., and Wariishi, H. (2008). “Paper-structured catalyst with porous fiber-network microstructure for autothermal hydrogen production,” Chem. Eng. J. 139, 408-415.
Koga, H., Umemura, Y., Ishihara, H., Kitaoka, T., Tomoda, A., Suzuki, R., and Wariishi, H. (2009). “Paper-structured fiber composites impregnated with platinum nanoparticles synthesized on a carbon fiber matrix for catalytic reduction of nitrogen oxides,” Appl. Catal. B: Environmental 90, 699-704.
SCAN standard methods SCAN-C 26:76 and SCAN-M 5:76.
Vu, D., Marquez, M., and Larsen, G. (2002). “A facile method to deposit zeolites Y and L onto cellulose fibers” Micropor. Mesopor. Mater. 55, 93-101.
Article submitted: August 1, 2012; Peer review completed: November 1, 2012; Revised version received and accepted: November 20, 2012; Published: November 27, 2012.
