Abstract
Agro-industrial waste is generated in large quantities, producing negative environmental impacts. For instance, in the distillation process of vinasses, up to 15 L are produced per alcohol produced. Therefore, it is necessary to search for ecological alternatives. Biological treatments are not recommended because vinasses contain compounds, such as melanoidins, which exert inhibitory activity against microorganisms. Thanks to this activity, melanoidins could be removed, recovered, and become a value-added product. In this study, Opuntia ficus-indica (OFI) mucilage, a natural biopolymer as coadjuvant, was used to improve the coagulation-flocculation process in the treatment of real samples of mezcal vinasses, after evaluating the individual effect of aluminum sulfate and ferric chloride. It was possible to eliminate 90% of color using ferric chloride, showing better removals than aluminum sulfate. However, the effect of ferric chloride plus OFI mucilage generated an adverse effect because the removal was under 17%. The individual effect of ferric chloride for chemical oxygen demand (COD) removal was 28%. This removal was improved by the addition of OFI mucilage, as it was able to increase removal to 84%. The natural coadjuvant was shown to be effective in the COD removal in the treatment of mezcal vinasse using the coagulation-flocculation process.
Download PDF
Full Article
Evaluation of Opuntia ficus-indica Potential as a Natural Coadjuvant for Vinasse Treatment
Crisel A. Mejía-Rivas,a Ana M. Bailón-Salas,b,δ Luis A. De la Peña-Arellano,a María D. J. Rodríguez-Rosales,a and Luis A. Ordaz-Díaz c,*
Agro-industrial waste is generated in large quantities, producing negative environmental impacts. For instance, in the distillation process of vinasses, up to 15 L are produced per alcohol produced. Therefore, it is necessary to search for ecological alternatives. Biological treatments are not recommended because vinasses contain compounds, such as melanoidins, which exert inhibitory activity against microorganisms. Thanks to this activity, melanoidins could be removed, recovered, and become a value-added product. In this study, Opuntia ficus-indica (OFI) mucilage, a natural biopolymer as coadjuvant, was used to improve the coagulation-flocculation process in the treatment of real samples of mezcal vinasses, after evaluating the individual effect of aluminum sulfate and ferric chloride. It was possible to eliminate 90% of color using ferric chloride, showing better removals than aluminum sulfate. However, the effect of ferric chloride plus OFI mucilage generated an adverse effect because the removal was under 17%. The individual effect of ferric chloride for chemical oxygen demand (COD) removal was 28%. This removal was improved by the addition of OFI mucilage, as it was able to increase removal to 84%. The natural coadjuvant was shown to be effective in the COD removal in the treatment of mezcal vinasse using the coagulation-flocculation process.
Keywords: Mezcal vinasse; Agro-industrial waste; Biopolymer: Coagulation-flocculation; Mucilage
Contact information: a: Chemical and Biochemical Engineering Department, Durango Institute of Technology (ITD), Blvd. Felipe Pescador 1830 Ote. Col. Nueva Vizcaya, 34080, Durango, Dgo., México; b: Facultad de Ciencias Forestales, Universidad Juarez del Estado de Durango, Río Papaloapan, Valle del Sur, 34120 Durango, Dgo., México; c: Environmental Engineering Technology, Universidad Politécnica de Durango, Carretera Dgo-México, km 9.5, Col. Dolores Hidalgo, Durango, Dgo. México; δ: Postdoctoral student; *Corresponding author: luis.ordaz@unipolidgo.edu.mx
GRAPHICAL ABSTRACT
INTRODUCTION
Agro-industrial wastes are produced worldwide in large quantities (approximately a thousand million tons per year), and their disposal and treatment present an environmental problem (Knob et al. 2014; Ahmed et al. 2020). The main liquid agroindustrial waste resulting from the production of bioethanol is called vinasses (Ahmed et al. 2020). For instance, the Mezcal is one of Mexico’s most representative products internationally (Secretaria de Economía 2015). The distillation stage generates up to 10 to 15 L of vinasse per liter of ethanol (García et al. 1997; Moraes et al. 2015). According to the COMERCAM (2018), 5,089,667 L of mezcal was produced in Mexico. This is equivalent to generating more than 76 million liters of mezcal vinasse.
The vinasse produces negative environmental impacts. The disposal of this untreated waste in water-bodies and soil represents a potential risk (dos Santos et al. 2019). Therefore, its treatment with developing ecological methods is necessary (Christofoletti et al. 2013).
Anaerobic, aerobic, and physicochemical processes have been used for vinasses treatment. However, the treatments have disadvantages, due to requiring dilution or pre-treatment for removing color (España-Gamboa et al. 2011). The phenolic compounds and melanoidins present in the vinasses possess antimicrobial activity, and they are toxic (Borja et al. 1993; Rufián-Henares and Morales 2007; Bouarab-Chibane et al. 2019). Johnson et al. (2019) reported that conventional biological processes are not efficient for the removal of melanoidins because they can only remove up to 7%. Morever, due to their microbial inhibiting activity, the color associated with these two compounds must be removed before using a biological process. Furthermore, there is the option of the recovery of melanoidins, which could become value-added products (Kaushik et al. 2018).
Within the physicochemical processes, coagulation and flocculation are the most common and economically viable (Matilainen et al. 2010). Additionally, they are effective in removing particulates, natural organic matters, ions, and others (Teh and Wu 2014). However, some chemical reagents used are considered expensive (Barrera-Díaz et al. 2018). Therefore, the search for natural alternatives that reduce treatment costs is fundamental to treat water systems without generating other pollution sources (Gomez-Maldonado et al. 2019). Natural polymers, such as polysaccharides, are widely available in nature and are highly biodegradable (Maćczak et al. 2020). Biopolymers have been explored, such as chitosan (Ferral-Pérez et al. 2016; Poznyak et al. 2019), a natural tannin-based flocculant (de Souza et al. 2013; Junior et al. 2019) for contaminants removal from vinasses. There are other natural polymers from agro-industrial sources to improve the flocculation efficiency. For instance, Opuntia ficus-indica (OFI) has been used to remove the dye, pesticides, turbidity, chemical oxygen demand (COD), and heavy metals from stagnant water port, cosmetic industry, municipal sewage, tannery, textile, paint industry, jeans laundry, and fabric dyeing mesh wastewaters (Nharingo and Moyo 2016). Opuntia ficus-indica is widely distributed and grows in various dry and semi-dry countries (Pimienta-Barrios and del Castillo 2002). Carbohydrates as l-arabinose, d-galactose, l-rhamnose, and d-xylose are present in OFI and are named mucilage (Sáenz et al. 2004). The considerable amount of these carbohydrates confers the ability of flocculation and/or coagulation (Othmani et al. 2020). Furthermore, this hydrocolloid has several advantages due to being relatively cheap, biodegradable, and non-toxic (Razavi 2019). However, it has not been studied for the removal of contaminants in vinasses.
The aim of this work was to study the effectiveness of a biopolymer based on OFI to eliminate the color and the COD present in mezcal vinasse as coadjuvant in the coagulation and flocculation process.
EXPERIMENTAL
Materials
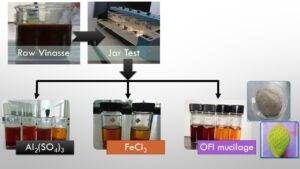
Three alternatives were analyzed, as shown in Table 1. Aluminum sulfate (Al2(SO4)3) and ferric chloride (FeCl3) were evaluated separately as coagulants. Then, based on the experimental results, the better-performing coagulant for the removal of color was chosen to evaluate its effect in combination with the coadjuvant of OFI mucilage, as alternative 3. Three levels were used for the pH factor for the alternative A and B, these levels were selected according to reported by Ryan et al. (2008). The pH for alternative 3 was kept constant, once the results of the first two alternatives were analyzed.
Table 1. Vinasse Treatment Alternatives
Methods
Collection and isolation of coadjuvant
Opuntia ficus indica (OFI) plant material was collected in the state of Durango, Mexico (24°28’36.0”N 104°04’02.7”W) in summer season. The leaves were deposited in a plastic bag and stored at 4 °C until processing. Afterward, the cuticle was removed, crushed in a blender, homogenized with water in the ratio of 1:1, and then filtered and centrifuged at 3500 rpm for 20 min (Rodríguez-González et al. 2004). The ethanolic extraction was performed according to Rivera-Corona et al. (2014) and then was dried at room temperature. Electrical conductivity (EC), pH, total solids (TS), and turbidity were determined.
Sampling and Physicochemical Characterization
The water samples were collected in a mezcal factory located in Nombre de Dios, Durango, Mexico. They were taken from an underground storage pit, and were stored at 4 °C before use.
Color, turbidity, temperature, pH, electrical conductivity (EC), chlorides, biochemical oxygen demand (BOD5), COD, TS, fixed solids (FS), volatile solids (VS), dissolved oxygen (DO), total nitrogen (TN), phosphorus (P), NH3, nitrates, sulfates, calcium, and zeta potential (ZP) were determined. Temperature, DO, EC, and pH were determined in situ with an HQ40d portable device (HACH Company, Loveland, CO, USA). The TS, FS, and VS were gravimetrically analyzed. The ZP was measured by a Zeta meter 3.0 (Zeta-Meter, Inc., Staunton, VA, USA). The BOD5 was determined by a manometric-respirometric method with a BODTrak™ II apparatus (Hach Company, Loveland, CO, USA). The COD was determined using the closed reflux (colorimetric) method. Using a photometer HI83225 (HANNA Instruments Inc., Woonsocket, RI, USA), chlorides, NH3, calcium, phosphorus, nitrates, and sulfates were determined. The color and turbidity were determined by a spectrophotometric method.
Jar Tests
With the aim of determining optimum doses, experiments were conducted by a jar test apparatus (PHIPPS & BIRD, PB-700 TM, Richmond, VA, USA) at room temperature, to compare the effectiveness of coagulation. To each jar, 500 mL of sample were added, having previously adjusted the pH with NaOH (Sigma-Aldrich Co., Darmstadt, Germany). The following conditions were used: the coagulation rate was 50 rpm and the coagulation time was a rapid agitation of 200 rpm for 5 min, a slow agitation of 100 rpm for 5 min, and a sedimentation time of 20 min.
Once the best coagulant alternative (A or B) and pH were found, alternative 3 was evaluated, OFI mucilage was added as coadjuvant until the maximum removal of the parameters was found. After settling, 20 mL of sample was taken from each vessel, and the measurement was carried out. Subsequently, color and COD were determined. All experiments were made in duplicate.
Statistical Analysis
Color removal and COD removal were selected as the response variables. Using Statistica 7 StatSoft, Inc. (Quest, Oklahoma City, OK, USA), a generalized linear model was generated for each alternative, which was validated taking into account the assumptions of normality and independence of residuals and homogeneity of variance. To determine the significant difference influences between treatments, the least significant difference (LSD) test was applied and the response surface technique was also applied.
RESULTS AND DISCUSSION
The OFI mucilage characterization indicated a pH of 5.11 ± 0.09, EC of 10.46 ± 0.75 mS/cm, TS of 5.29 ± 0.12 ppt, an ivory white color, and turbidity of 532 ± 23 NTU. The pH coincided with the natural pH of the mucilage reported by Espino-Díaz et al. (2010). The EC was close to that reported by Gebresamuel and Gebre-Mariam (2011) that characterized a species obtained from Ethiopia.
Physicochemical characterization during the mezcal vinasse sampling is shown in Table S1. It was sampled three times corresponding to the months of June, July, and August. A low DO content (0.16 to 0.23 mg/L) was due to samples being taken in the underground storage pit and the fact that the vinasse came from an anaerobic process. As is characteristic of this type of effluent, a low pH (3.94) (Cruz-Salomón et al. 2017; Robles-González et al. 2018; García-Becerra et al. 2019), similar values of EC (2.08 to 4.20 mS/cm) to those reported by Robles-González et al (2018), high organic load, however lower than those reported by Robles-González et al. (2018) and García-Becerra et al. (2019) for mezcal and tequila vinasses, respectively, were observed. There were more than 2 times higher TS than those reported in other studies for mezcal vinasse (García-Becerra et al. 2019). The volatile solids content, up to 62,890 mg/L, was close to that reported by Moran-Salazar et al. (2016) in sugarcane vinasse. The low pH, EC, and chemical compounds can modify the physicochemical properties in the receptor bodies (soil, rivers, or lakes) (Christofolett et al. 2013). Furthermore, the low pH favors the dissolution of heavy metals in soils (García et al. 1997). P, NH3, and nitrates of up to 95, 1000, and 340 mg/L, respectively were observed. High nutrient concentrations, such as phosphorus and nitrogen, can cause eutrophication in water-bodies (Vlyssides et al. 1997). A sulfates content of 200 mg/L was found, which can impair the fertility, structure, and porosity of the soil due to the high content of salts in the vinasse (Tejada et al. 2009). Additionally, turbidity of 2970 NTU and color of 3440 Pt-Co concentrations were detected. Turbidity and color associated with the presence of suspended solids and melanoidins, respectively, both of which can affect photosynthetic processes and aquatic life in water-bodies (Fitzgibbon et al. 1995). The color in the stillage, in addition to being attributed to the content of melanoidins, is due to the presence of phenolic compounds (España-Gamboa et al. 2017). Phenol is a recalcitrant compound (Othman et al. 2008) and the melanoidins are considered the most recalcitrant dye (Johnson et al. 2019). Likewise, phenolic and polyphenolic compounds in vinasses can inhibit microorganisms (Freitas et al. 2018; Ao et al. 2020).
Derived from the results obtained from the jar test, three equations were generated to explain the behavior of color removal using the three alternatives, and another two equations were generated for the COD removal. These models are capable of predicting the optimal doses or explaining the behavior of the coagulants studied in this work.
Alternative A: Aluminum Sulfate
Color removal
With a significance level of 0.05, the univariate test of importance (Table S2) shows effects of aluminum sulfate doses. The pH and pH² were shown to have a significant difference with a 95% confidence interval and p < 0.05, which are key factors for color removal in mezcal vinasse. The finding coincided with that reported by Ryan et al. (2008) for molasses, due to pH variations that can help the color removal
The least significant difference (LSD) test (Table S3), shows that pH levels of 5, 8, and 11 had a minimum significant difference; pH 11 produced a greater removal of color using aluminum sulfate.
To obtain color removals greater than 50% doses, more than 1000 mg/L of aluminum sulfate was required (Table S4).
The model that explains the behavior of color removal in mezcal vinasse using aluminum sulfate is shown in Eq. 1. This model shows an adjustment coefficient of 0.96 (Table S5). The model was validated; it was observed that it did not violate any of the assumptions: the residuals behaved normally (Fig. A1) and the residues were not self-correlated, so they were independent (Table S6).
Colorremo(%)=-58.6604 + 0.0082x + 32.771y – 7.7869-7x2 + 0.0001xy – 2.2749y2 (1)
In Eq. 1, x is the aluminum sulfate doses (mg/L) and y is the pH.
Figure 1 shows the 3D contour plot, showing the point where the maximum values of color removal were reached using aluminum sulfate. Based on Eq. 1, the optimal doses of aluminum sulfate were 5590, 5780, and 5790 mg/L using pH levels of 5, 8, and 11, respectively. The best pH was 8, because it was possible to achieve a color removal of 83% (Fig. A2).
Fig. 1. 3D contour plot for color removal in mezcal vinasse using aluminum sulfate
COD Removal
For the COD removal using alternative A, both the dose and the pH were key factors (Table S7) with a sinificance level of 0.05.
There were significant statistical differences between the pH levels studied, showing a better removal with higher (pH=11) (Table S8).
According to Table S9, there were no statistically significant differences between doses of 100 and 1000 mg/L of aluminum sulfate achieving 46% removal of COD.
The model to explain the behavior of COD removal in mezcal vinasse using aluminum sulfate is shown in Eq. 2, which shows an adjustment coefficient of 0.88 (Table S10). The validation of the assumptions of normality and independence is shown in Fig. S3 and Table S11, respectively.
CODrem(%)=-10.7067-0.0002x+12.2298y+5.1885-8x2+2.8709-5xy-0.5744y2 (2)
In Eq. 2, y is the pH and x is thealuminum sulfate doses (mg/L).
In Fig. 2, there is an area in which nearly 60% removal COD was obtained. These were found when the pH was 11 and the aluminum sulfate doses were around 10,000 mg/L. At a higher dose and pH, a greater COD removal was obtained. The optimal dose of aluminum sulfate was 10,000 mg/L, with pH levels of 5, 8, and 11, which led to 62, 77, and 82% COD removal, respectively (Fig. S4).
Fig. 2. 3D contour plot for COD removal in mezcal vinasse using aluminum sulfate
Alternative B: Ferric Chloride
Color removal
Table S12 shows that the doses, the pH, and the interactions had significant differences with p < 0.05. This means that the color removal under these experimental conditions was dominated by the ferric chloride dose and the pH.
In Table S13 it is apparent that between cell numbers 7 and 2, 4 and 1, and 3 and 6 there was no significant statistical difference. The treatment with negative effect was 10,000 mg/L and pH = 8. It was observed that with pH 11 it was possible to find removals close to 90% with the three doses.
The model to explain the behavior of color removal in mezcal vinasse using ferric chloride showed an adjustment coefficient of 0.96 (Table S14). The model was validated, the residuals behave normally (Fig. S5) and residues are not self-correlated, so they are independent (Table S15).
Colorrem(%)=446.551-0.0075x-109.270y+1.5471-7x2+0.0002xy+7.0927y2 (3)
In Eq. 3, x is the ferric chloride doses (mg/L) and y is the pH.
The 3D contour plot for color removal in mezcal vinasse using ferric chloride (Fig. 3) shows that the optimal dose of ferric chloride was 100 mg/L, however, the pH that had the most effect was 11.
Fig. 3. Contour plot for color removal in mezcal vinasse using ferric chloride
Most colloids in aqueous solution have a negative charge, producing a colloidal stable solution (Derjaguin 1989). This stability is reduced thanks to charge neutralization, and ionic layer compression, which reduces the repulsive forces between colloidal particles (Johnson et al. 2019). Phenolic compounds and high molecular weight nitrogen compounds like melanoidins are related to color (España-Gamboa et al. 2017). Fe (III) a metal cation are hydrated in water, these hydroxo-metal complexes are readily adsorbed at interfaces; hence, the colloids become destabilized, allowing the coagulation (O’melia 1972). Therefore, in the coagulation process of mezcal vinasses, ferric chloride neutralized the colloidal particles charges that were related to color.
Ferric chloride was shown to be efficient for color removal. These results do not coincide with the studies by Ferral-Pérez et al. (2016), because in that study the authors mention that with doses of 500 mg/L there was no positive effect for the removal of color by treating tequila vinasse, a similar effluent.
COD removal
Univariate tests of significance (Table S16) showed that the important factors for COD removal using ferric chloride were: dosage, pH, and interaction (doses * pH).
There were statistically significant differences between the different pH values (Table S17). However, alternative B was only able to remove 28% of the COD. In comparison with the aluminum sulfate, it was possible to remove twice that of alternative B; however, to achieve this, a pH of 11 and a dose of 10,000 mg/L was required. The COD removal using ferric chloride was lower than that reported by Campos-Diaz et al. (2017), treating tequila vinasse at pH 12.
According to Table S18, the 100 mg/L doses best removed COD. However, the coagulation of mezcal stillage with ferric chloride for the removal of COD was not effective. It was higher than that reported by Ferral-Pérez et al. (2016) because those authors only reached removals close to 5% for tequila vinasse treated with ferric chloride.
In the 3D contour plot (Fig. 4), there is an area in which nearly 30% COD removal was obtained. This was found when the pH increased from 8 to 11 and ferric chloride doses were around 100 and 10,000 mg/L. Using doses close to 100 mg / L and pH of 11 was possible to achieve the highest removals (Fig. S6). Compared to alternative A, ferric chloride was not more effective in removing COD.
Fig. 4. 3D contour plot for COD removal in mezcal vinasse using ferric chloride
Alternative C: Best Coagulant and Mucilage
The color parameter is associated with the presence of melanoidin (Fitzgibbon et al. 1995). Therefore, the coagulant that best removed the color was chosen. Table S19 shows that the highest color removals were found at a pH of 11. Of these treatments, there were no statistically significant differences between doses of 100 and 1000 mg/L of ferric chloride. Thus, the lowest dose was chosen. For the alternative C evaluation, the ferric chloride was chosen because it has a good effect on color removal and for economic purposes a near dose of 100 mg/L.
Ferric chloride turned out to be effective for low dose color removal but not for COD removal (Fig. 4); it was chosen to study the effect of mucilage as a coadjuvant on color and COD removals. A balanced A x B factorial design was used, where A was the mucilage dose and B was the ferric chloride doses with 3 levels, and the pH was kept constant (pH = 11).
Color Removal
The mucilage, ferric chloride doses, and interaction between both are important factors to remove color in mezcal vinasse (Table S20).
With α = 0.05 in the mean difference test (Table S21), it shows that the effect of mucilage as a coadjuvant had a negative effect on color removal. Due to the use of ferric chloride in alternative B, under this same pH (11) (Table S13) is close to 90% of color removal. Quite contrary to this behavior, the use of mucilage is capable of increasing the color (-8.8822) (Table S21).
The model below explains the behavior for color removal (Eq. 4) and shows an adjusted coefficient of determination of 0.99 (Table S22). The model does not violate any of the assumptions: the residuals behaved normally (Fig. S7), and the residues were not self-correlated, so they were independent (Table S23).
Colorrem(%)=2826.440-58.8906x+1.0945y+0.3092x2-0.019xy+0.0032y2 (4)
In Eq. 4, x is the FeCl3 (mg/L) and y is the mucilage dose (mg/L).
As shown in Fig. 5, using doses close to the optimal in alternative B there was a redissolution (green zone). The observed reduction in coagulation activity using OFI mucilage can be explained by too low or too high doses (Israelachvili 2011), which causes their redispersal, increasing the concentration (Drifford et al. 1996).
Fig. 5. 3D contour plot for color removal in mezcal vinasse using ferric chloride plus mucilage
COD Removal
With a significance level of 0.05, the univariate significance test (Table S24) showed that ferric chloride doses, mucilage doses, and ferric chloride doses * mucilage doses were important factors for COD removal in mezcal vinasse.
The effect of ferric chloride plus OFI mucilage reached a removal greater than 84% (Table S25) using 90 mg/L and 150 mg/L. This treatment was more effective than the one used by Carvajal-Zarrabal et al. (2012) for tequila vinasse using polyglutamic acid combined with sodium hypochlorite and sand filtration. Ferral-Pérez et al. (2016) used 300 mg/L of chitosan to remove COD achieving similar results as that of alternative C (90 mg/L of ferric chloride and 150 mg/L mucilage. Campos-Diaz et al. (2017) used a dual process (biological-advanced oxidation) achieving removals of 99%, however, this process required 90 days, so alternative C allows for a relatively fast-treated effluent.
The model that predicted the behavior of COD removal using ferric chloride plus OFI mucilage is shown in Eq. 5,
CODrem(%)=400.3038+0.2948x-6.1354y-7.7537-5x2-0.0028xy+0.0283y2 (5)
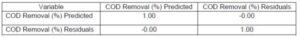
where y is the ferric chloride doses (mg/L) and x is the OFI mucilage doses (mg/L). The model shows an adjusted coefficient of determination of 0.99 (Table S26), not violating the assumptions of normality (Fig. S8) and independence (Table S27).
The best COD removals were obtained with doses of 90 mg/L (Fig. 6). The positive effect for the removal of COD using alternative C was that the mucilage contains galacturonic acid (Sáenz et al. 2004) an active ingredient that provides the coagulation capability (Miller et al. 2008).
Fig. 6. 3D contour plot for COD removal using ferric chloride plus OFI mucilage
The coagulation-flocculation using ferric chloride plus OFI mucilage was more efficient than the use of aluminum sulfate, with doses 11 times less of ferric chloride. Further, it increased the removal of the individual effect of ferric chloride from 30 to 84%. Both the aluminum sulfate and ferric chloride ions are positively charged. However, the OFI mucilage may have a polyvalent character. As shown by the mucilages of M. malabathricum and Zea mays that have an affinity to trivalent and divalent cations respectively (Watanabe et al. 2008). Therefore, future research suggests tested by means of zeta potential measurements to confirm.
CONCLUSIONS
- It was possible to eliminate 90% of color using ferric chloride, showing better removals than aluminum sulfate.
- The effect of ferric chloride plus OFI mucilage generated an adverse effect because the color removal was under 17%.
- The natural coadjuvant was shown to be effective in the COD removal in the treatment of mezcal vinasse using the coagulation-flocculation process, because the individual effect of ferric chloride for COD removal was 30%. This removal was improved by the addition of OFI mucilage, as it was able to increase removal to 84%.
ACKNOWLEDGMENTS
The authors gratefully appreciate the support of the Consejo Nacional de Ciencia y Tecnologia (CONACyT) for the support of the Sistema Nacional de Investigadores (SNI) as well as for the postdoctoral scholarship (130780/20) received by one of the authors.
REFERENCES
Ahmed, P. M., De Figueroa, L. I. C., and Pajot, H. F. (2020). “Dual purpose of ligninolytic-basidiomycetes: Mycoremediation of bioethanol distillation vinasse coupled to sustainable bio-based compounds production,” Fungal Biology Reviews 34(1), 25-40. DOI: 10.1016/j.fbr.2019.12.001
Ao, T., Luo, Y., Chen, Y., Cao, Q., Liu, X., and Li, D. (2020). “Towards zero waste: A valorization route of washing separation and liquid hot water consecutive pretreatment to achieve solid vinasse based biorefinery,” Journal of Cleaner Production 248, Article ID 119253. DOI: 10.1016/j.jclepro.2019.119253
Barrera-Díaz, C. E., Balderas-Hernández, P., and Bilyeu, B. (2018). “Electrocoagulation: Fundamentals and prospectives,” in: Electrochemical Water and Wastewater Treatment Butterworth-Heinemann, Oxford, United Kingdom, pp. 61-76. DOI: 10.1016/B978-0-12-813160-2.00003-1
Borja, R., Martin, A., Luque, M., and Duran, M. M. (1993). “Kinetic study of anaerobic digestion of wine distillery wastewater,” Process Biochemistry 28(2), 83-90. DOI: 10.1016/0032-9592(93)80011-5
Bouarab-Chibane, L., Forquet, V., Lantéri, P., Clément, Y., Léonard-Akkari, L., Oulahal, N., Degraeve, P., and Bordes, C. (2019). “Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure–activity relationship) models,” Frontiers In Microbiology 10, Article number 829. DOI: 10.3389/fmicb.2019.00829
Campos-Diaz, K. E., Álvarez Cruz, J. L., Lira Rodriguez, M. L., and Bandala, E. R. (2017). “Coupled inverse fluidized bed bioreactor with advanced oxidation processes for treatment of vinasse,” AIMS Geosciences 3(4), 538-551. DOI: 10.3934/geosci.2017.4.538
Carvajal-Zarrabal, O., Nolasco-Hipólito, C., Barradas-Dermitz, D. M., Hayward-Jones, P. M., Aguilar-Uscanga, M. G., and Bujang, K. (2012). “Treatment of vinasse from tequila production using polyglutamic acid,” Journal of Environmental Management 95, S66-S70. DOI: 10.1016/j.jenvman.2011.05.001
Christofoletti, C. A., Escher, J. P., Correia, J. E., Marinho, J. F. U., and Fontanetti, C. S. (2013). “Sugarcane vinasse: Environmental implications of its use,” Waste Management 33(12), 2752-2761. DOI: 10.1016/j.wasman.2013.09.005
COMERCAM (2018). “Mezcal: The liquid culture of Mexico,” Consejo Mexicano Regulador del Mezcal, (http://www.crm.org.mx/PDF/INF_ACTIVIDADES/INFORME2018.pdf), Accessed 28 Jan 2021.
Cruz-Salomón, A., Gordillo, R. M., Rosales-Quintero, A., Ventura-Canseco, L. M. C., Rivera, S. L., and Carrasco-Cervantes, J. (2017). “Biogas production from a native beverage vinasse using a modified UASB bioreactor,” Fuel 198, 170-174. DOI: 10.1016/j.fuel.2016.11.046
de Souza, R. P., Girardi, F., Slusarski-Santana, V., Fernandes-Machado, N. R. C., and Gimenes, M. (2013). “Vinasse treatment using a vegetable-tannin coagulant and photocatalysis,” Acta Scientiarum Technology 35(1), 89-95. DOI: 10.4025/actascitechnol.v35i1.11011
Derjaguin, B. V. (1989). Theory of Stability of Colloids and Thin Films, Consultants Bureau, New York.
dos Santos, J. F., Canettieri, E. V., Souza, S. M. A., Rodrigues, R. C. L. B., and Martínez, E. A. (2019). “Treatment of sugarcane vinasse from cachaça production for the obtainment of Candida utilis CCT 3469 biomass,” Biochemical Engineering Journal, 148, 131-137. DOI: 10.1016/j.bej.2019.04.009
Drifford, M., Dalbiez, J., Delsanti, M., and Belloni, L. (1996). “Structure and dynamics of polyelectrolyte solutions with multivalent salts,” Berichte der Bunsengesellschaft für Physikalische Chemie 100(6), 829-835. DOI: 10.1002/bbpc.19961000624
España-Gamboa, E., Mijangos-Cortes, J. O., Barahona-Perez, L. F., Maldonado, J. D., Hernández-Zarate, G., and Alzate-Gaviria, L. (2011). “Vinasses: Characterization and treatments,” Waste Management and Research 29(12), 1235-1250. DOI: 10.1177/0734242X10387313
España-Gamboa, E., Vicent, T., Segura, X. F., Maldonado, J. D., Canto-Canché, B., and Alzate-Gaviria, L. (2017). “Pretreatment of vinasse from the sugar refinery industry under non-sterile conditions by Trametes versicolor in a fluidized bed bioreactor and its effect when coupled to an UASB reactor,” Journal of Biological Engineering 11(1), article no. 6. DOI: 10.1186/s13036-016-0042-3
Espino‐Díaz, M., Ornelas‐Paz, J. d. J., Martínez‐Téllez, M. A., Santillán, C., Barbosa‐Cánovas, G. V., Zamudio‐Flores, P. B., and Olivas, G. I. (2010). “Development and characterization of edible films based on mucilage of Opuntia ficus‐indica (L.),” Journal of Food Science 75(6), E347-E352. DOI: 10.1111/j.1750-3841.2010.01661.x
Ferral-Pérez, H., Bustillos, L. G. T., Méndez, H., Rodríguez-Santillan, J. L., and Chairez, I. (2016). “Sequential treatment of tequila industry vinasses by biopolymer-based coagulation/flocculation and catalytic ozonation,” Ozone: Science and Engineering 38(4), 279-290. DOI: 10.1080/01919512.2016.1158635
Fitzgibbon, F. J., Nigam, P., Singh, D., and Marchant, R. (1995). “Biological treatment of distillery waste for pollution‐remediation,” Journal of Basic Microbiology 35(5), 293-301. DOI: 10.1002/jobm.3620350504
Freitas, P. V., da Silva, D. R., Beluomini, M. A., da Silva, J. L., and Stradiotto, N. R. (2018). “Determination of phenolic acids in sugarcane vinasse by HPLC with pulse amperometry,” Journal of Analytical Methods in Chemistry 2018, Article ID 4869487. DOI: 10.1155/2018/4869487
García, I. G., Venceslada, J. L. B., Peña, P. R. J., and Gómez, E. R. (1997). “Biodegradation of phenol compounds in vinasse using Aspergillus terreus and Geotrichum candidum,” Water Research 31(8), 2005-2011. DOI: 10.1016/S0043-1354(97)00014-6
García-Becerra, M., Macías-Muro, M., Arellano-García, L., and Aguilar-Juárez, O. (2019). “Bio-hydrogen production from tequila vinasses: Effect of detoxification with activated charcoal on dark fermentation performance,” International Journal of Hydrogen Energy 44(60), 31860-31872. DOI: 10.1016/j.ijhydene.2019.10.059
Gebresamuel, N., and Gebre-Mariam, T. (2011). “Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia spp.) obtained from Mekelle, Northern Ethiopia,” Biomaterials and Nanobiothechnology 3(1), 79-86. DOI: 10.4236/jbnb.2012.31010
Gomez-Maldonado, D., Erramuspe, I. B. V., and Peresin, M. S. (2019). “Natural polymers as alternative adsorbents and treatment agents for water remediation,” BioResources 14(4), 10093-10160. DOI: 10.15376/biores.14.4.Gomez-Maldonado
Israelachvili, J. N. (2011). Intermolecular and Surface Forces (3rd Ed.), Academic Press, Amsterdam, Netherlands. DOI: 10.1016/C2009-0-21560-1
Johnson, I., Ali, M. A. S., and Kumar, M. (2019). “Cyanobacteria/microalgae for distillery wastewater treatment-past, present and the future,” in: Microbial Wastewater Treatment, Elsevier, Amsterdam, Netherlands, pp. 195-236. DOI: 10.1016/B978-0-12-816809-7.00010-5
Junior, A. T., Hasan, S. D. M., and Sebastien, N. Y. (2019). “Optimization of coagulation/flocculation treatment of brewery wastewater employing organic flocculant based of vegetable tannin,” Water, Air, and Soil Pollution 230(8), Article number 202. DOI: 10.1007/s11270-019-4251-5
Kaushik, A., Basu, S., Raturi, S., Batra, V. S., and Balakrishnan, M. (2018). “Recovery of antioxidants from sugarcane molasses distillery wastewater and its effect on biomethanation,” Journal of Water Process Engineering 25, 205-211. DOI: 10.1016/j.jwpe.2018.08.003
Knob, A., Fortkamp, D., Prolo, T., Izidoro, S. C., and de Almeida, J. M. (2014). “Agro-residues as alternative for xylanase production by filamentous fungi,” BioResources 9(3), 5738-5773. DOI: 10.15376/biores.9.3.5738-5773
Maćczak, P., Kaczmarek, H., and Ziegler-Borowska, M. (2020). “Recent achievements in polymer bio-based flocculants for water treatment,” Materials 13(18), Article ID 3951. DOI: 10.3390/ma13183951
Matilainen, A., Vepsäläinen, M., and Sillanpää, M. (2010). “Natural organic matter removal by coagulation during drinking water treatment: A review,” Advances in Colloid and Interface Science 159(2), 189-197. DOI: 10.1016/j.cis.2010.06.007
Miller, S. M., Fugate, E. J., Craver, V. O., Smith, J. A., and Zimmerman, J. B. (2008). “Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment,” Environmental Science & Technology 42(12), 4274-4279. DOI: 10.1021/es7025054
Moraes, B. S., Zaiat, M., and Bonomi, A. (2015). “Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives,” Renewable and Sustainable Energy Reviews 44, 888-903. DOI: 10.1016/j.rser.2015.01.023
Moran-Salazar, R. G., Sanchez-Lizarraga, A. L., Rodriguez-Campos, J., Davila-Vazquez, G., Marino-Marmolejo, E. N., Dendooven, L., and Contreras-Ramos, S. M. (2016). “Utilization of vinasses as soil amendment: Consequences and perspectives,” SpringerPlus 5(1), Article number 1007. DOI: 10.1186/s40064-016-2410-3
Nharingo, T., and Moyo, M. (2016). “Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review,” Journal of Environmental Management 166, 55-72. DOI: 10.1016/j.jenvman.2015.10.005
O’melia, C. R. (1972). “Coagulation and flocculation,” in: The Scientific Basis of Flocculation, Ives, K. J. (eds.), NATO Advanced Study Institutes Series (Series E: Applied Science), vol 27, Springer, Dordrecht, 61-109. DOI: 10.1007/978-94-009-9938-1_10
Othman, N. B., Ayed, L., Assas, N., Kachouri, F., Hammami, M., and Hamdi, M. (2008). “Ecological removal of recalcitrant phenolic compounds of treated olive mill wastewater by Pediococcus pentosaceus,” Bioresource Technology 99(8), 2996-3001. DOI: 10.1016/j.biortech.2007.06.017
Othmani, B., Rasteiro, M. G., and Khadhraoui, M. (2020). “Toward green technology: A review on some efficient model plant-based coagulants/flocculants for freshwater and wastewater remediation,” Clean Technologies and Environmental Policy 22, 1025-1040. DOI: 10.1007/s10098-020-01858-3
Pimienta-Barrios, E., and del Castillo, R. F. (2002). “Reproductive biology,” in: Cacti: Biology and Uses, P. S. Nobel (ed.), University of California Press, Berkeley, CA, USA, pp. 75-90.
Poznyak, T. I., Chairez Oria, I., and Poznyak, A. S. (2019). “Combination of physical-chemical methods and ozonation. Ozonation and Biodegradation”, In Environmental Engineering, 199–246. DOI: 10.1016/b978-0-12-812847-3.00017-2
Razavi, S. M. (2019). Emerging Natural Hydrocolloids: Rheology and Functions, John Wiley and Sons, Hoboken, NJ, USA.
Rivera-Corona, J. L., Rodríguez-González, F., Rendón-Villalobos, R., García-Hernández, E., and Solorza-Feria, J. (2014). “Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition,” LWT-Food Science and Technology 59(2), 806-812. DOI: 10.1016/j.lwt.2014.06.011
Robles-González, V., Poggi-Varaldo, H. M., Galíndez-Mayer, J., and Ruiz-Ordaz, N. (2018). “Combined treatment of mezcal vinasses by ozonation and activated sludge,” Water Environment Research 90(11), 1985-1996. DOI: 10.2175/106143017×15054988926433
Rodríguez-González, S., Martínez-Flores, H. E., Órnelas-Nuñez, J. L., and Garnica-Romo, M. G. (2004). “Optimización de la extracción del mucílago de nopal (Opuntia ficus-indica) [Optimization of the extraction of nopal mucilage (Opuntia ficus-indica)],” in: XIV Congreso Nacional de Biotecnologia y Bioingenieria [XIV National Congress of Biotechnology and Bioengineering], 22 June 2011, Juriquilla, Mexico.
Rufián-Henares, J. A., and Morales, F. J. (2007). “Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities,” Food Research International 40(8), 995-1002. DOI: 10.1016/j.foodres.2007.05.002
Ryan, D., Gadd, A., Kavanagh, J., Zhou, M., and Barton, G. (2008). “A comparison of coagulant dosing options for the remediation of molasses process water,” Separation and Purification Technology 58(3), 347-352. DOI: 10.1016/j.seppur.2007.05.010
Sáenz, C., Sepúlveda, E., and Matsuhiro, B. (2004). “Opuntia spp mucilage’s: A functional component with industrial perspectives,” Journal of Arid Environments 57(3), 275-290. DOI: 10.1016/S0140-1963(03)00106-X
Secretaria de Economía. (2015). “Se amplía zona de denominación de origen del Mezcal [Mezcal designation of origin area is expanded],” (https://www.gob.mx/se/articulos/se-amplia-zona-de-denominacion-de-origen-del-mezcal), Accessed 28 Jan 2021.
Teh, C. Y., and Wu, T. Y. (2014). “The potential use of natural coagulants and flocculants in the treatment of urban waters,” in: Chemical Engineering Transactions: 17th Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction (PRES2014), P. Varbanov, J. Klemes, P. Y. Liew, and J. Y. Yong (eds.), Associazione Italiana Di Ingegneria Chimica (AIDIC), Milan, Italy, pp. 1603-1608. DOI: 10.3303/CET1439268
Tejada, M., García-Martínez, A. M., and Parrado, J. (2009). “Effects of a vermicompost composted with beet vinasse on soil properties, soil losses and soil restoration,” Catena, 77(3), 238-247. DOI: 10.1016/j.catena.2009.01.004
Vlyssides, A. G., Israilides, C. J., Loizidou, M., Karvouni, G., and Mourafeti, V. (1997). “Electrochemical treatment of vinasse from beet molasses,” Water Science and Technology 36(2-3), 271-278. DOI: 10.1016/S0273-1223(97)00398-3
Watanabe, T., Misawa, S., Hiradate, S., and Osaki, M. (2008). “Characterization of root mucilage from Melastoma malabathricum, with emphasis on its roles in aluminum accumulation,” New Phytologist 178(3), 581-589. DOI: 10.1111/j.1469-8137.2008.02397.x
Article submitted: April 20, 2021; Peer review completed: July 11, 2021; Revised version received and accepted: July 13, 2021; Published: July 15, 2021.
DOI: 10.15376/biores.16.3.6031-6056
APPENDIX
Supplementary
The Appendix contains 10 pages with 8 figures and 27 tables.
Table S1. Characterization of the Mezcal Vinasse Sample Taken From an Underground Storage Pit (Mean ± SD, n = 3)
Table S2 Univariate Test of Significance for Color Removal (%) using Aluminum Sulfate
Table S3. LSD Test, Variable Color Removal Using Different pH (Aluminum Sulfate)
Table S4. LSD Test, Variable Color Removal Using Different Doses (Aluminum Sulfate)
Table S5. Test of the SS Whole Model vs. SS Residual (Color Removal Using Aluminum Sulfate)
Fig. S1. Validation of the color removal model using aluminum sulfate, Test of Kolmogorov-Smirnov, Lilliefors, and Shapiro-Wilk. Normal distribution
Table S6. Independence Test, Correlation Between Predicted and Residual Values for Color Removal Model Using Aluminum Sulfate
Fig. S2. Maximum color removal in relation to the pH 5, 8 and 11 using 5590, 5780, and 5790 mg/L of aluminum sulfate, respectively
Table S7. Univariate Test of Significance for COD Removal (%) Using Aluminum Sulfate
Table S8. LSD Test, Variable COD Removal Using Different pH (Aluminum Sulfate)
Table S9. LSD Test, Variable COD Removal Using Different Doses (Aluminum Sulfate)
Table S10. Test of the SS Whole Model vs. SS Residual (COD Removal Using Aluminum Sulfate)
Fig. S3. Validation of the COD removal model using aluminum sulfate, Test of Kolmogorov-Smirnov, Lilliefors and Shapiro-Wilk. Normal distribution
Table S11. Independence Test, Correlation Between Predicted and Residual Values for COD Removal Model Using Aluminum Sulfate
Fig. S4. Maximum COD removal in relation to the pH using 10,000 mg/L of aluminum sulfate
Table S12.. Univariate Test of Significance for COD Removal (%) Using Ferric Chloride
Table S13. LSD Test, Variable Color Removal Using Different Ferric Chloride Doses and pH
Table S14. Test of the SS Whole Model vs. SS Residual (Color Removal Using Ferric Chloride)
Fig. S5.. Validation of the color removal model using ferric chloride, Test of Kolmogorov-Smirnov, Lilliefors, and Shapiro-Wilk. Normal distribution
Table S15. Independence Test, Correlation Between Predicted and Residual Values for Color Removal Model Using Ferric Chloride
Table S16. Univariate Test of Significance for COD Removal (%) Using Ferric Chloride
Table S17. LSD Test, Variable COD Removal Using Different pH (Ferric Chloride)
Table S18.. LSD Test, Variable COD Removal Using Different Ferric Chloride Doses
Fig. S6. Maximum color removal in relation to the pH using 100 mg/L of ferric chloride
Table S19. LSD Test, Variable Color Removal Using Different pH, Doses, and Coagulant
Table S20. Univariate Test of Significance for Color Removal (%) Using Ferric Chloride and Mucilage
Table S21. LSD Test, Variable Color Removal Using Ferric Chloride and Mucilage
Table S22. Test of the SS Whole Model vs. SS Residual (Color Removal Using Ferric Chloride Plus Mucilage)
Fig. S7. Validation of the color removal model using ferric chloride plus mucilage, Test of Kolmogorov-Smirnov, Lilliefors, and Shapiro-Wilk. Normal distribution
Table S23. Independence Test, Correlation Between Predicted and Residual Values for Color Removal Model Using Ferric Chloride Plus Mucilage
Table S24. Univariate Test of Significance for COD Removal (%) Using Ferric Chloride and Mucilage
Table S25. LSD Test, Variable COD Removal Using Ferric Chloride and Mucilage
Table S26. Test of the SS Whole Model vs. SS Residual (COD Removal Using Ferric Chloride Plus Mucilage)
Fig. S8. Validation of the COD removal model using ferric chloride plus mucilage, Test of Kolmogorov-Smirnov, Lilliefors, and Shapiro-Wilk. Normal distribution
Table S27. Independence Test, Correlation Between Predicted and Residual Values for COD Removal Model Using Ferric Chloride Plus Mucilage