Abstract
The combination of hemicellulose pre-hydrolysis by dilute acid or steam-explosion followed by kraft pulping was one approach evaluated in this work to generate pulps from radiata pine wood chips amenable to saccharification by enzymatic hydrolysis. Dilute acid (combined severity factor, CS = 1.67) and steam explosion (severity factor, log Ro = 4.09) treatments were able to solubilize approximately 53% and 63% of the original hemicelluloses content in wood, respectively. Extracted wood chips were subjected to kraft cooking (170 °C, 16-18% active alkali, 30% sulfidity and 1200 H-factor) to produce pulps that were further saccharified by cellulases. Lignin removal increased with increasing active alkali, affording delignification levels 28% for dilute acid and 68% for steam explosion extraction pretreatment pulps. Enzymatic digestibility of P. radiata pulps were low, and only samples pretreated by steam explosion reached glucan-to-glucose conversion near to 75%; this treatment was 31% and 37% higher than that obtained with wood chips that were pretreated by dilute acid extraction.
Download PDF
Full Article
Evaluation of Combined Dilute Acid-Kraft and Steam Explosion-Kraft Processes as Pretreatment for Enzymatic Hydrolysis of Pinus radiata Wood Chips
Pablo Reyes,a,b,* Nicole Márquez,b Eduardo Troncoso,a Carolina Parra,b Regis Teixeira Mendonça,b,c and Jaime Rodríguez b,c
The combination of hemicellulose pre-hydrolysis by dilute acid or steam-explosion followed by kraft pulping was one approach evaluated in this work to generate pulps from radiata pine wood chips amenable to saccharification by enzymatic hydrolysis. Dilute acid (combined severity factor, CS = 1.67) and steam explosion (severity factor, log Ro = 4.09) treatments were able to solubilize approximately 53% and 63% of the original hemicelluloses content in wood, respectively. Extracted wood chips were subjected to kraft cooking (170 °C, 16-18% active alkali, 30% sulfidity and 1200 H-factor) to produce pulps that were further saccharified by cellulases. Lignin removal increased with increasing active alkali, affording delignification levels 28% for dilute acid and 68% for steam explosion extraction pretreatment pulps. Enzymatic digestibility of P. radiata pulps were low, and only samples pretreated by steam explosion reached glucan-to-glucose conversion near to 75%; this treatment was 31% and 37% higher than that obtained with wood chips that were pretreated by dilute acid extraction.
Keywords: Dilute acid; Steam explosion; Hemicelluloses; Kraft pulping; Enzymatic hydrolysis
Contact information: a: Consorcio Tecnológico BioEnercel S.A., Universidad de Concepción, Casilla 160-C Chile; b: Laboratorio de Recursos Renovables, Centro de Biotecnología, Universidad de Concepción, Casilla 160-C, Chile; c: Facultad de Ciencias Forestales, Universidad de Concepción, Casilla 160-C, Chile; * Corresponding author: preyesc@udec.cl
INTRODUCTION
The need to find alternatives to the fossil fuels has opened new opportunities for the use of renewable resources, such as lignocellulosic biomass (LCB), for producing biofuels, biomaterials, and chemicals. The current approach for the use of LCB in a comprehensive manner is the biorefinery, which is a facility that integrates fractionation and conversion processes for treating LCB and its components (van Heiningen 2006).
Various fractionation methods for processing LCB have been developed in the past decades; these include: steam, acid, or alkali treatments (Cara et al. 2006; Al-Dajani et al. 2009; Kemppainen et al. 2012; Martin Sampedro et al. 2014); and organosolv, fungal, and combinations of these processes (Muñoz et al. 2007; Araque et al. 2008; Fissore et al. 2010). Enzymatic hydrolysis of pretreated materials is used for the production of fermentable sugars, which can be converted into bio-based fuels and chemicals.
LCB pretreatments disrupt and partially open up the lignocellulose structure by removing lignin and hemicelluloses. This action is required to expose cellulose to increase its saccharification during the enzymatic hydrolysis process (Mosier et al. 2005).
Cellulosic bioethanol production from sugar cane bagasse, corn, wheat, and rice straw has been extensively studied; however, it is still a challenge to produce it from forest biomass (e.g., wood chips), especially from softwood under low severity conditions. Pinus radiata is the main forest species cultivated in Chile. This pine has a high growth rate. However, the structure of P. radiata inhibits the ability of enzymes to access the carbohydrates to convert them into monosaccharides. Only organosolv pretreatment has been successfully shown to pretreat radiata pine, so it is often suggested to use it in combined treatment methods.
To achieve biomass saccharification, a combination of different pretreatments have been proposed as an alternative for improving sugar production. Recently, Reyes et al. (2015) evaluated the hemicellulose pre-extraction associated with kraft pulping as a way to produce bleachable-grade pulps. The removal of 27% of the hemicelluloses was detrimental to the strength properties and viscosities of the pulp fibers. However, this work showed that this disadvantage could be useful for other applications of the pulps, such as biofuel conversion. In this case, it would be important to evaluate the combined process as a way to overcome the recalcitrance of the lignocellulose biomass for bioethanol production. Pre-extraction hydrolysis can promote the solubilization of hemicelluloses and kraft pulping can delignify the residual material.
For the first stage of combined pretreatment, steam explosion pretreatment offers several attractive features when compared to other fractionation technologies: lower environmental impact, lower capital investment, higher energy efficiency, and fewer hazardous process chemicals and process conditions (Jacquet et al. 2012). In steam explosion pretreatment, the biomass is exposed to pressurized steam followed by rapid depressurization. This pretreatment favors the breakdown of the lignocellulosic structure, the hydrolysis of hemicelluloses, and the depolymerization of the lignin. These actions increase the pore sizes in the fiber walls. Acid-catalyzed hemicellulose hydrolysis is another pretreatment that is able to break-down the long carbohydrate chains to oligomers and monomeric sugars, and it is effective at dissolving hemicelluloses (Lavarack et al. 2002). During the aforementioned pretreatments, several products are formed, such as monosaccharides (e.g., xylose, arabinose, mannose, etc.) and dehydrated sugar products (e.g., furfural, hydroxymethylfurfural) while leaving lignin and cellulose as insoluble residues (Garrote et al. 2001).
Kraft delignification is used for the complete biomass fractionation. The objective of kraft pulping is to chemically separate the fibers in wood and dissolve most of the lignin contained in the fiber walls. Fiber separation is achieved by breaking down and dissolving the lignin in the middle lamella that holds the fibers together. The main active agents in the kraft process are hydroxide and hydrosulfide anions, which are present in the cooking liquor (Sjöström 1993). These agents cause the cleavage of structural linkages between the lignin monomer units, which in turn leads to an increase in the internal surface area and pore sizes within the fiber wall. These actions improve the accessibility of enzymes to the cellulose for saccharification.
On the basis on this literature review, it is hypothesized that the extraction of hemicelluloses with dilute acid (DA) and steam-explosion (SE) pretreatments could be a feasible alternative to combine with kraft pulping to generate a cellulosic substrate for enzymatic hydrolysis, especially when the raw material is P. radiata, which is difficult to pretreat by other methods. It is expected that the combined pretreatment may reduce the severity of the pretreatment process, thus improving cellulose saccharification. Changes in the morphology and chemical structure of the extracted wood were investigated to understand the enzymatic hydrolysis efficiency of the combined pretreatments.
EXPERIMENTAL
Raw Material
Wood chips from an 11-year old Pinus radiata D. Don tree were provided by a pulp mill located in the Biobío Province, Southern Chile. The wood chips were approximately 2.0 × 2.5 × 0.5 cm in size and were air-dried to a moisture level of 10% (w/w). The chips were stored in plastic bags at room temperature until use.
Extraction of Hemicelluloses
For the extraction of hemicelluloses, two treatments were evaluated: steam explosion and dilute acid treatment. The experimental procedures for each are described as follows.
Dilute acid treatment
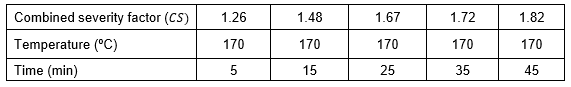
Wood chips were extracted with 1.0% w/w H2SO4 in a rotary digester (model AU/E-27 (Regmed, Brazil)) equipped with four independent 1.5 L vessels. Each vessel was loaded with 100 g of wood chips (dry basis), and 400 mL aqueous solution. The mixture was heated to 170 ºC at a rate of 2.5 ºC/min from room temperature. The cooking time ranged between 5 to 45 min at the target temperature. After each reaction, the material was cooled to room temperature and filtered to separate liquid and solid fractions. Solids were air-dried and the moisture content in the dried solids was determine. For each reaction, the combined severity factor (CS) was calculated according to Chum et al. (1990),![]()
where t is the reaction time in minutes, is the hydrolysis temperature in ºC, and is a reference temperature most often 100 ºC. The value of 14.75 is an empirical parameter related to activation energy and temperature. The pH term in Eq. (1) is the initial pH of the aqueous solution used.
Table 1. Dilute Acid Conditions
Steam Explosion
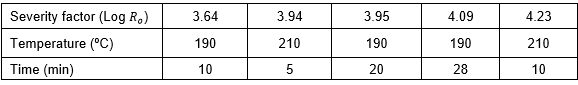
The treatment was performed in a custom-made steam-explosion system with a 5-L stainless steel reaction vessel. The vessel is connected to a blow tank where the material is discharged after the steaming reaction is completed. In each experiment, 200 g of P. radiata chips were treated at temperatures of 190 to 210 oC at residence times ranging from 5 to 28 min. The severity factor was calculated according to Overend and Chornet (1987),
![]()
where t is reaction time in minutes, is the hydrolysis temperature in ºC, and is a reference temperature most often 100 ºC. The value of 14.75 is an empirical parameter related to activation energy and temperature.
After each reaction, the steam-exploded material was recovered and filtered for liquid and solid separation. Solids were air-dried and the moisture content in the dried solids was determined. The mass of the wood chips used and of the recovered solids were used to determine the mass loss due to this pretreatment procedure.
Table 2. Steam Explosion Conditions
Kraft Delignification
Kraft pulping of pretreated wood chips was performed in a rotary digester (model AU/E-27, Regmed, Brazil) equipped with four independent 1.5 L vessels. Each vessel was loaded with 100 g of wood chips (dry basis) and 400 mL of white liquor with active alkali (AA) of 16% or 18% and 30% sulphidity (calculated on dry wood basis and expressed as NaOH equivalents). The reactor heating rate was 2.1 ºC/min from room temperature, the cooking temperature was 170 ºC, and the H-factor was 1200. After each reaction, the black liquor was drained and the pulps were washed with tap water. The total yield was determined from the measured wet mass and moisture content of the washed solid substrate. Samples were stored in plastic bags at 4 °C.
Characterization of Wood and Pulp Samples
The wood chips were milled in a knife mill and sieved through a 45/60 mesh screen. Milled wood was extracted with a 90% acetone solution for 16 h to determine the amount of extractives. Hemicellulose-extracted wood chips were also milled and sieved but not extracted with acetone. Milled wood samples were hydrolyzed with 72% sulfuric acid at 30 °C for 1 h (300 mg of sample and 3 mL of sulfuric acid). The acid was diluted to 4% (by the addition of 84 mL of water) and the mixture heated at 121 °C (1 atm) for 1 h. The residual material was cooled and filtered through porous glass filter (number 4). The solids were dried to constant weight at 105 °C and the dried mass was determined as insoluble lignin (Mendonça et al. 2008). The soluble lignin concentration in the filtrate was determined by the measurement of the absorbance at 205 nm and using the value of 110 L/g cm as absorption coefficient. The concentration of monomeric sugars in the soluble fraction was determined by high-performance liquid chromatography (HPLC) (Merck Hitachi, Germany). A BIO-RAD HPX-87H column was used with the HPLC, which was operated at 45 °C and eluted at 0.6 mL/min with 5 mM H2SO4. An IR detector was used (operated at 30 ºC).
Scanning Electron Microscopy (SEM) Analysis
Scanning electron microscope (SEM) analysis was performed using a JEOL JSM-638 LV (Joel, USA) with a voltage of 15 and 20 kV. The external morphology and orientation of the fibers was analyzed. Images were obtained at a magnification of 100X.
FT-IR Analysis
Fourier transform infrared spectroscopy (FT-IR) was performed by using a Perkin Elmer spectrometer 2000 FT-IR (USA) equipped with a triglycine sulfate detector and KBr beam splitter. The spectra (4000 to 400 cm-1) were recorded with a resolution of 4 cm-1 and 64 scans per sample. Approximately 2.0-mg samples were prepared by mixing with 120 mg of spectroscopic grade KBr then pressed to produce 13-mm-diameter pellets.
Enzymatic Hydrolysis
Enzymatic hydrolysis of pulps was performed using a cellulase mixture from Novozymes (Denmark): cellulase NS-22128 (71 FPU/mL) supplemented with β– glucosidase NS-22118 (370 CBU/mL). The enzyme dosage utilized was 20 FPU and 20 CBU per gram of pretreated material (dry weight). Each hydrolysis experiment was carried out in 125-mL Erlenmeyer flasks containing 3 g of pulp (dry weight) and 30 mL of 50 mM sodium-acetate buffer at a pH of 4.8 (final consistency of 10%). The Erlenmeyer flaks were incubated for 96 h at 50 °C, 150 rpm in a rotatory shaker. Samples were taken for sugar analysis and withdrawn after 0, 24, 48, 72, and 96 h. All experiments were run in triplicates and the average data presented. Concentrations of sugars in the supernatants were determined using the HPLC method (as mentioned above). Glucans-to-glucose conversions were calculated using a 0.9 multiplication factor to account for the loss of water from the carbohydrate polymer. The glucose yield of the substrate was calculated according to the Eq. 3.

RESULTS AND DISCUSSION
Chemical Composition
The chemical composition of P. radiata used in this work is shown in Table 3. Wood chips of P. radiata were pretreated with dilute acid (DA) and steam explosion (SE) extraction to remove hemicelluloses prior to kraft pulping. The yield of solids obtained from dilute acid extraction ranged between 76.5% and 91.2%, while the yield of solids obtained from steam explosion extraction was even lower, with values from 66.7% to 80.7%. In both extractions (DA and SE), the amount of material dissolved during the extraction increased with increasing process severity factor. The yield decrease after extractions was due to the solubilization of hemicelluloses and lignin (Leschinsky et al. 2009; Borrega et al. 2011). Long pre-hydrolysis time degraded higher amounts of hemicelluloses, thus reducing system efficiency.
Table 3. Chemical Composition of Pinus radiata Wood Chips
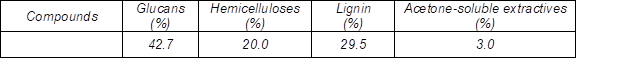
The compositions of pretreated materials after extraction (i.e., cellulose, hemicellulose, and lignin) are depicted in Fig. 1. The content of cellulose in DA-pretreated wood varied between 36.7 to 41.3%, representing a retention of glucans of 88% to 99%. The SE-pretreated material showed that there was a decrease in cellulose content during the process; the retention of glucans was between 80.6 to 94.6%, and decreased with increasing process severity factor. The dissolution of acetyl groups from the hemicelluloses in the water decreased the pH of the liquor and catalyzed extraction. As a result, most of the amorphous hemicelluloses and part of the lignin could be dissolved in water (Saha and Cotta 2008). Low concentrations of hemicelluloses are observed in the residual wood when the severity of extraction was increased. The hemicellulose content in DA pretreated material varied between 3.8 to 5.3% w/w, and in SE pretreated material the hemicellulose content was 2.8 to 10.6% w/w. Lignin did not undergo major changes during pretreatment and its retention in the solids was above 92%.
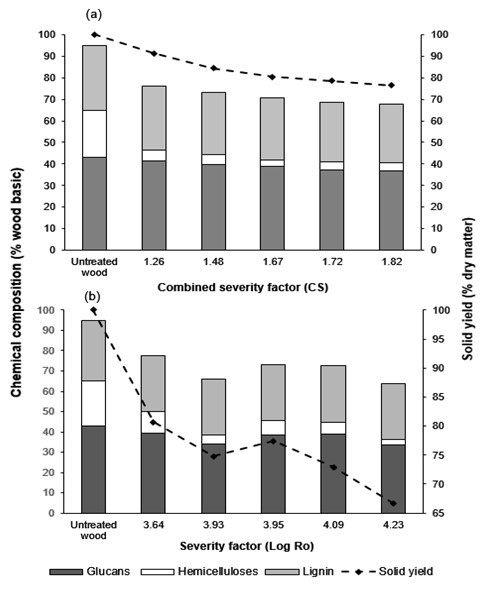
Fig. 1. Remaining glucans, hemicelluloses, and total lignin in the solid fraction from: (a) dilute acid and (b) steam explosion extraction
FT-IR Spectra
Fourier transform infrared spectroscopy (FT-IR) analysis was utilized to investigate the effects of DA-pretreatment and SE-pretreatment on the chemical structure of the biomass (Fig. 2). Characteristic assignments of hemicelluloses at 1738 cm-1, corresponding to the C=O linkages of acetyl groups and other carbonyl groups present in the branches of the hemicelluloses, decreased in intensity until disappearing when the time of extraction increased for both methods evaluated (DA and SE). This information correlated with the content of hemicelluloses determined in extracted wood (Fig. 1).
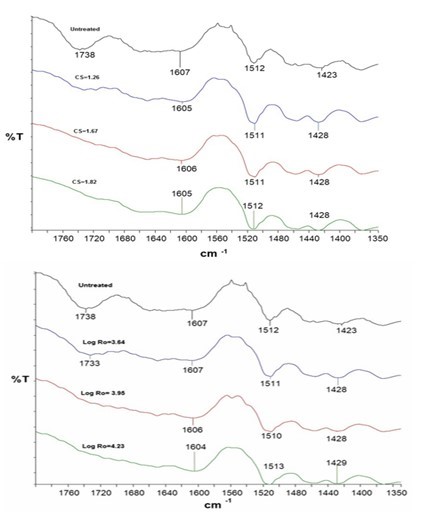
Fig. 2. Infrared spectra of extracted wood (top) dilute acid and (down) steam explosion
The region between 1607 and 1512 cm-1 in the spectra corresponded to vibrations of aromatic ring and are used to identify lignin. In DA and SE extractions, there was an increase in the intensity of bands at 1607 cm-1 as the extraction severity factor increased. This may be caused by the cleavage of β–O-4 structures in lignin. Under acidic pretreatment conditions, the predominant acidolysis reactions with lignin are the fragmentation of aryl ether linkages (e.g., a–O-4 and β–O-4 linkages) and acid-catalyzed condensation (Kumar et al. 2009; Shuai et al. 2010). However, no observations of any displacement to higher wavenumber values or an increase in the intensity of the band at 1512 cm-1 that could indicate a higher degree of condensation in the lignin.
The absorption bands at approximately 1430 cm-1 and 897 cm-1 are very sensitive to the crystalline structure of cellulose, and are often used to determine the crystallinity index (O’Connor et al. 1958). Moreover, the displacement of these bands also indicates changes to the structure of cellulose. In both methods of extraction, a displacement was observed from 1423 cm-1 of untreated wood to 1428 cm-1 for extracted wood. When this band appeared at 1420 cm-1, it was attributed to amorphous cellulose and crystalline cellulose II, whereas the 1430 cm-1 band is attributed to crystalline cellulose I.
Extracted woods have been reported to show a displacement of the band at 1430 cm-1, which indicated an increase in the ratio of crystalline-to-amorphous cellulose (Nelson and O’Connor 1964; Wang et al. 2009). Decreasing intensity at 1244/1232 cm-1 (which are attributed to C-O bending vibration in hemicelluloses) suggested that the hemicellulose content decreased, which was in accordance to the results observed from the chemical composition analyses.
Microscopic Characterization of Pretreated Material
Scanning electron microscopy (SEM) images revealed how the compact and regular surface structure of the pretreated material changed as the combined severity factor (CS) of the pretreatments increased from 1.26 to 1.82 (Fig. 3). It was clear that some cell tissues had been destroyed during the hydrolysis process, and that the connections between the fibers were loosened. Changing the fiber length, width, or lumen diameter of fiber cells in biomass will collectively affect substrate particle size, resulting in changes of the substrate external surface area. Disruption of physical and chemical interactions among cellulose, hemicelluloses and lignin can influence layer and porous structures within fiber cell walls, leading to changes in substrate internal surface area (Ju et al. 2013).
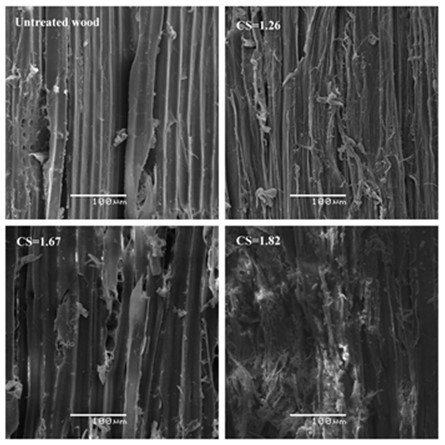
Fig. 3. Surface images obtained by SEM of P. radiata extracted with dilute acid (DA) at different combined severity factors (CS)
Figure 4 showed that SE extraction produced more noticeable physical changes in the structure of the fibers than DA, with the presence of cracks and small debris on the fiber surfaces. The sudden depressurization led to an “explosion” of the steam inside the lignocellulosic matrix, which promotes breakdown and defibrillation of its structure, hydrolysis of the hemicelluloses, and depolymerisation/repolymerization of lignin (Ruiz et al. 2008). As the severity factor of steam extraction was further increased, the SEM micrographs of the wood showed that any residual fiber structure had been lost and now had the form of an amorphous cellulosic material, which is similar to that observed by Gourlay et al. (2012). The decompression forces the fibrous material to explode into separated fibers and fiber bundles, generating a solid fraction with a more open structure (Martín-Sampedro et al. 2011), which may enhance the efficient diffusion of cooking liquor into the fibers. At low severity, the surface area shows a compact structure and the fibers are arranged in bundles and debris. With increasing treatment severity, apparent changes to the surfaces were observed (Log Ro = 4.23), which included the presence of cracks and ridges, exposure of internal cell walls, and disappearance of cohesion within the fibers.
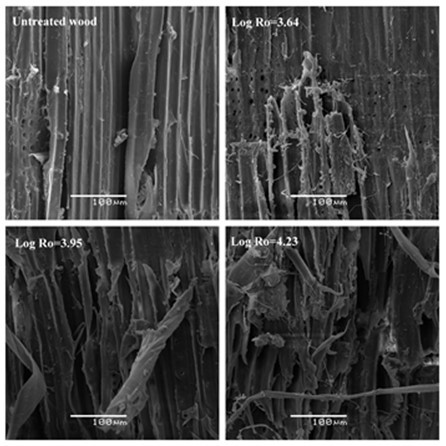
Fig. 4. Surface images of P. radiata pretreated with steam explosion (SE) obtained by SEM. Arrows indicate splits, delamination, cracks, and changes in the structure of the fibers
Kraft Delignification
One of the reaction conditions was selected to evaluate how the pre-extracted material performs under kraft delignification. P. radiata wood chips were extracted with dilute acid (DA) at 170 ºC for 25 min (CS = 1.67) and steam explosion (SE) at 190 ºC for 28 min (Log Ro 4.09). These conditions were selected because the amount of cellulose removed was low and the amounts of hemicelluloses solubilized were similar. Kraft pulp from pre-extracted chips were obtained in yields between 56% and 59% depending on the AA used and the pretreatment method used to extract the hemicelluloses (Table 4). The pulp mass balance of dilute acid/kraft (DA1.67/kp) and steam explosion/kraft (SE4.09/kp) indicated that cellulose was primarily retained in control and SE4.09/kp combined treatments. Only the DA1.67/kp combined treatment exhibited a noteworthy loss of cellulose of around 25% for both AA levels examined. Hemicelluloses were not detected in pulps generated, which indicated that the residual hemicelluloses and part of the cellulose were dissolved/degraded during the kraft pulping of the extracted chips. Lignin presented variations in the content depending of the combined pretreatment, SE4.09/kp showed delignification degrees of 47% for AA 16 and 68% for AA 18. DA1.67/kp for both AA levels showed the least amount of delignification versus SE4.09/kp, with amounts of delignification of 15% and 28% for AA16 and 18, respectively. The lower percentage of delignification for DA1.67/kp and SE4.09/kp pretreatments could indicate that lignin condensation occurred along with the acid degradation of carbohydrates in the extraction pretreatment. The condensed lignin precipitated onto the fiber and resisted delignification from wood (Borrega et al. 2011).
Table 4. Chemical Composition of Kraft Pulp after Dilute Acid and Steam Explosion Extraction on Original Wood Dry Basis
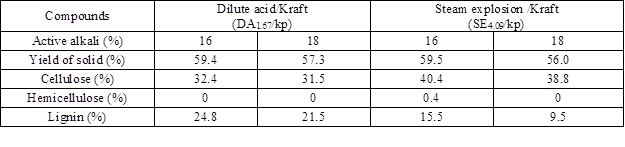
The higher degree of delignification for the SE4.09/kp also was facilitated by the greater diffusion of cooking liquor into the fibers, a consequence of the more open structure of the extracted wood of SE-pretreated. Leschinsky et al. (2008) indicate that the removal of hemicelluloses results in the loss of biomass structural integrity, improved diffusion of pulping chemicals, and faster delignification under milder conditions. The reagents penetrate the cell wall through the lumen, degrading the most accessible lignin in the secondary cell wall first and leaving the remaining lignin in the middle lamella, which is more recalcitrant (Siqueira et al. 2011).
Enzymatic Hydrolysis
The enzymatic digestibility of the various P. radiata pulps were evaluated. The conversion of glucans to glucose was monitored for 96 h of enzymatic hydrolysis (Fig. 5). The enzymatic digestibility of the SE4.09/kp treated with AA 16 and 18 showed yields of 58.4% and 75.1%, respectively, which corresponded to the low levels of lignin in these pulps. Conversely, the enzymatic digestibility of pulps produced by DA1.67/kp was very low for both AA evaluated. The conversion values varied between 38% and 44.2% for these pulps. This low percentage of conversion is probably due to the new structural features of the pretreated material, which had a higher content of residual lignin. It is known that lignin can act as a barrier and/or as an inhibitor for enzymes, making saccharification more difficult in the samples (Rahikainen et al. 2011).
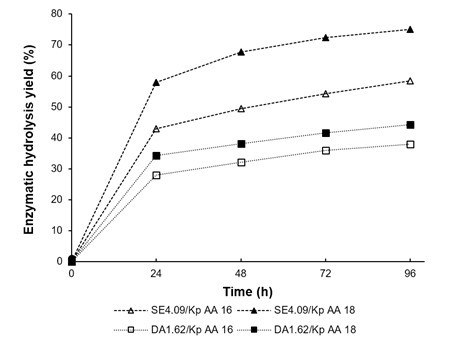
Fig. 5. Enzymatic hydrolysis yield obtained for P. radiata after dilute acid/kraft and steam explosion/kraft treatment after 96 h
Several studies showed that the lignin content negatively affects enzymatic digestibility (Alvira et al. 2010; Soares and Gouveia 2013), possibly acting as a physical barrier to restrict the access of cellulases to cellulose chains (Chang and Holtzapple 2000; Laureano-Perez et al. 2005). This is particularly true when the residual lignin remains homogeneously distributed in the fibers than when heterogeneously redistributed as droplets, which allows a better enzymatic access to the cellulose (Araya et al. 2015). The sudden depressurization applied in the reaction performed at SE4.09/kp can favor this redistribution of the lignin in the fibers in comparison with DA1.67/kp. Recently, Nakagame et al. (2010) tested the inhibitory effect of softwood, hardwood, and grass lignins in the hydrolysis of microcrystalline cellulose (Avicel) and observed that the lignin-rich hydrolysis residue from softwood had a stronger inhibitory effect on the hydrolysis than the lignins from other sources. Consequently, the inhibitory effect of lignin appears to be a major challenge in the utilization of softwood materials via enzymatic hydrolysis.
CONCLUSIONS
- Part of the labile hemicelluloses from radiata wood chips was extracted using dilute acid and steam explosion pretreatment methods. Mass balance data for cellulose retained in the extracted wood chips showed that both hemicelluloses extraction treatments solubilized a marginal fraction of the cellulose present, which enabled subsequent kraft pulping.
- Low delignification at the same AA was observed in pulps from wood chips extracted with dilutes acid versus steam explosion. This may be due to the consumption of part of the original alkali added during the cooking step by polysaccharide and lignin degradation products formed during the dilute acid extraction. The degradation of polysaccharide and lignin is a critical phenomenon. It was possible that lignin condensation occurred during the extraction pretreatments when the severities levels were high, and especially with the acidic conditions. Throughout fractionation, the first stage was considered the key process for determining the yields of biomass components.
- Glucan-to-glucose conversion in the majority of pulps was low. Only wood extracted with steam explosion carried out at AA 18 showed a percentage of conversion near to 75%; this value was 31% higher that pulp from extracted wood chips with dilute acid.
- The combination of steam explosion with kraft pulping was an effective method to remove hemicelluloses and lignin, leaving more accessible material for bioconversion to ethanol.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Consortium Bioenercel S.A, Project INNOVA-Chile 208-7320 and Project FONDECYT 1130693.
REFERENCES CITED
Araya, F., Troncoso, E., Teixeira Mendonça, R., Freer, J., Rencoret, J., and Del Río, J. C. (2015). “Structural characteristics and distribution of lignin in Eucalyptus globulus pulps obtained by a combined autohydrolysis/alkaline extraction process for enzymatic saccharification of cellulose,” J. Chil. Chem. Soc. 60(2), 2954-2960. DOI: 10.4067/S0717-97072015000200020.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Biores. Technol. 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093
Al-Dajani, W. W., Tschirner, U. W., and Jensen, T. (2009). “Pre-extraction of hemicelluloses and subsequent kraft pulping Part II: Acid- and autohydrolysis,” TAPPI J. 8(9), 30-37.
Araque, E., Parra, C., Freer, J., Rodríguez, J., Mendonça, R., Contreras, D., and Baeza, J. (2008). “Evaluation of organosolv pretreatment for the conversion of Pinus radiata D. Don to ethanol,” Enzyme Microb. Technol. 43(2), 114-119. DOI: 10.1016/j.enzmictec.2007.08.006
Borrega, M., Nieminen, K., and Sixta, H. (2011). “Effects of hot water extraction in a batch reactor on the delignification of birch wood,” BioResources 6(2), 1890-1903. DOI:10.15376/biores.6.2.1890-1903
Cara, C., Ruiz, E., Ballesteros, I., Negro, M. J., and Castro, E. (2006). “Enhanced enzymatic hydrolysis of olive tree wood by steam explosion and alkaline peroxide delignification,” Process Biochem. 41(2), 423-429. DOI: 10.1016/j.procbio.2005.07.007
Chang, V., and Holtzapple, M. (2000). “Fundamental factors affecting biomass enzymatic reactivity,” in: Twenty-First Symposium on Biotechnology for Fuels and Chemicals, Humana Press, pp. 5-37. DOI: 10.1007/978-1-4612-1392-5_1
Chum, H., Johnson, D., Black, S., and Overend, R. (1990). “Pretreatment-catalyst effects and the combined severity parameter,”Appl. Biochem. Biotechnol. 24(1), 1-14. DOI: 10.1007/BF02920229.
Fissore, A., Carrasco, L., Reyes, P., Rodríguez, J., Freer, J., and Mendonça, R. (2010). “Evaluation of a combined brown rot decay–chemical delignification process as a pretreatment for bioethanol production from Pinus radiata wood chips,” J. Ind. Microbiol. Biotechnol. 37(9), 893-900. DOI: 10.1007/s10295-010-0736-3.
Garrote, G., Dominguez, H., and Parajo, J. C. (2001). “Study on the deacetylation of hemicelluloses during the hydrothermal processing of Eucalyptus wood,” Holz Roh. Werkst. 59(1-2), 53-59. DOI: 10.1007/s001070050473.
Gourlay, K., Arantes, V., and Saddler, J. N. (2012). “Use of substructure-specific carbohydrate binding modules to track changes in cellulose accessibility and surface morphology during the amorphogenesis step of enzymatic hydrolysis,” Biotechnol. Biofuels 5(1), 1754-6834. DOI: 10.1186/1754-6834-5-51.
Jacquet, N., Vanderghem, C., Danthine, S., Quiévy, N., Blecker, C., Devaux, J., and Paquot, M. (2012). “Influence of steam explosion on physicochemical properties and hydrolysis rate of pure cellulose fibers,” Bioresour. Technol. 121, 221-227. DOI: 10.1016/j.biortech.2012.06.073
Ju, X., Grego, C., and Zhang, X. (2013). “Specific effects of fiber size and fiber swelling on biomass substrate surface area and enzymatic digestibility,” Bioresour. Technol. 144, 232-239. DOI: 10.1016/j.biortech.2013.06.100.
Kemppainen, K., Inkinen, J., Uusitalo, J., Nakari-Setälä, T., and Siika-aho, M. (2012). “Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark,” Bioresour. Technol. 117, 131-139. DOI: 10.1016/j.biortech.2012.04.080.
Kumar, R., Mago, G., Balan, V., and Wyman, C. E. (2009). “Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies,” Bioresour. Technol. 100(17), 3948-3962. DOI: 10.1016/j.biortech.2009.01.075.
Laureano-Perez, L., Teymouri, F., Alizadeh, H., and Dale, B. E. (2005). “Understanding factors that limit enzymatic hydrolysis of biomass,” Appl. Biochem. Biotechnol. 121, 1081-1099.
Lavarack, B. P., Griffin, G. J., and Rodman, D. (2002). “The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products,” Biomass Bioenerg. 23(5), 367-380. DOI: 10.1016/S0961-9534(02)00066-1.
Leschinsky, M., Sixta, H., and Patt, R. (2009). “Detailed mass balances of the autohydrolysis of Eucalyptus globulus at 170 ºC,” BioResources 4(2), 687-703. DOI: 10.15376/biores.4.2.687-703.
Leschinsky, M., Zuckerstatter, G., Weber, H. K., Patt, R., and Sixta, H. (2008). “Effect of autohydrolysis of Eucalyptus globulus wood on lignin structure. Part 1: Comparison of different lignin fractions formed during water prehydrolysis,” Holzforschung 62(6), 645-652. DOI: 10.1515/HF.2008.117.
Martin-Sampedro, R., Eugenio, M. E., Revilla, E., Martin, J. A., and Villar, J. C. (2011). “Integration of kraft pulping on a forest biorefinery by the addition of a steam explosion pretreatment,” BioResources 6(1), 513-528. DOI: 10.15376/biores.6.1.513-528.
Martin-Sampedro, R., Eugenio, M., Moreno, J., Revilla, E., and Villar, J. C. (2014). “Integration of a kraft pulping mill into a forest biorefinery: Pre-extraction of hemicellulose by steam explosion versus steam treatment,” Bioresour. Technol. 153, 236-244. DOI: 10.1016/j.biortech.2013.11.088.
Mendonca, R. T., Jara, J. F., Gonzalez, V., Elissetche, J. P., and Freer, J. (2008). “Evaluation of the white-rot fungi Ganoderma australe and Ceriporiopsis subvermispora in biotechnological applications,” J. Ind. Microbiol. Biotechnol. 35(11), 1323-1330. DOI: 10.1007/s10295-008-0414-x.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pretreatment of lignocellulosic biomass,” Bioresour. Technol. 96(6), 673-686. DOI: 10.1016/j.biortech.2004.06.025.
Muñoz, C., Mendonça, R., Baeza, J., Berlin, A., Saddler, J., and Freer, J. (2007). “Bioethanol production from bio-organosolv pulps of Pinus radiata and Acacia dealbata,” J. Chem. Technol. Biotechnol. 82(8), 767-774. DOI: 10.1002/jctb.1737.
Nakagame, S., Chandra, R. P., and Saddler, J. N. (2010). “The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis,” Biotechnol. Bioeng. 105(5), 871–879. DOI: 10.1002/bit.22626.
Nelson, M. L., and O’Connor, R. T. (1964). “Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II,” J. Appl. Polym. Sci. 8(3), 1325-1341. DOI: 10.1002/app.1964.070080323.
O`Connor, R.T., DuPré, E. F., and Mitcham, D. (1958). “Applications of infrared adsorption spectroscopy to investigations of cotton and modified cottons. Part I: Physical and crystalline and oxidation,” Text. Res. J. 28(5), 382-392. DOI: 10.1177/004051755802800503.
Overend, R. P., and Chornet, E. (1987). “Fractionation of Lignocellulosic by steam-aqueous pretreatments,” Philos. T. Roy. Soc. A. 321(1561), 523-536. DOI: 10.1098/rsta.1987.0029
Rahikainen, J., Mikander, S., Marjamaa, K., Tamminen, T., Lappas, A., Viikari, L., and Kruus, K. (2011). “Inhibition of enzymatic hydrolysis by residual lignins from softwood – Study of enzyme binding and inactivation on lignin-rich surface,” Biotechnol. Bioeng. 108(12), 2823-2834. DOI: 10.1002/bit.23242.
Reyes, P., Ferraz, A., Pereira, M., Rodríguez, L., and Mendonça, R. T. (2015). “Chemithermomechanical and kraft pulping of Pinus radiata wood chips after the hydrothermal extraction of hemicelluloses.” Holzforschung 69(1), 33-40. DOI: 10.1515/hf-2013-0235.
Ruiz, E., Cara, C., Manzanares, P., Ballesteros, M., and Castro, E. (2008). “Evaluation of steam explosion pre-treatment for enzymatic hydrolysis of sunflower stalks,” Enzyme Microb. Technol. 42(2), 160-166. DOI: 10.1016/j.enzmictec.2007.09.002.
Saha, B. C., and Cotta, M. A. (2008). “Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol,” Biomass Bioenerg. 32(10), 971-977. DOI: 10.1016/j.biombioe.2008.01.014.
Shuai, L., Yang, Q., Zhu, J. Y., Lu, F. C., Weimer, P. J., Ralph, J., and Pan, X. J. (2010). “Comparative study of SPORL and dilute-acid pretreatments of spruce for cellulosic ethanol production,” Bioresour. Technol. 101(9), 3106-3114. DOI: 10.1016/j.biortech.2009.12.044
Siqueira, G., Milagres, A. M. F., Carvalho, W., Koch, G., and Ferraz, A. (2011). “Topochemical distribution of lignin and hydroxycinnamic acids in sugar-cane cell walls and its correlation with the enzymatic hydrolysis of polysaccharides,” Biotechnol. Biofuels 4, 7. DOI: 10.1186/1754-6834-4-7.
Sjöström, E. (1993) Wood Chemistry. Fundamental and Applications, Academic Press Inc., London, UK.
Soares, M. L., and Gouveia, E.R. (2013). “Influence of the alkaline delignification on the simultaneous saccharification and fermentation (SSF) of sugar cane bagasse,” Bioresour. Technol. 147, 645-648. DOI: 10.1016/j.biortech.2013.08.103
Van Heiningen, A. (2006). “Converting a kraft pulp mill into an integrated forest biorefinery,” Pulp Pap. Can. 107(6), 38-43.
Wang, K., Jiang, J. X., Xu, F., and Sun, R. C. (2009). “Influence of steaming pressure on steam explosion pretreatment of Lespedeza stalks (Lespedeza crytobotrya): Part 1. Characteristics of degraded cellulose,” Polym. Degrad. Stabil. 94(9), 1379-1388. DOI: 10.1016/j.polymdegradstab.2009.05.019.
Article submitted: June 24, 2015; Peer review completed: October 13, 2015; Revised version received and accepted: November 7, 2015; Published: November 24, 2015.
DOI: 10.15376/biores.11.1.612-625
