Abstract
Biomaterials from olive tree pruning are an abundant agricultural residue in various Mediterranean regions. A suggested use of this residue is its separation in a main fraction (trunks and stems with diameter > 1 cm) and a residual fraction (leaves and stems with diameter 1 < cm), using biorefinery procedures. The main fraction is cooked with ethanol, giving rise to a pulp, which can be used either in paper or in bioethanol production if before pulping the main fraction is subjected to a hydrothermal treatment. Pulping with 70% ethanol concentration, 185 °C for 80 min resulted in a pulp with a yield of 46.30% and a content of holocellulose, α-cellulose, and lignin of 77.17%, 62.49%, and 21.73%, respectively. The paper sheets obtained had a breaking length of 1168 m, a burst index of 0.44 kN /g, a tear index of 2.25 mN.m2/g, and a brightness of 43.66%. The pulp converted into bioethanol (by simultaneous hydrolysis and fermentation) achieved a conversion of 70 g bioethanol/100 g potential bioethanol. The residual fraction of olive tree prunings was subjected to combustion to produce thermal energy. The heating value was 18700 kJ/kg, the flame temperature range was 1094 to 2013 ºC, and the dew point temperature range of the flue gases was 47 to 53 °C.
Download PDF
Full Article
ETHANOL PULPING AS A STAGE IN THE BIO-REFINERY OF OLIVE TREE PRUNINGS
Ana Requejo, Alejandro Rodríguez, Zoilo González, Fátima Vargas, and Luis Jiménez*
Biomaterials from olive tree pruning are an abundant agricultural residue in various Mediterranean regions. A suggested use of this residue is its separation in a main fraction (trunks and stems with diameter > 1 cm) and a residual fraction (leaves and stems with diameter 1 < cm), using biorefinery procedures. The main fraction is cooked with ethanol, giving rise to a pulp, which can be used either in paper or in bioethanol production if before pulping the main fraction is subjected to a hydrothermal treatment. Pulping with 70% ethanol concentration, 185 °C for 80 min resulted in a pulp with a yield of 46.30% and a content of holocellulose, α-cellulose, and lignin of 77.17%, 62.49%, and 21.73%, respectively. The paper sheets obtained had a breaking length of 1168 m, a burst index of 0.44 kN /g, a tear index of 2.25 mN.m2/g, and a brightness of 43.66%. The pulp converted into bioethanol (by simultaneous hydrolysis and fermentation) achieved a conversion of 70 g bioethanol/100 g potential bioethanol. The residual fraction of olive tree prunings was subjected to combustion to produce thermal energy. The heating value was 18700 kJ/kg, the flame temperature range was 1094 to 2013 ºC, and the dew point temperature range of the flue gases was 47 to 53 °C.
Keywords: Biorefinery; Olive Tree Prunings; Pulp, Paper; Bioethanol; Combustion.
Contact information: Chemical Engineering Department, Campus de Rabanales, Building Marie Curie (C-3), University of Córdoba, 14071 Córdoba, Spain. * Corresponding author: iq1jiall@uco.es
INTRODUCTION
Olive tree prunings are very abundant in the Mediterranean area, particularly in Spain. These biomaterials come from logging, which occurs every year or every two years, depending on weather conditions and the soil nature. This felling operation is necessary for suitable growth and development of trees and optimal production of olives (Pastor and Humanes 2010). The olive tree prunings are an agricultural waste which must be removed to avoid contamination of soils, plague invasion, and interference with agricultural work. Currently, olive tree prunings are removed by burning in the same cropland or by shredding the prunings and scattering them on the ground. These removal solutions are costly because they entail a lot of labour, and furthermore they are not free of problems, such as risk of fires, air pollution, plague proliferation, etc. (Sánchez et al. 2002; Rodriguez et al. 2010). For these reasons, removal of olive tree prunings is interesting, based on a biorefinery scheme of lignocellulosic materials (Ruiz et al. 2009; Rodriguez et al. 2010 López et al. 2010; Spinelli and Picchi 2010; Ballesteros et al. 2011; Cara et al. 2012), so that agricultural waste becomes an agricultural by-product, obtaining several products of commercial value, which in turn makes farms more profitable.
Lignocellulosic materials (hardwoods and softwoods; agricultural, agro-food and forestry residues; and non-wood plants) have been traditionally used for the production of cellulosic pulp for paper or other uses or for the production of heat energy (through combustion) or energy products (via gasification and pyrolysis). The cellulosic pulp industry (the most current consumer of lignocellulosic resources) now faces the problem of a scarce supply of conventional raw materials (hardwood and softwood) in several regions of the world (FAO 2012). Thus, the possibility of using alternative feedstocks at the same time, such as agricultural residues which do not have the same value as waste products, is being investigated. Furthermore, this process avoids economic losses for farmers who have to get rid of such residues.
Traditionally in the production of pulp, only the rich cellulose fraction (wood from trunks and branches) is used, discarding the use of the residual fraction (mixture of stems, branches of small size, and leaves), which normally is burned to produce energy. In addition, the main fraction, which is richer in cellulose, is only used to obtain α-cellulose, missing two important components: hemicellulose and lignin. An attempt to achieve an integral exploitation of the natural resources suggests the need to introduce new production lines to take advantage of all the components of these resources by fractioning them (Stephen et al. 2010; Hoekman et al. 2011; Romaní et al. 2011). Thus, after the separation operations of the main and residual fractions of lignocellulosic materials, and other operations of conditioning from these fractions (bark removal, chipping, removal of foreign materials, etc.), a way to achieve an integral exploitation of them consists of their processing separately. The main fraction can be subjected to a hydrothermal treatment to degrade the hemicelluloses to monomers and oligomers that may have different applications (food additives, medicines, ethanol, xylitol, furfural, etc.) (Moure et al. 2006; Quing and Wyman 2011; Ballesteros et al. 2011). Subsequently, the resulting solid fraction of the hydrothermal treatment is subjected to a suitable pulping with the purpose of separating the cellulose (used for the making pulp for paper or other uses) from the lignin, which can be used for the production of various products with high commercial value, such as resins, polyurethanes, acrylates, epoxies, composites, etc. (Rodriguez et al. 2010). Alternatively, the separated cellulose in the pulping process can be subjected to a process of simultaneous hydrolysis and fermentation to obtain bioethanol, which can be used as fuel (Wang and Cheng 2011). Moreover, the residual fraction of the raw material can be used for energy production by conversion processes of its energy such as combustion process (Overend and Wright 2008; López et al., 2010; Spinelli and Picchi, 2010; Saidur et al. 2011; Li et al. 2012).
The consecutive hydrothermal and pulping treatment gives rise to moderate amounts of monomers and oligomers from the hemicelluloses, as well as pulp for paper having worse characteristics than those obtained directly from original raw materials (Sánchez et al. 2011). This suggests the use of olive tree prunings through its separation in a main fraction and other residual fraction; the main fraction can be pulped with ethanol to obtain pulp for paper, or it may be subjected to hydrothermal treatment and subsequently to pulping with ethanol, and can use the result pulp for bioethanol production, by simultaneous hydrolysis and fermentation. The residual fraction is subjected to combustion to produce thermal energy.
EXPERIMENTAL
Raw Material and Characterization Methods
Olive tree (Olea europaea) prunings were collected in Córdoba (South of Spain). Air-dried olive tree prunings were separated into two fractions: a) a main fraction consisting of stems above 1 cm diameter, and b) a residual fraction consists of leaves and stems with a diameter less than 1 cm.
The content in holocellulose, lignin, α-cellulose, ethanol–benzene extractives, and ashes of the two fractions of olive prunings were determined in accordance with the following TAPPI standards: T-9 m-54, T-222, T-203 0S-61, T-204, and T-211, respectively.
The fiber lengths of the raw material were determined by using a projection microscope Visopan. Elemental analysis was made using the Dumas method with a Eurovector “EA 3000” in the Spectroscopy Unit at the NIR/MIR Central Service for Research Support of the University of Córdoba (Spain). The calorific values were determined according to EN/TS 14918:2005 (E) solid biofuels method and UNE 164001 EX standards (UNE Standards, 1990) by using a Parr 6200 Isoper ibol Calorimeter.
Pulping
Main fractions of the olive tree pruning samples were pulped with ethanol-water in a stainless steel reactor of 2 L (Parr Instruments Company, Moline, Illinois) fitted with two six-blade turbine impellers. The vessel was heated with an external fabric mantle to reach the operating temperature. The heating rate to reach the working temperature was 17 °C/min. Time zero was taken to be when the system reached the preset temperature. After a suitable breakdown of this main fraction of olive tree prunings to get sizes between 1 and 5 cm in length and width, respectively, and a thickness between 0.5 and 1 cm, these were mixed with ethanol and water, to achieve a liquid/solid ratio (by weight) of 8:1 (on dry basis, considering the olive tree pruning moisture as water). Concentrations of ethanol in water between 60% and 80% (w/w) were used. Operational temperatures ranged from 175 and 195 °C, and the process time ranged from 40 and 120 minutes. At the end of the treatments, the media were cooled, and the pulped solids were recovered by filtration and washed (first with ethanol-water and then with distilled water). Pulp samples were subjected to chemical analysis using the same methods indicated for the raw material.
Formation and Characterization of Paper Sheets
Paper sheets were obtained with an Enjo-F39-71 former (T205- Sp-95 method, TAPPI Standards) and analyzed for breaking length, burst index, tear index, and brightness in accordance with the following methods: T-494, T-403, T-414, and T-525, respectively.
Hydrothermal-Saccharification-Fermentation process
The production of bioethanol from the main fraction of the olive tree prunings involves a hydrothermal treatment and a subsequent pulping, followed by subjection of the resulting solid fraction (hydrothermed-pulp) to simultaneous saccharification and fermentation (SSF).
The hydrothermal treatment consisted of mixing the main fraction of olive tree prunings with water (liquid/solid ratio = 8) and heating to 196 °C for 12 min. A solid fraction obtained was pulped to 185 °C, using an ethanol concentration of 60% for 60 min. Hydrothermal treatment and pulping were carried out in the reactor described above (pulping session). SSF was performed under the following conditions: Liquid/solid ratio = 8 g/g hydrothermed pulp (oven-dry basis); enzyme/solid ratio = 10 FPU/g (oven-dry basis); and β-glucosidase/cellulose activity ratio = 5 IU/FPU. The commercial enzyme concentrates employed in this work (“Celluclast 1.5 L” cellulases from Trichoderma reesei and “Novozym” β-glucosidase from Aspergillus niger) were provided by Novozymes (Madrid, Spain), and the strain Saccharomyces cerevisiae CECT-1170 was obtained from the Spanish Collection of Type Cultures, Valencia, Spain.
The SSF was carried out in 250 mL Erlenmeyer flasks placed in an orbital shaker at 120 rpm (pH = 5). Suspension containing water and hydrothermed-pulp was autoclaved (121 ºC, 15 min), separately from the nutrients and thermostated at 35 ºC. The SSF experiment started with yeast inoculation and enzyme addition. Preparing 100 mL of media required 10 mL of inocula (leading to an initial yeast cell concentration about 1.0 g/L) and 10 mL of nutrient solution (5 g peptone/L, 3 g yeast extract/L, and 3 g malt extract/L). A given time, sample was withdrawn from the media and centrifuged (500 rpm, 5 min). Supernatant was assayed for ethanol by HPLC (Requejo et al. 2012a).
RESULTS AND DISCUSSION
Physicochemical Characterization of the Olive Prunings
Table 1 shows the results related to the content of the main components, the elemental analysis, and the heating values.
Table 1. Elemental Analysis and Components Analysis of Olive Tree Pruning
The carbon content of the main fraction of olive tree prunings was higher than other agricultural residues (from 42.5 to 46.2% for sunflower stalks, cotton stalks, wheat straw, and vine shoots). The content of carbon for the residual fraction was lower than the considered agricultural residues, except in the case of sunflower. The hydrogen content of both fractions was higher than those of sunflower stalks (5.9%) and similar to those of other agricultural residues considered (6.1 to 6.4%). The nitrogen content of the main fraction was less than the mentioned agricultural residues (0.5 to 1.3%). For the residual fraction, the nitrogen content was similar to that of wheat straw (0.5%) and lower than those of the other agricultural wastes considered. Finally, the sulfur content was low, as in the agricultural residues studied (Jimenez et al. 1991).
The holocellulose content of the olive tree prunings was higher than those agricultural residues considered (60.8 to 64.1%), higher than agro-industrial residues (60.3 and 64.1% for sunflower seed husk and olive marc), higher than eucalyptus residues (61.8%), and similar to olive stones (67.6%) and holm oak residues (66.4%). The α-cellulose content of main fraction of olive tree prunings was higher than those agricultural residues considered (37.2 to 38.4%), and higher than the residual fraction of olive tree prunings (38.48%). The lignin contents were similar to those of cotton stalks (18.3%), eucalyptus residues (17.9%), sunflower seed husk (17.3%), and olive stones (19.1%); higher than those of wheat straw (14.5%), sunflower stalks (14.1%), olive marc (13.3%), and holm oak residues (13.9%); and lower than those of vine shoots (21.6%). Extractable contents were lower than those of the materials considered (13.4 to 17.9%), except in the case of olive stones (12.2%) which was similar to the residual fraction from olive tree prunings. The ash content was lower than those of most of the materials considered (3.7 to 9.5%), and these values were similar to the percentages of olive stones, holm oak, and eucalyptus residues (1.1 to 2.4%) (Jiménez and González, 1991; Jiménez et al. 1991). Lengths of fiber of the two fractions of the olive tree prunings as well as other agricultural residues are presented in Table 2. As seen, the average length of the fibers of the main fraction of the olive tree prunings was lower than those of wheat straw and sunflower stalks, and greater than the vine shoots, which was higher than that corresponding to the residual fraction of the olive tree prunings. As shown, the main fraction had a similar fiber length to the hardwood, as Eucalyptus globulus (1.05 mm).
Table 2. Fiber Lengths of Several Agricultural Residues
Energetic Characterization of the Olive Prunings
Heating values of the two factions of the olive prunings (Table 1) were of the same magnitude as those found in the literature (Jiménez et al. 1991; Jiménez and González 1991; González et al. 2011) for different lignocellulosic materials (wheat straw: 18088 kJ/kg, sunflower stalks: 16296 kJ/kg, vine shoots: 17941 kJ/kg, cotton stalks: 17857 kJ/kg, orange tree: 18626 kJ/kg, olive stones: 19967 kJ/kg, olive marc: 21055 kJ/kg, holm oak residues: 20565 kJ/kg, and eucalyptus residues: 20184 kJ/kg).
To determine the flame temperature during the combustion of a material, the following need to be considered: a) The reagents (raw material and the necessary air), to the reference temperature (298ºC), react giving products of combustion. b) Products sharply raise its temperature until reaching the final temperature (flame temperature) c) Regarding the products of reaction and the incombustible material, the temperature will increase until the flame temperature, depending on the heat generated in the combustion. On the other hand, there are heat losses from the system, and the combustion usually takes place with an excess of air. Taking these factors into consideration, an energy balance on the basis of a kg of combustible material is as follows (Jiménez et al. 1991),
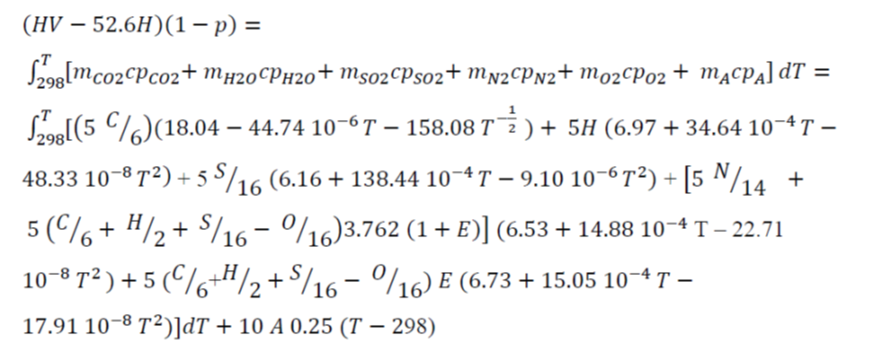
where T is flame temperature (ºC), HV is the heating values (kJ/kg), p is the heat loss (parts per unit), mCO2, mH2O, mSO2, mN2, mO2 and mA are the mol/(kg combustible material) of CO2, H2O, SO2, N2, O2, and ash, respectively; cpCO2, cpH2O, cpSO2, cpN2, cpO2, and cpA (Jiménez et al. 1991) are the molar specific heats of CO2, H2O vapor, SO2, N2, O2, and ash, respectively (J/ºC.mol). E is the air excess in combustion (parts per unit), and C, H, S, N, O, and A are the mass percentages of carbon, hydrogen, sulfur, nitrogen, oxygen, and ashes of combustible material.
The values of flame temperature were found when this equation was solved, which are presented in Table 3.
Table 3. Flame Temperature (ºC) as a Function of the Heat Losses and the Excess Air Used in the Combustion from the Olive Tree Pruning
The high values of flame temperature demonstrated the possibility of using these materials in the production of steam.
For the determination of the dew point, it is necessary to know the mole fraction of water vapor in the flue gases to find the pressure of the water vapor (in mm of Hg), by the following equation:
Tables of the water vapor pressure were used.
For a fuel whose percentages of carbon, hydrogen, sulfur, oxygen, and nitrogen, being C, H, S, O, and N, respectively, burned with an excess of air E (parts per unit), the mole fraction of vapor in the combustion gases (XH2O) is given by (Jiménez et al. 1991):
The calculated values of the dew point temperatures are presented in Table 4. As noted, these values were low, thus avoiding condensation in chimneys and exhaust fumes, as well as preventing corrosion that could result from condensation. Anyway, in the event of such condensation, the phenomenon would not be very serious, given the small sulfur content of the material considered. This is an additional advantage for which these fuels can be regarded clean.
Finally, the air/fuel ratio (AFR, kg air/kg fuel) used in the combustion of the olive tree prunings is given by Jiménez et al. (1991),
where E, C, H, S, and O have the meaning as already indicated earlier.
Table 4 also presents the values of AFR for the olive tree prunings.
Table 4. Dew Point Temperature (ºC) of the Flue Gases of Olive Prunings and Air/Fuel Ratio Used in the Combustion, Depending on the Excess Air Used
Table 5 compares the heating values, unit cost of the fuel (Requejo et al. 2012b), and cost of the heat units obtained by combustion of the different fuels. As can be seen, the units of energy obtained by combustion of olive tree prunings were cheaper than those obtained from mineral coal and even much cheaper than those obtained from fossil fuel fluids. Moreover, it should be pointed out that some of the advantages of the studied lignocellulosic residues are their renewability, the release of very small amounts of sulfur dioxide in combustion gases, and lower amounts of ash compared to the solid fossil fuel, so they can be regarded as good competitors with fossil fuels.
Table 5. Comparison of Heating Values and Energy Costs Obtained by Combustion of Various Fuels
Pulping
The main fraction of olive tree prunings is pulped with ethanol, as indicated in Experimental. Table 7-1 presents the values of the dependent variables (pulp yield, contents of holocellulose, α-cellulose and lignin, and paper sheet properties – breaking length, burst index, tear index, and brightness-) for three experiments, under the conditions that are also specified in the table. As noted, the pulp yield and lignin content decreased with an increase in values of operational variables (temperature, time, and ethanol concentration), while the contents of holocellulose and α-cellulose increased. With regard to the stretch properties of the paper sheets, the higher values correspond to the pulp obtained under median values of the operation variables. This fact may be due to a degradation of cellulose fibers caused by a high temperature (195 °C) sustained for a long time (120 min), as noted by other authors (Gilarranz et al. 1998). The brightness decreased by increasing the values of the operation variables.
Tables 7-1 to 7-4 show results found by other authors for the pulping of olive tree prunings through various processes. Comparing experimental results found for the ethanol pulping, with those provided by Jiménez et al. (2001) (Table 7-1), it is apparent that values were lower for the pulp yield and the content of α-cellulose and lignin, and higher for the content of holocellulose. This fact can be explained by the different operational procedure: in this work, the mixture of the olive tree prunings was performed by heating with ethanol and water in a reactor at 17 ºC/min, while in the data provided by Jiménez et al. (2001), the heating rate was much lower (4 °C/min), which makes the combined effect of temperature-time (severity factor) much greater and, therefore, the delignification more intense.
Results found for the soda pulping (Table 7-1, Jiménez et al. 1999) showed that for equal values of temperature and time, the yield and content of holocellulose were lower than those found for the ethanol pulping, and α-cellulose, and lignin contents were similar in both pulps (ethanol and soda), since the values of α-cellulose and lignin were similar to the values for the ethanol pulping. This indicates a higher degradation of the hemicelluloses in the soda pulping process. With regard to the values of the stretch properties and the brightness, these values were smaller for the soda pulping, except in the case of the tear index, which was higher in the soda pulping.
At the same values of temperature and time, the pulp yield in the kraft pulping (Table 7-1, Jimenez et al. 2000a) was higher than in the ethanol pulping when those values of active alkali, sulfidity, and liquid/solid ratio were low (5, 8, and 4, respectively); but if the values of these three operational variables were increased, the yields and lignin content were minor. The contents of holocellulose and α-cellulose of the ethanol pulp were greater than the corresponding kraft pulp obtained with identical values of temperature and time and low values of active alkali, sulfidity, and liquid/solid ratio. But if the values of these last three variables of operation increased, the values of the holocellulose and α-cellulose content surpassed those obtained with the ethanol process. The breaking length and burst index values were lower in the case of the ethanol process, but the brightness was greater. If refining is carried out in the kraft pulp (López et al. 2000), the values of the stretch properties of the paper sheets will increase significantly, as stated in Table 7-2.
Concerning sulfite pulping (Table 7-2, Jimenez et al. 2000b), similar conclusions to those for kraft pulping were reached. With respect to the ethanol pulping: lignin and pulp yield were lower and the holocellulose and α-cellulose were higher for the ethanol pulp compared to values for sulfite pulp; both pulps were obtained with identical values of temperature and time, low value of liquid/solid ratio and low concentrations of sulfite and anthraquinone, respectively. But when values of sulfite and anthraquinone concentra-tions, as well as the liquid/solid ratio were increased, the opposite occurred. Regarding the properties of paper sheets, it was observed that the values were higher when pulp was obtained with sulfite to high concentration and with high values of the concentration of anthraquinone and liquid/solid ratio.
Table 7-1. Characterization of Pulps and Paper Sheets Corresponding to Olive Tree Prunings Obtained by Various Pulping Processes
Table 7-2. Continuation
Table 7-3. Continuation
Table 7-4. Continuation
For low values of temperature and time (175 °C and 40 min) and for high values (185 °C and 80 min), the pulp yields were higher for the ethanol pulp, relative to the ethanolamine-soda pulp (Table 7-3, Jimenez et al. 2004a). The content of holocellulose, α-cellulose, and lignin for ethanol pulp were intermediate to the ethanolamine-soda pulp, under different conditions of concentrations of soda and ethanolamine and liquid/solid ratio. With regard to the properties of the paper sheets, the breaking length and tear index were always higher for the ethanol pulp, while burst index was always less (Jimenez et al. 2008).
Finally, by comparing the obtained results in this work with those for ethylene glycol-soda (Table 7-4, Jiménez et al. 2004c), it can be observed that the pulp yield was higher in the ethanol pulp when the temperature and time were low (175 °C and 40 min). But if the values of these operational variables were increased (185 °C and 80 min), the yield of ethanol pulp was lower. The contents of holocellulose, α-cellulose, and lignin from the ethanol pulp were checked and had intermediate values between those corresponding to low and high values of the concentrations of ethylene glycol and soda and liquid/solid ratio in the ethylene glycol-soda pulping; but when the values of temperature and time were increased, the values of holocellulose, α-cellulose, and lignin content from ethanol pulp decreased relative to the ethylene glycol-soda pulp. On the other hand, the values of the breaking length and tear index always were higher for the ethanol pulp (Jimenez et al. 2004b).
In summary, it can be concluded that the obtained ethanol pulp at a temperature of 185 °C and 80 minutes provided a greater value of the pulp yield than the pulps obtained through other procedures that operate with low or average concentrations of reagents, except in the case of the pulp obtained with the ethylene glycol-soda process. A similar conclusion can be considered for the holocellulose and α-cellulose, which had higher values than those obtained through different processes considered, operating with low or average concentrations of reagents; the opposite happened with the content of lignin, which was lower. Concerning the properties of the paper sheets of ethanol pulp, the value of the breaking length was exceeded only by the kraft pulp by the corresponding to the kraft pulp, the value of burst index was only surpassed by the ethanolamine-soda pulp, and brightness only surpassed by the value of the sulfite pulp obtained under high concentrations of reagents; the higher tear index value corresponded to the ethanol pulp.
Bioethanol Production
An alternative to the use of olive tree prunings for paper pulp production is the use of the pulp for bioethanol production. This was obtained by subjecting the pulp to the process of simultaneous hydrolysis and fermentation. The hydrolysis can be carried out with commercial enzymes and fermentation with Saccharomyces cerevisiae (Requejo et al. 2012).
To facilitate the enzymatic hydrolysis of cellulose of pulp, it is desirable to remove the hemicelluloses before the pulping through a hydrothermal treatment. In this way, the solid from hydrothermal treatment is enriched in α-cellulose, thus providing its transformation to bioethanol by simultaneous hydrolysis and fermentation.
Operating as indicated in the Experimental part, the concentration of bioethanol will grow over time to remain constant when the moment is approximately 72 hours. The maximum value entered for the concentration of bioethanol (Bmax) was 28.0 g bioethanol/L (Requejo et al. 2012a). This result is comparable with the literature reported for bioethanol production from this feedstock and is close to the threshold reported for economic feasibility (Zhang and Lynd 2010).
The bioethanol conversion (defined as “g bioethanol/100 g potential bioethanol”) is a key parameter to assess the efficiency of a given process. The bioethanol conversion (BC) can be calculated as a function of Bmax using the equation,
where Bmax is the maximum bioethanol concentration achieved in the experiment, CE is the α-cellulose content of pulp (60.9 g cellulose/100 g oven-dry pulp), 92/162 is the stoichiometric factor for bioethanol production from cellulose (g bioethanol/g cellulose), ρ is the density of liquors (average value, 1005 g/L), L/S is the liquid-to-solid ratio (fixed at 8 g/g), and Li is the lignin content of pulp (29.2 g lignin/100 g oven-dry HPS). The bioethanol conversion calculated was 70.1 g bioethanol/100 g potential bioethanol.
This result compared well with the literature and confirmed the efficiency of the scheme hydrothermal-organosolv pulping for the development of second generation bioethanol biorefineries (Garrote et al. 2008; Zhang and Lynd 2010).
CONCLUSIONS
The total use of olive tree prunings can be carried out with separation of the material into two fractions: a main fraction (trunks and stems with diameter > 1 cm) and another residual (leaves and stems with diameter 1 < cm).
Pulp can be obtained from the main fraction, which can be used for paper or bioethanol production. Pulping with ethanol to 60%, to 185 °C for 80 min obtains a pulp with a yield of 46.30% and the contents of holocellulose, α-cellulose, and lignin of 77.17%, 62.49%, and 21.73%, respectively. The paper sheets obtained had a breaking length of 1168 m, a burst index of the 0.44 kNg, a tear index of 2.25 tear mNm2g, and a brightness of 43.66%. On the other hand the main fraction may be subjected to a hydrothermal treatment and to a pulping with ethanol, and the obtained pulp being subject to a simultaneous hydrolysis and fermentation process to obtain bioethanol with a conversion of the 70 g bioethanol/100 g potential of bioethanol.
The residual fraction can be used as fuel, with a heating value of 18700 kJ/kg, a flame temperature of 1094 to 2013 ºC and a dew point temperature of combustion gases of 47 to 53ºC.
ACKNOWLEDGMENTS
The authors are grateful to Ecopapel, S.L. (Écija, Sevilla, Spain) and ENCE (Huelva, Spain) for their support, to Spain’s DGICyT for funding this research within the framework of the Projects CTQ-2010-19844-C02-01, TEP-6261 and TRA-2009-0064.
REFERENCES CITED
Ballesteros, I., Ballesteros, M., Cara, C., Sáez, F., Castro, E., Manzanares, P., Negro, M. J., and Oliva, J. M. (2011). “Effect of water extraction on sugars recovery from steam exploded olive tree pruning,” Bioresource Technology 102(11), 6611-6616.
Cara, C., Ruiz, E., Carvalheiro, F., Moura, P., Ballesteros, I., Castro, E., and Girio, F. (2012). “Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis,” Industrial Crops & Products 40, 225-231.
FAO. 2012. http://faostat.fao.org. Accessed March, 2012.
Garrote, G, Yáñez, R., Alonso, J. L., and Parajó, J. C. (2008). “Coproduction of oligosaccharides and glucose from corncobs by hydrothermal processing and enzymatic hydrolysis,” Ind. Eng. Chem. Res. 47, 1336-1345.
Gilarranz, M.A., Oliert, M., Rodríguez, F., and Tijero, L. (1998). “Ethanol water pulping. Cooking variables optimization,” Can. J. Chem. Eng. 76, 253-260.
González, Z., Rosal, A., Requejo, A., and Rodríguez, A. (2011). “Production of pulp and energy using orange tree prunings,” Bioresour. Technol. 102, 9330-9334.
Hoekman, S. K., Broch, A., and Robbins, C. (2011). “Hydrothermal carbonization (HTC) of lignocellulosic biomass,” Energy Fuels 25, 1802-1810.
Jiménez, L., Bonilla, J. L., and González, F. (1991). “Physical and chemical characterization of agricultural wastes,” Afinidad 48, 39-44.
Jiménez, L., and González, F. (1991). “Study of the physical and chemical properties of lignocellulosic residues with a view to the production of fuels,” Fuel 70, 947-950.
Jiménez, L., Pérez, I., de la Torre, M. J., and García, J. C. (1999). “The effect of processing variables won the soda pulping of olive tree wood,” Bioresour. Technol. 69, 95-102.
Jiménez, L., Pérez, I., de la Torre, M. J., and García, J. C. (2000a). “Kraft pulping of olive wood trimmings: Influence of process variables,” Tappi J. 83(5), 1-8.
Jiménez, L., Pérez, I., de la Torre, M. J., and García, J. C. (2000b). “Influence of process variables on the properties of pulp and paper sheets obtained by sulphite pulping of olive tree wood,” Wood Sci. Technol. 34, 135-149.
Jiménez, L., Pérez, I., García, J. C., and Rodríguez, A. (2001). “Influence of process variables in the ethanol pulping of olive tree trimmings,” Bioresour. Technol. 78, 63-69.
Jiménez, L., Rodríguez, A., Calero, A. M., and Eugenio, M. E. (2004a). “Use of ethanolamine-soda-water mixtures for pulping olive wood trimmings,” Chem. Eng. Res. Des. 82, 1037-1042.
Jiménez, L., Rodríguez, A., Pérez, I., Calero, A. M., and Ferrer, J. L. (2004b). “Ethylene glycol/soda organosolv pulping of olive tree trimmings,” Wood Fiber Sci. 36, (3), 423-431.
Jiménez, L., Rodríguez, A., Díaz, M. J., López, F., and Ariza, J. (2004c). “Organosolv pulping of olive tree trimmings by use of ethylene glycol/soda/water mixtures,” Holzforschung 58, 122-128.
Jiménez, L., Rodríguez, A., Serrano, L., and Moral, A. (2008). “Organosolv ethanolamine pulping of olive wood. Influence of the process variables on the strength properties,” Biochem. Eng. J. 39, 230-235.
López, F., Ariza, J., Pérez, I., and Jiménez, L. (2000). “Influence of the operating conditions on the properties of paper sheets obtained by kraft pulping of olive tree wood,” Bioresour. Technol. 72, 147-151.
López, F. J., Pinzi, S., Ruiz, J. J., López, A., and Dorado, M. P. (2010). “Economic viability of the use of olive tree pruning as fuel for heating systems in public institutions in South Spain,” Fuel 89, 1386-1391.
Li, H. N., Qun, C., Zhang, H. X., Finney, K. N., Sharifi, V., and Swithenbank, J. (2012). “Evaluation of a biomass drying process using waste heat from process industries: A case study,” Appl. Thermal Eng. 35, 71-80.
Moure, A., Gullón, P., Domínguez, H., and Parajó, J. C. (2006). “Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals,” Process Biochem. 41, 1913-1923.
Overend, R. P., and Wright, L. L. (2008). “Biomass energy,” Energy Conversion 3, 18-24.
Pastor Muñoz-Cobos, M., and Humanes Guillén, J. (1989). “Poda del olivo, moderna olivicultura,” Ed. Agrícola España, S.A. Government of Andalusia. The Andalusian Department of Fish and Agriculture.
Qing, Q., and Wyman, C. E. (2011). “Hydrolysis of different chain length xylooligomers by cellulose and hemicellulose,” Bioresour. Technol. 102, 1359-1366.
Requejo, A., Peleteiro, S., Rodríguez, A., Garrote, G., and Parajó, J. C. (2012a). “Second-genaration bioethanol from residual Woods biomass,” Energy Fuels 25(10), 4803-4810.
Requejo, A., Peleteiro, S., Garrote, G., Rodríguez, A., and Jiménez, L. (2012b). “Biorefinery of olive pruning using various processes,” Bioresour. Technol. 111, 301-307.
Rodríguez, A., Rosal, A., and Jiménez, L. (2010). “Biorefinery of agricultural residues by fractionation of their components through hydrothermal and organosolv processes,” Afinidad 67, 14-19.
Romaní, A., Garrote, G., López, F., and Parajó, J. C. (2011). “Eucalyptus globulus wood fractionation by autohydrolysis and organoslv delignification,” Bioresour. Technol. 102, 5896-5904.
Ruiz, E., Cara, C., Castro, E., Moura, P., Carvalheiro, F., and Gírio, F. (2009). “Fermentability of selected oligosaccharides from olive tree pruning by Bifidobacterium adolescentis,” New Biotechnology 25, S248-S249.
Saidur, R., Abdelaziz, E. A., Demirbas, A., Hossain, M. S., and Mekhilef, S. (2011). “A review on biomass as a fuel for boilers,” Renewable & Sustainable Energy Reviews 15(5), 2262-2289.
Sánchez, S., Moya, A. J., Moya, M., Romero, I., Torrero, R., Bravo, M., and San Miguel, M. P. (2002). “Aprovechamiento del residuo de poda del olivar mediante conversión termoquímica,” Ingeniería Química, 194-202. Diputación Provincial de Jaén (Spain).
Sánchez, R., Rodríguez, A., García, J. C., Rosal, A., and Jiménez, L. (2011). “Exploitation of hemicellulose, cellulose and lignin from Hesperaloe funifera,” Bioresour. Technol. 102, 1308-1315.
Spinelli, R., and Picchi, G. (2010). “Industrial harvesting of olive tree pruning residue for energy biomass,” Bioresource Technology 101(2), 730-735.
Stephen, J. D., Mabee, W. E., and Saddler, J. N. (2010). “Biomass logistics as a determinant of second-generation biofuel facility scale, location and technology selection,” Biofuels, Bioprod. Biorefin. 239(4), 503-518.
Wang, Z., and Cheng, J. J. (2011). “Lime pretreatment of coastal bermudagrass for bioethanol production,” Energy Fuels 25, 1830-1836.
Zhang, J., and Lynd, L. R. (2010). “Ethanol production from paper sludge by simultaneous saccharification and co-fermentation using recombinant xylose-fermenting microorganisms,” Biotechnol. Bioeng. 107, 235-244.
Article submitted: March 28, 2012; Peer review completed: May 15, 2012; Revised version received and accepted: May 23, 2012; Published: June 4, 2012.
