Abstract
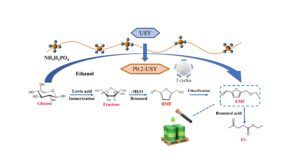
5-Ethoxymethylfurfural (EMF) can be considered as a potential biofuel because of its excellent combustion properties, such as high energy density and low carbon smoke emissions. In this study, Ultra-stable Y (USY) zeolite was modified with NH4H2PO4 and then used as an efficient solid catalyst for the catalytic synthesis of EMF via ethanolysis of glucose First, the NH4H2PO4-modified USY was characterized by FT-IR, XRD, BET, and NH3-TPD. The effect of reaction temperature, reaction time, substrate concentration, and catalyst loading on the yield of EMF was investigated. The P0.2-USY optimal EMF yield was 39.6 mol%, which increased by 20.7% compared to USY, and still had better activity after being reused for 5 cycles. Moreover, the pseudo-homogeneous first-order kinetics model was developed to elucidate the kinetics of EMF formation from glucose, and the kinetics results showed that the activation energy of EMF formation (64.2 kJ⋅mol-1) was lower than that of humins formation (73.2 kJ⋅mol-1). Finally, the ethanolysis pathway was proposed based on the product distribution.
Download PDF
Full Article
Ethanolysis of Glucose into Biofuel 5-Ethoxymethyl-Furfural Catalyzed by NH4H2PO4 Modified USY Zeolite
Yihang Chen,a Xuanyu Liang,a Kutumova Aliya,a Zhangbin Zheng,a Chao He,a Youzhou Jiao,a Hongge Tao,a Chun Chang,b and Guizhuan Xu a,c,*
5-Ethoxymethylfurfural (EMF) can be considered as a potential biofuel because of its excellent combustion properties, such as high energy density and low carbon smoke emissions. In this study, Ultra-stable Y (USY) zeolite was modified with NH4H2PO4 and then used as an efficient solid catalyst for the catalytic synthesis of EMF via ethanolysis of glucose First, the NH4H2PO4-modified USY was characterized by FT-IR, XRD, BET, and NH3-TPD. The effect of reaction temperature, reaction time, substrate concentration, and catalyst loading on the yield of EMF was investigated. The P0.2-USY optimal EMF yield was 39.6 mol%, which increased by 20.7% compared to USY, and still had better activity after being reused for 5 cycles. Moreover, the pseudo-homogeneous first-order kinetics model was developed to elucidate the kinetics of EMF formation from glucose, and the kinetics results showed that the activation energy of EMF formation (64.2 kJ⋅mol-1) was lower than that of humins formation (73.2 kJ⋅mol-1). Finally, the ethanolysis pathway was proposed based on the product distribution.
DOI: 10.15376/biores.18.2.2707-2725
Keywords: 5-Ethoxymethylfurfural; Ethanolysis; Kinetics; Pathway
Contact information: a: College of Mechanical and Electrical Engineering, Henan Agricultural University, Zhengzhou 450002, China; b: School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China; c: Henan Key Laboratory of Green Manufacturing of Biobased Chemicals, Puyang 457000, China;
* Corresponding author: xuguizhuan@henau.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
Fuels and chemicals are indispensable products for modern society, and they are mainly produced from non-renewable fossil resources (Gómez Millán et al. 2019a). With the decline of fossil resources and growing environmental problems, the development of renewable energy sources is imperative (Gómez Millán et al. 2019b; Wu et al. 2020). Lignocellulose, as an abundant and inexpensive neutral carbon source, can be converted into high-value platform chemicals and biofuels, such as xylitol, furfural, glucaric acid, 5-hydroxymethylfurfural (5-HMF), ethyl levulinate(EL), 2,5-furandicarboxylic acid (FDCA), etc. (Chen et al. 2020a; Liu and Abu-Omar 2021; Liu et al. 2021; Peixoto et al. 2021). As a new chemical and biofuel, 5-ethoxymethylfurfural (EMF) has attracted much attention. EMF has an energy density of 30.3 MJ·L-1, which is similar to that of gasoline (31.9 MJ·L-1). It has excellent combustion properties, such as high oxidative stability and low toxicity, which could reduce exhaust gases, smoke emissions, and particulate pollution (Li et al. 2016a,b; Xu et al. 2017; Liu and Wang 2019). Additionally, EMF can be used as a flavor and aroma additive in food and beverage (Liu and Wang 2019).
As an abundant and inexpensive monosaccharide, glucose is an ideal raw material for EMF production via one-pot ethanolysis. During ethanolysis, the catalyst is essential for the cascade reactions of glucose isomerization to fructose, fructose dehydration to HMF, and HMF etherification to EMF (Zheng et al. 2021; Wang et al. 2021; Wang et al. 2022a). Liquid or solid acid catalysts can be used. Although liquid acids are widely available and inexpensive, they can cause equipment corrosion and environmental pollution, and they are difficult to recover and reuse (Balakrishnan et al. 2012). Solid acid catalysts have the advantages of being separated easily from the reaction, not corroding equipment, superior recyclability, and excellent thermal stability. Thus, the application of solid acid catalysts in EMF production is a research hotspot (Zhang and Huber 2018).
Solid acid catalysts used in EMF production mainly include zeolites, heteropolyacid-based hybrid catalysts, and sulfonate functionalized catalysts, as well as some others (Wang et al. 2017; Nie et al. 2022). Zeolites are molecular sieve catalysts, which are natural or synthetic chemicals with a network structure consisting of highly dispersed microporous channels (Chen et al. 2019). However, the unique micropore structure of molecular sieves restricts the diffusion of reactants and affects the catalytic performance (Liu and Wang 2018). The modification of zeolite to change the pore size and acid sites is important for biomass conversion reactions. Bai et al. (2018) reported the application of hierarchical meso-/microporous lamellar MFI-Sn/Al zeolites in EMF production. The MFI-Sn/Al zeolites contained both Lewis acidic Sn and Al sites and Brønsted acidic Al-O(H)-Si, which catalyzes the conversion of glucose to EMF with a yield of 44%. Rao et al. (2020) produced sufficient Brønsted and Lewis acidic sites in SBA-15 by loading tin-modified heteropoly silicate (SnSTA), resulting in 41% EMF yield from glucose and 72% EMF yield from fructose. Morales et al. (2017) modified SBA-15 with aromatic sulfonic acid to catalyze the glucose conversion in a dimethyl sulfoxide (DMSO)/ethanol medium at 116 °C for 4 h, with an EMF yield of 63.4%. Although some progress has been made in the preparation of EMF from glucose using modified catalysts, the concentration of substrate is usually low (≤50 g/L), and the reaction time is long (≥ 1 h) (Morales et al. 2017). Therefore, it is important to develop efficient solid acid catalysts suitable for high substrate concentration.
The ratio of Lewis acid to Brønsted acid is an important factor in EMF selectivity (Yu et al. 2021). It might be an effective strategy to promote EMF production by regulating the ratio of Lewis / Brønsted acid of solid acid catalysts. Ultra-stable Y (USY) zeolite is an efficient solid acid catalyst for EMF synthesis (Zheng et al. 2021; Wang et al. 2022a). Modification of USY is expected to improve the EMF production from biomass. Sulfuric acid, nitric acid, phosphoric acid, and citric acid have been used to modify USY. Phosphoric acid and nitric acid adjust the micropore and mesopore distribution of USY, and citric acid increases the L acid content of USY (Heda et al. 2020; Pande et al. 2018).
Preliminary experiments showed that USY modified by ammonium dihydrogen phosphate (NH4H2PO4) has a positive effect on the ethanolysis of fructose. EMF yield was 82.3%, which was increased by 11.6% Inspired by this result, the NH4H2PO4-modified USY was generated to promote the ethanolysis of glucose for EMF production (Scheme 1). In this work, the characteristics of modified USY and its catalytic performance on the EMF formation from glucose were investigated. A pseudo-homogeneous first-order kinetics model was developed to elucidate the kinetics of EMF formation. Possible reaction pathways were proposed based on the product distribution. This study inspires EMF production by ethanolysis of lignocellulosic biomass.
Scheme 1. P0.2-USY-catalyzed glucose alcoholysis
EXPERIMENTAL
Materials
Glucose (>99%), fructose (>99%), cellulose (>99%), EL (>99%), and HMF (>98%) were purchased from Aladdin Reagent (Shanghai, China). Corn stover was provided by a local farm (Zhengzhou, China). EMF (>97%) was obtained from Sigma-Aldrich (Shanghai, China). USY (NKF-7Ⅱ) was purchased from Nankai University Catalyst Co., Ltd (Tianjin, China); the surface area, Si/Al ratio, and Na2O content of USY were 600 m2·g-1, 5.3, and 1.8 wt%, respectively. NH4H2PO4 (>99%) and ethanol with chemical purity were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Preparation of Modified USY
The modified substances were loaded on USY by the impregnation method. First, 1.0 g of USY was added to 20 mL of NH4H2PO4 aqueous solution with different concentrations. It was macerated at 25 °C for 2 h, filtered, and washed three times with deionized water. The catalyst was dried at 105 °C for 4 h. The dried catalyst was calcined at 550 °C for 4 h, cooled, and collected.
Characterization of Catalyst
A Bruker D8 Advance XRD diffractometer (Karlsruhe, Germany) was used to characterize the crystal structure of the USY solid molecular sieve. The samples were tested under Cu Kα radiation source with the following conditions: tube current of 35 mA, tube voltage of 40 kV, diffraction angle 2θ ranging from 4 to 60°, and scanning speed of 3°/min. The changes in functional groups before and after the catalyst modification were analyzed using a Fourier-transform infrared (FTIR) (iS10 Nicolet-IR 200, Madison, WI, USA) spectrometer with 4 cm−1 resolution, and the scanning range was from 400 to 4000 cm−1. The pore volume, pore size, and specific surface area of the catalysts were analyzed using a fully automated BELSORP mini II specific surface area and porosity analyzer (Osakan Kaupunki, Japan). The catalyst was characterized using a micropolitics Auto Chem II 2920 fully automated programmed temperature rise chemisorption instrument (Norcross, GA, USA). A tensor 27 Fourier infrared spectrometer (Bruker) was used to characterize the B/L acid amounts of the USY samples.
Ethanolysis of Glucose
A certain amount of glucose and catalyst were mixed with 40 mL (40% of the maximum capacity of the reactor) of anhydrous ethanol and placed in an autoclave with a magnetic stirring device, sealed, and heated. The stirring speed was kept at 250 rpm during the heating phase to mix the substrate with the solvent and catalyst. When the temperature reached the set temperature, the time was set to zero and the stirring speed was adjusted to 500 rpm. At the end of the ethanolysis reaction, the reactor was placed in a cold water bath to terminate the ethanolysis reaction. The solid and liquid phases of the reaction products were separated by vacuum filtration and the liquid phase was filtered using a 0.45 μm filter for further analysis.
Products Analysis
EMF, fructose, and glucose were quantified by high-phase liquid chromatography (HPLC) equipped with a refractive index detector (RID), HPX-87H (300 mm × 7.8 mm) with a mobile phase of 0.005 mol·L-1 sulfuric acid solution, a column temperature of 65 °C, and a flow rate of 0.6 mL/min. GC equipped with a flame ionization detector and a TG-5MS capillary column was used to test EL, and the temperature program was, the initial temperature 60 ℃, holding for 3 min, ramping up to 240 ℃ with a rate of 10 ℃/min, and then holding 8 min, and then ramping up to 280 ℃, holding for 2 min with a rate of 10 ℃/min. The inlet temperature and detector temperature were both 250 ℃, and the carrier gas was nitrogen with a rate of 1.0 mL/min. The EL was determined by the internal standard method, and 200 mg/L of n-octanol was selected as the internal standard compound to prepare EL standard solutions of different concentrations. The EL standard solutions were determined according to the above chromatographic conditions as well as the method, respectively. Under the above gas chromatographic conditions, the retention time of EL was about 8.9 min, and that of the internal standard compound n-octanol was 8.1 min. The linear regression equation was derived from the concentration ratio and peak area ratio of EL and n-octanol at different concentrations, and according to the regression equation, the EL concentration was obtained. The liquid products distribution was performed on GC-MS, with an HP-5MS capillary column (30 m × 0.32 mm × 0.50 µm), and a flame ionization detector (Agilent Technologies 7890B, USA). The temperature program was, initial temperature 40 °C for 2 min, ramping up to 220 °C at a rate of 10 °C/min for 8 min, and helium was used as carrier gas.
The conversion of glucose, the yield of EMF, and the yield of EL were calculated by Eqs. 1, 2, and 3, respectively.
RESULTS AND DISCUSSION
Screen and Characterization of Catalyst
USY zeolite was modified by different NH4H2PO4 loadings in the experiments, and the catalytic performance of USY and modified USY on the EMF yield was first investigated. Figure 1 shows that EMF yield increased with the increase of the NH4H2PO4 concentration. The USY modified by 0.2 wt.% concentration of NH4H2PO4 showed a good catalytic performance. When the NH4H2PO4 concentration was more than 0.2 wt.%, the higher concentration resulted in a lower EMF yield. Therefore, 0.2 wt.% can be used as a suitable NH4H2PO4 concentration, and the modified USY named P0.2-USY was screened as the optimal modified catalyst for further investigation.
Fig. 1. Effect of modified USY with different NH4H2PO4 on EMF yield. Reaction conditions: 60 mL ethanol, 100 g·L−1, 200 °C, 10 min, 1.8 wt.%
Fig. 2. X-ray diffraction of catalyst
USY and the modified P0.2-USY were analyzed by XRD. As shown in Fig. 2, characteristic peaks at 10.16°, 11.92°, 15.68°, 18.74°, 20.46°, 23.74°, and 27.16° were observed (Li et al. 2014). These identical peaks suggested that the structure of the catalysts did not change. There was a slight decrease in the peak height of modified P0.2-USY, which was likely due to reduced crystallinity and a partial collapse of the USY framework structure caused by calcination (Pande et al. 2018; García et al. 2016).
The physical parameters of the catalysts were analyzed by the BET method, and the results of pore volume, pore size, and specific surface area are shown in Table 1. The pore size of the USY was changed from 0.6 nm to 0.7 nm after modification. The pore volume slightly increased (1.9%), while the specific surface area was reduced by approximately 2%.
Table 1. BET Analysis Results of Catalyst
The FTIR spectra of P0.2-USY and USY are shown in Fig. 3. The catalyst showed a characteristic peak of the surface hydroxyl group between 3400 and 3450 cm-1. The peaks observed at 1045, 790, and 450 cm-1 are Si-O bonds in zeolites (Buttersack and Laketic 1994), where the peak at 1045 cm-1 is an O-Si-O asymmetric stretching vibration peak (Kim et al. 2011; Verboekend et al. 2012). There was no obvious change in the position of the characteristic peaks, indicating that the silica-alumina ratio of the molecular sieve was unchanged with the modification.
Fig. 3. FT-IR spectra of catalyst
The NH3-TPD spectra of USY and P 0.2-USY are shown in Fig. 4. The desorption peaks between 150 and 250 °C characterize the weak acid center, and the desorption peaks between 350 and 450 °C characterize the medium to strong acid center. The central temperature of the weak acid center changed from 183.2 to 197 °C after modification, and that of the strong acid center changed from 398.6 °C to 405.7 °C after the modification. The shift of temperatures indicated that P0.2-USY adsorbed ammonia more strongly after modification compared to USY, reflecting that the modified substance enhanced the acidity of USY. The peak of P0.2-USY was much higher than that of USY also confirmed the result.
Fig. 4. NH3-TPD spectra of catalyst
Py-IR results of USY and P0.2-USY are shown in Fig. 5. The absorbance peak located at 1540 cm-1 was the typical Brønsted acid peak (Fals et al. 2020), and the absorbance peak at 1450 cm-1 corresponded to Lewis acid (Oruji et al. 2018). The peak around 1490 cm-1 was formed by pyridine adsorption in Brønsted acid and Lewis acid (García et al. 2016; He et al. 2022). The ratio of Lewis/Brønsted acid increased from 0.51 to 0.82 for weak acids (200 °C), and 0.19 to 0.36 for medium strong acids (350 °C) (Table 2).
Fig. 5. Py-IR spectra of catalyst
Table 2. Py-IR Analysis Results of Catalyst
Effects of Reaction Conditions on Glucose Ethanolysis
The effects of reaction conditions on glucose ethanolysis, including EMF, fructose, EL yield, and glucose conversion, are shown in Fig 6. The effect of catalyst dosage was investigated first, and the result was shown in Fig. 6a. Within the range of catalyst dosage of 1.65% to 2.25%, glucose conversion was nearly 100%, indicating that glucose reacted thoroughly at the catalyst dosages. However, the yield of EMF and EL increased with the increase of catalyst dosage from 1.65% to 1.95% and decreased with the increase of catalyst dosage from 1.95% to 2.25%.
Fig. 6. Effect of reaction conditions on the conversion of glucose to EMF catalyzed by P0.2-USY. Reaction conditions: 60 mL ethanol. (a) Substrate concentration 100 g·L−1,200 °C, 10 min, (b) substrate concentration 100 g·L−1, 200 °C, P0.2-USY 1.95 wt%, (c) 200 °C, 10 min, P0.2-USY 1.95 wt%, (d) 10 min, substrate concentration 100 g·L−1, P0.2-USY 1.95 wt%
When 1.95% of the catalyst was used, the EMF yield increased to a maximum value of 39.6 mol% and the yield of EL increased to 12.5 mol%. Similarly, fructose content increased with the catalyst dosage increasing from 1.95% to 2.25%. The presence of fructose indicated that there was still fructose isomerized from glucose unreacted under the reaction conditions.
The effect of reaction time on glucose alcoholysis to EMF is shown in Fig. 6b. The EMF yield was approximately 32 mol% at zero time. The glucose conversion was 100% and constant with the further passage of reaction time. After a short reaction time of 10 min, the highest EMF yield of 39.6 mol% was achieved. After that, the yield of EMF decreased sharply at 15 min and maintained nearly the same value from 15 to 40 min, indicating that a short reaction time of 10 min was sufficient for EMF synthesis from glucose catalyzed by the modified USY. Furthermore, the fructose yield increased when the reaction time increased from zero to 25 min, suggesting that the isomerization of glucose to fructose was likely to have occurred within 25 min. However, the yield of EL grandly increased as the reaction time increased, indicating that the extension of reaction time was favorable for EL accumulation (Wang et al. 2022b).
The effect of substrate concentration on the reaction is shown in Fig. 6c. To realize the ethanolysis of glucose with high substrate concentration to prepare EMF, a high substrate concentration range from 100 to 140 g·L−1 was chosen for the ethanolysis experiments. As shown in Fig. 6c, both the EMF yield and glucose conversion gradually decreased with the increase of the substrate concentration, and the maximum yield of EMF and glucose conversion can be achieved at a substrate concentration of 100 g·L−1. Contrarily, the fructose content increased with the increase in substrate concentration, indicating that more fructose was produced. EL yield increased as the substrate concentration increased from 100 to 130 g·L−1. However, when the substrate concentration was 140 g·L−1, the EL yield decreased significantly, which might be caused by insufficient acid sites in the reaction system.
The effect of reaction temperature on glucose alcoholysis is illustrated in Fig. 6d. The EMF yield increased when the temperature was increased from 160 to 200 °C, and the maximum EMF yield was obtained at 200 °C. The glucose conversion was nearly constant from 160 to 200 °C. Similarly, the EL yield increased with the increase in temperature, indicating that high temperature can promote the synthesis of EL. However, the content of fructose decreased sharply as the temperature increased. Based on the above results, the optimal reaction conditions for glucose ethanolysis into EMF catalyzed by P0.2-USY were 1.95 wt.% of catalyst dosage, 100 g·L−1 of substrate concentration, reaction temperature 200 °C, and reaction time 10 min. Under the optimal reaction conditions, the maximum EMF yield was 39.6 mol%, which increased by 20.7% compared with the result catalyzed by USY (32.8 mol%).
The catalytic performance of modified Y-type zeolite (P0.2-USY) was tested using other biomass derivatives as substrates (Table 3). The EMF yield of fructose (82.3%) was significantly higher than that of glucose (39.6%). The fructose conversion to EMF is formed by Brønsted acid-driven dehydration, while the isomerization of glucose to fructose is a Lewis-catalyzed reaction and is the rate-limiting step (Wang et al. 2022a). Due to the van der Waals forces, and intermolecular hydrogen bonding that cellulose has, chemical bond breaking is more difficult (Peixoto et al. 2021). The main components of corn stover are holy carbohydrates and lignin, and the structure is difficult to destroy. P0.2-USY catalyzed corn stover can still obtain 10.8% EMF.
Table 3. Product Distribution From the Conversion of Different Substrates Over the P0.2-USY
Reusability and Characterization of P0.2-USY
The reusability of the P0.2-USY zeolite catalyst was tested by cyclic tests. After the reaction, the catalyst was filtered and recovered, dried overnight at 105 °C, and roasted at 500 °C for 4 h in a muffle furnace. As shown in Figure 7, the EMF yield was 28.6% after 5 repetitions, showing the good catalytic activity of P0.2-USY. The decrease in EMF yield may be due to the reduced P0.2-USY pore space and the loss of acid sites. The characterization of catalyst P0.2-USY-R5 was performed, and no significant changes in grain size and morphology were observed after 5 cycles (Figures 2 and 3). As shown in Fig. 4, the change in the acidity of P0.2-USY after recycling was characterized by NH3-TPD. Both the weak and strong acid peaks decreased after reused for 5 cycles of P0.2-USY, indicating the loss of acid sites. The result was also verified by Py-IR, as shown in Fig. 5. Both Lewis acid and Brønsted acid sites were reduced, which is one of the reasons for the decrease in catalytic activity. The Lewis/Brønsted ratio increased (Table 2), which is due to the loss of more Brønsted acid.
Fig. 7. Reusability of catalyst; reaction conditions: 200 °C, 100 g/, 10 min, catalyst dosage 1.95 wt%
Catalytic Pathway
To elucidate the reaction pathway of the glucose methanolysis into EMF catalyzed by P0.2-USY, GC-MS was used to identify the products, and the results are shown in Fig. 8. The identified components are shown in Table 4.
Fig. 8. GC-MS atlas of glucose products
The liquid phase products catalyzed by USY and P0.2-USY were mainly EMF, EL, 1,6-anhydro-β-D-glucose (LG), and ethyl-D-glucopyranoside (EDGP). Based on the previous reports, three reaction routes for the ethanolization of glucose to EMF were summarized, as shown in Fig. 9. In path Ⅰ, glucose was isomerized to fructose by Lewis acid, and fructose was dehydrated and converted to HMF, and then etherified to EMF, which was also the main reaction route in the process of glucose ethanolysis (Tao et al. 2021). According to path Ⅱ, glucose was firstly converted to EDGP, and then it was isomerized to ethyl-D-fructofuranoside (EDFF), which will then be etherified into EMF (Liu and Wang 2018). Besides, one can consider path Ⅲ, in which glucose isomerization to fructose is reacted with ethanol in an acidic solution to generate EDFF, then dehydration to produce EMF (Zhang et al. 2018). Based on GC-MS results (Table 4), P0.2-USY catalyzes the reaction pathway of glucose in ethanol to produce EMF in path I. P0.2-USY has a higher Lewis/ Brønsted acid ratio, which promotes the isomerization of glucose and benefits the production of EMF (He et al. 2022).
Fig. 9. The postulated reaction pathway of preparing EMF from glucose
Table 4. GC-MS Analysis Results of Glucose Ethanolysis into EMF
Kinetic Study of Glucose Conversion Reaction Catalyzed by P0.2-USY
In the ethanolysis process catalyzed by P0.2-USY, the conversion of glucose to EMF was the main reaction pathway. As discussed above, longer reaction times and higher temperature resulted in EL production and EMF decomposition, and some unknown by-products and humins were produced at the same time (Wang et al. 2022a). In this study, a pseudo-homogeneous first-order kinetics model was developed to reveal the glucose ethanolysis to EMF. As shown in Fig. 10, two parallel reactions existed in the reaction process: (1) ethanolysis of glucose to EMF and (2) conversion of glucose to unknown products and humins.
Fig. 10. Reaction kinetics model
The ethanolysis of glucose to EMF can be simplified to a first-order reaction, and the differential Eqs. 4 through 6 are obtained as follows,
where CG is the glucose concentration (g·L−1) and the initial concentration is recorded as CG0; CEMF is the concentration of EMF (g·L−1); k1 and k2 are the reaction rate constants (min-1) of EMF formation and by-products, respectively; k is the degradation reaction rate constant of glucose (min-1), and t is the reaction time (min).
By solving Eq. 6, the EMF yield can be expressed as follows,
Figure 10 shows the variation of EMF yield with time, and the kinetic parameters were fitted by MATLAB based on the data in Fig. 11. The results are listed in Table 5. The rate constant increased with the increase of the temperature, indicating that high temperature promoted the ethanolysis of glucose. The rate constant for the conversion of glucose to EMF (k1) was greater than the conversion of glucose to humins (k2). Based on the reaction rate constant and the Arrhenius equation (Eq. 8), the activation energy (Ea, kJ⋅mol−1) and exponential factor (A, min−1) of each reaction stage was obtained. R is the gas constant (8.314 J mol−1 ·K−1), and T is the temperature (K).
Fig. 11. (a) EMF yield over P0.2-USY at different temperatures. Reaction conditions: Substrate concentration 100 g·L−1, Catalyst 1.95 wt% (b) EMF yield over USY and P0.2-USY. Reaction conditions: Substrate concentration 100 g·L−1, Catalyst 1.95 wt%
The activation energies and pre-exponential factors of the reactions catalyzed by P0.2-USY are shown in Table 5. The value of Ea1 (64.2 kJ⋅mol−1) was lower than Ea2 (73.2 kJ⋅mol−1), showing that apparent activation energy for the conversion of glucose to EMF was lower than that for the conversion of glucose to humins and other by-products. This result indicated that EMF can be generated more easily than humins when P0.2-USY was used as the catalyst for glucose ethanolysis. As described in Fig. 12, the experimental data were in keeping with the theoretical data from the kinetics model, indicating that the kinetics of EMF formation was appropriately described by the kinetics model.
As shown in Table 6, the k1 with P0.2-USY (0.2945 min-1) catalyst was significantly higher than k1 with USY(0.2103 min-1) because P0.2-USY had a higher Lewis/ Brønsted acid ratio, which was more favorable for further alcoholysis of glucose isomerization to obtain EMF.
Table 5. Reaction Rate Constants and Kinetic Parameters for Glucose to EMF with P0.2-USY
Table 6. Reaction Rate Constants for Glucose to EMF with USY and P0.2-USY at 200 °C
Fig. 12. Relation between predicted and experimental EMF yields
Comparison with Literature
To evaluate the advantages of the P0.2-USY catalyst, a comparison with recent study results of EMF preparation from glucose is shown in Table 6. In the ethanol medium (Table 7, entries 1 to 4), EMF yields were in the range of 32.8 to 41% in the presence of different catalysts. In the ethanol/water medium (Table 6, entries 5-8), the EMF yields were in the range of 18 to 33% with a small amount of HMF. Furthermore, the addition of DMSO to the reaction system (Table 6, entries 9-13) resulted in a long reaction time. In comparison, the high efficiency of the ethanol/THF reaction medium (Table 6, entries 9-13) may be attributed to the stabilization effect of THF on the EMF (Zhang et al. 2020). As shown in Table 6, the P0.2-USY showed a good catalytic performance, a comparable EMF yield can be obtained under the conditions of high substrate concentration and short reaction time, which can provide a strategy for the efficient production of EMF from glucose with high concentration.
Table 7. Comparison of Different Methods for the Synthesis of EMF from Glucose
CONCLUSIONS
The one-pot ethanolysis of glucose into 5-ethoxymethyl furfural (EMF) catalyzed by NH4H2PO4 modified Ultra-stable Y (USY) zeolite P0.2-USY was investigated.
- P0.2-USY exhibited a higher Lewis/Brønsted acid ratio which was effective for cascade isomerization-dehydration-etherification reactions.
- P0.2-USY had a larger pore size and pore capacity, and it was possible to obtain a 39.6 mol% EMF yield at a high substrate concentration of 100 g/L, which is 20.7% higher than that catalyzed by USY.
- The kinetic study showed that EMF formation had a higher reaction rate and smaller energy barrier than that of by-product formation.
- P0.2-USY catalyzed fructose and corn straw can achieve better EMF yields which have good feasibility in biorefinery schemes.
- The recycling test showed that P0.2-USY remained better activity after reused for 5 cycles.
ACKNOWLEDGMENTS
This study is supported by the National Natural Science Foundation of China (U1904122; 22178328), the Program of Processing and Efficient Utilization of Biomass Resources (GZS2022007) and the Henan Provincial Science and Technology Research Project (222102320059).
Bai, Y., Wei, L., Yang, M., Chen, H., Holdren, S., Zhu, G., Tran, D. T., Yao, C., Sun, R., Pan, Y., and Liu, D. (2018). “Three-step cascade over a single catalyst: Synthesis of 5-(ethoxymethyl)furfural from glucose over a hierarchical lamellar multi-functional zeolite catalyst,” Journal of Materials Chemistry A 6(17), 7693-7705. DOI: 10.1039/c8ta01242c
Balakrishnan, M., Sacia, E. R., and Bell, A. T. (2012). “Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(Alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates,” Green Chemistry 14(6), 1626-1634. DOI: 10.1039/c2gc35102a
Buttersack, C., and Laketic, D. (1994). “Hydrolysis of sucrose by dealuminated Y-zeolites,” Journal of Molecular Catalysis 94(3), 283-290. DOI: 10.1016/0304-5102(94)00158-8
Chen, B., Xu, G., Chang, C., Zheng, Z., Wang, D., Zhang, S., Li, K., and Zou, C. (2019). “Efficient one-pot production of biofuel 5-ethoxymethylfurfural from corn stover: Optimization and kinetics,” Energy and Fuels 33(5), 4310-4321. DOI: 10.1021/acs.energyfuels.9b00357
Chen, B., Yan, G., Chen, G., Feng, Y., Zeng, X., Sun, Y., Tang, X., Lei, T., and Lin, L. (2020a). “Recent progress in the development of advanced biofuel 5-ethoxymethyl-furfural,” BMC Energy 2(1), 1-13. DOI: 10.1186/s42500-020-00012-5
Chen, Y., Guan, W., Zhang, Y., Yan, C., Li, B., Wei, Y., Pan, J., and Yan, Y. (2020b). “One-pot synthesis of the biofuel 5‑ethoxymethylfurfural from carbohydrates using a bifunctional catalyst prepared through a pickering HIPE template and pore-filled strategy,” Energy and Fuels 34(11), 14264-14274. DOI: 10.1021/acs.energyfuels.0c02942
Fals, J., García, J. R., Falco, M., and Sedran, U. (2020). “Performance of equilibrium FCC catalysts in the conversion of the SARA fractions in VGO,” Energy and Fuels 34(12), 16512-16521. DOI: 10.1021/acs.energyfuels.0c02804
García, J. R., Falco, M., and Sedran, U. (2016). “Impact of the desilication treatment of y zeolite on the catalytic cracking of bulky hydrocarbon molecules,” Topics in Catalysis 59(2-4), 268-277. DOI: 10.1007/s11244-015-0432-7
Gómez Millán, G., Hellsten, S., Llorca, J., Luque, R., Sixta, H., and Balu, A. M. (2019a). “Recent advances in the catalytic production of platform chemicals from holocellulosic biomass,” ChemCatChem 11(8), 2022-2042. DOI: 10.1002/cctc.201801843
Gómez Millán, G., Phiri, J., Mäkelä, M., Maloney, T., Balu, A. M., Pineda, A., Llorca, J., and Sixta, H. (2019b). “Furfural production in a biphasic system using a carbonaceous solid acid catalyst,” Applied Catalysis A 585. DOI: 10.1016/j.apcata.2019.117180
Guo, H., Duereh, A., Hiraga, Y., Qi, X., and Smith, R. L. (2018). “Mechanism of glucose conversion into 5-ethoxymethylfurfural in ethanol with hydrogen sulfate ionic liquid additives and a Lewis acid catalyst,” Energy and Fuels 32(8), 8411-8419. DOI: 10.1021/acs.energyfuels.8b00717
He, Y., Zhang, L., Liu, Y., Yi, S., Yu, H., Zhu, Y., and Sun, R. (2022). “Sulfated complex metal oxides solid acids with dual Brønsted-Lewis acidic property for production of 5-ethoxymethylfurfural from biomass-derived carbohydrates,” Chemical Engineering Journal 429, 132279. DOI: 10.1016/j.cej.2021.132279
Heda, J., Niphadkar, P., Mudliar, S., and Bokade, V. (2020). “Highly efficient micro-meso acidic H-USY catalyst for one step conversion of wheat straw to ethyl levulinate (biofuel additive),” Microporous and Mesoporous Materials 306, article 110474. DOI: 10.1016/j.micromeso.2020.110474
Kim, S. B., You, S. J., Kim, Y. T., Lee, S. M., Lee, H., Park, K., and Park, E. D. (2011). “Dehydration of D-xylose into furfural over H-zeolites,” Korean Journal of Chemical Engineering 28(3), 710-716. DOI: 10.1007/s11814-010-0417-y
Li, H., Yang, S., Riisager, A., Pandey, A., Sangwan, R. S., Saravanamurugan, S., and Luque, R. (2016). “Zeolite and zeotype-catalysed transformations of biofuranic compounds,” Green Chemistry 18(21), 5701-5735. DOI: 10.1039/c6gc02415g
Li, L., Quan, K., Xu, J., Liu, F., Liu, S., Yu, S., Xie, C., Zhang, B., and Ge, X. (2014). “Liquid hydrocarbon fuels from catalytic cracking of rubber seed oil using USY as catalyst,” Fuel 123, 189-193. DOI: 10.1016/j.fuel.2014.01.049
Liu, X., and Wang, R. (2018). “Upgrading of carbohydrates to the biofuel candidate 5-ethoxymethylfurfural (EMF),” International Journal of Chemical Engineering 2018, 2316939. DOI: 10.1155/2018/2316939
Liu, X., and Wang, R. (2019). “5-Ethoxymethylfurfural-a remarkable biofuel candidate,” Biomass, Biofuels, Biochemicals 355-375. DOI: 10.1016/B978-0-444-64307-0.00013-5
Liu, B., and Abu-Omar, M. M. (2021). “Lignin extraction and valorization using heterogeneous transition metal catalysts,” Advances in Inorganic Chemistry 77, 137-174. DOI: 10.1016/bs.adioch.2021.02.001
Liu, J., Zhang, C., Song, D., Guo, Y., and Leng, J. (2021). “Efficient transformation of 5-hydroxymethylfurfural to ethyl levulinate over the Brønsted acidic ionic liquid functionalized dendritic fibrous nanosilica spheres,” Microporous and Mesoporous Materials 326, 111354. DOI: 10.1016/j.micromeso.2021.111354
Morales, G., Paniagua, M., Melero, J. A., and Iglesias, J. (2017). “Efficient production of 5-ethoxymethylfurfural from fructose by sulfonic mesostructured silica using DMSO as co-solvent,” Catalysis Today 279, 305-316. DOI: 10.1016/j.cattod.2016.02.016
Mori, Y., Katayama, Y., Shikata, T., and Kasuya, N. (2020). “Synthesis of 5-ethoxy-methylfurfural from saccharides using combined metal-surfactant catalyst in ethanol/dimethyl sulfoxide,” Research on Chemical Intermediates 46(1), 609-620. DOI: 10.1007/s11164-019-03980-4
Nie, Y., Hou, Q., Qian, H., Bai, X., Xia, T., Lai, R., Yu, G., Rehman, M. L. U., and Ju, M. (2022). “Synthesis of mesoporous sulfonated carbon from chicken bones to boost rapid conversion of 5-hydroxymethylfurfural and carbohydrates to 5-ethoxymethyl-furfural,” Renewable Energy 192, 279-288. DOI: 10.1016/j.renene.2022.04.105
Oruji, S., Khoshbin, R., and Karimzadeh, R. (2018). “Preparation of hierarchical structure of Y zeolite with ultrasonic-assisted alkaline treatment method used in catalytic cracking of middle distillate cut: The effect of irradiation time,” Fuel Processing Technology 176(January), 283-295. DOI: 10.1016/j.fuproc.2018.03.035
Pande, A., Niphadkar, P., Pandare, K., and Bokade, V. (2018). “Acid modified H-USY zeolite for efficient catalytic transformation of fructose to 5-hydroxymethyl furfural (biofuel precursor) in methyl isobutyl ketone-water biphasic system,” Energy and Fuels 32(3), 3783-3791. DOI: 10.1021/acs.energyfuels.7b03684
Peixoto, A. F., Ramos, R., Moreira, M. M., Soares, O. S. G. P., Ribeiro, L. S., Pereira, M. F. R., Delerue-Matos, C., and Freire, C. (2021). “Production of ethyl levulinate fuel bioadditive from 5-hydroxymethylfurfural over sulfonic acid functionalized biochar catalysts,” Fuel 303(February), 121227. DOI: 10.1016/j.fuel.2021.121227
Srinivasa Rao, B., Dhana Lakshmi, D., Krishna Kumari, P., Rajitha, P., and Lingaiah, N. (2020). “Dehydrative etherification of carbohydrates to 5-ethoxymethylfurfural over SBA-15-supported Sn-modified heteropolysilicate catalysts,” Sustainable Energy and Fuels 4(7), 3428-3437. DOI: 10.1039/d0se00509f
Thombal, R. S., and Jadhav, V. H. (2016). “Application of glucose derived magnetic solid acid for etherification of 5-HMF to 5-EMF, dehydration of sorbitol to isosorbide, and esterification of fatty acids,” Tetrahedron Letters 57(39), 4398-4400. DOI: 10.1016/j.tetlet.2016.08.061
Tao, C., Peng, L., Zhang, J., and He, L. (2021). “Al-modified heteropolyacid facilitates alkyl levulinate production from cellulose and lignocellulosic biomass: Kinetics and mechanism studies,” Fuel Processing Technology 213 (March). DOI: 10.1016/j.fuproc.2020.106709.
Verboekend, D., Vilé, G., and Pérez-Ramírez, J. (2012). “Hierarchical y and USY zeolites designed by post-synthetic strategies,” Advanced Functional Materials 22(5), 916-928. DOI: 10.1002/adfm.201102411
Wang, J., Zhang, Z., Jin, S., and Shen, X. (2017). “Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst,” Fuel 192, 102-107. DOI: 10.1016/j.fuel.2016.12.027
Wang, S., Chen, Y., Jia, Y., Xu, G., Chang, C., Guo, Q., Tao, H., Zou, C., and Li, K. (2021). “Experimental and theoretical studies on glucose conversion in ethanol solution to 5-ethoxymethylfurfural and ethyl levulinate catalyzed by a Brønsted acid,” Physical Chemistry Chemical Physics 23, 19729-19739. DOI: 10.1039/d1cp02986j
Wang, S., Chen, Y., Jia, Y., Wang, C., Xu, G., Jiao, Y., He, C., Chang, C., and Guo, Q. (2022a). “DFT and dynamic analysis of glucose alcoholysis conversion to 5-ethoxy methylfurfural and ethyl levulinate,” Fuel 326(March), 125075. DOI: 10.1016/j.fuel.2022.125075
Wang, Y., Shi, J., Chen, X., Chen, M., Wang, J., and Yao, J. (2022b). “Ethyl levulinate production from cellulose conversion in ethanol medium over high-efficiency heteropoly acids,” Fuel 324(May). DOI: 10.1016/j.2022.124642.
Wu, Q., Zhao, B., Liu, S., Yu, S., Huang, L., and Ragauskas, A. J. (2020). “From cellulose to 1,2,4-benzenetriolviacatalytic degradation over a wood-based activated carbon catalyst,” Catalysis Science and Technology 10(10), 3423-3432. DOI: 10.1039/d0cy00424c
Xin, H., Zhang, T., Li, W., Su, M., Li, S., Shao, Q., and Ma, L. (2017). “Dehydration of glucose to 5-hydroxymethylfurfural and 5-ethoxymethylfurfural by combining Lewis and Brønsted acid,” RSC Advances 7(66), 41546-41551. DOI: 10.1039/c7ra07684c
Xu, G., Chen, B., Zheng, Z., Li, K., and Tao, H. (2017). “One-pot ethanolysis of carbohydrates to promising biofuels: 5-ethoxymethylfurfural and ethyl levulinate,” Asia-Pacific Journal of Chemical Engineering 12(4), 527-535. DOI: 10.1002/apj.2095
Yang, Y., Hu, C., and Abu-Omar, M. M. (2012). “Conversion of glucose into furans in the presence of AlCl 3 in an ethanol-water solvent system,” Bioresource Technology 116, 190-194. DOI: 10.1016/j.biortech.2012.03.126
Yu, D., Liu, X., Jiang, J., Liu, Y., Tan, J., and Li, H. (2021). “Catalytic synthesis of the biofuel 5-ethoxymethylfurfural (EMF) from biomass sugars,” Journal of Chemistry 2021, 9015481. DOI: 10.1155/2021/9015481
Yu, X., Gao, X., Peng, L., He, L., and Zhang, J. (2018). “Intensified 5-ethoxymethylfurfural production from biomass components over aluminum-based mixed-acid catalyst in co-solvent medium,” ChemistrySelect 3(47), 13391-13399. DOI: 10.1002/slct.201803059
Zhang, Z., and Huber, G. W. (2018). “Catalytic oxidation of carbohydrates into organic acids and furan chemicals,” Chemical Society Reviews 47(4), 1351-1390. DOI: 10.1039/c7cs00213k
Zhang, J., Dong, K., Luo, W., and Guan, H. (2018). “Catalytic Upgrading of Carbohydrates into 5-ethoxymethylfurfural using SO3H functionalized hyper-cross-linked polymer based carbonaceous materials,” Fuel 234(December). DOI:10.1016/j.fuel.2018.07.060
Zhang, L., Tian, L., Xi, G., Sun, R., and Zhao, X. (2020). “Catalytic valorization of expired fructan-rich food into the biofuel 5-ethoxymethylfurfural via a restaurant food waste-derived carbonaceous solid acid,” Waste and Biomass Valorization 11(11), 6223-6233. DOI: 10.1007/s12649-019-00904-6
Zhang, L., Liu, Y., Sun, R., and Yi, S. (2021). “Sulfonic acid-functionalized PCP(Cr) catalysts with Cr3+ and -SO3H sites for 5-ethoxymethylfurfural production from glucose,” RSC Advances 11(54), 33969-33979. DOI: 10.1039/d1ra05103b
Zheng, Z., Wang, C., Chen, Y., Wang, S., Guo, Q., Chang, C., Tao, H., and Xu, G. (2021). “One-pot efficient conversion of glucose into biofuel 5-ethoxymethylfurfural catalyzed by zeolite solid catalyst,” Biomass Conversion and Biorefinery. DOI: 10.1007/s13399-021-01660-1
Article submitted: November 7, 2022; Peer review completed: December 10, 2022; Revised version received: January 4, 2023; Accepted: February 5, 2023; Published: February 9, 2023.
DOI: 10.15376/biores.18.2.2707-2725
