Abstract
This study investigated the extraction and properties of an arabinogalactan polysaccharide from frost grape (FGP) as a potential alternative to gum arabic (GA). Collection date, solvent:feed ratio (S:F), chip size, C-18 filtration, ultrafiltration, freeze drying versus spray drying, methanol pre-extraction, and water absorption were examined. Sugar composition, elemental analysis, dietary fiber content, emulsification activity index (EAI), emulsification stability index (ESI), and viscosity were used to evaluate the extracts. Exudates collected in March from live stems were viscous with high percentage solids and FGP, while May collections were watery with low percentage solids and FGP. Frost grape stems were collected, chipped, and classified by size. The extraction system utilized pressure or vacuum to increase contact between the chips and extraction water. A S:F ratio of ca. 24% gave an excellent yield of FGP. Chips between 1.8 and 3.8 mm gave the highest mass yields. Pre-extracting the chips with methanol and C-18 filtration of the water extract both yielded a lighter product. The EAI for the FGP was higher than that for GA; however, its ESI was lower. Ultrafiltration of the crude extract separated glucose, fructose, and sucrose from the FGP. FGP with glucose, fructose, and sucrose adsorbed water and became darker.
Download PDF
Full Article
Extraction, Purification and Characterization of an Arabinogalactan from Frost (Riverbank) Grape (Vitis riparia Michx.) Stems
Fred J. Eller,a,* Steven F. Vaughn,a Neil P. J. Price,b James A. Kenar,a Michael A. Jackson,b Mark A. Berhow,a Korey J. Brownstein,a and Gordon W. Selling c
This study investigated the extraction and properties of an arabinogalactan polysaccharide from frost grape (FGP) as a potential alternative to gum arabic (GA). Collection date, solvent:feed ratio (S:F), chip size, C-18 filtration, ultrafiltration, freeze drying versus spray drying, methanol pre-extraction, and water absorption were examined. Sugar composition, elemental analysis, dietary fiber content, emulsification activity index (EAI), emulsification stability index (ESI), and viscosity were used to evaluate the extracts. Exudates collected in March from live stems were viscous with high percentage solids and FGP, while May collections were watery with low percentage solids and FGP. Frost grape stems were collected, chipped, and classified by size. The extraction system utilized pressure or vacuum to increase contact between the chips and extraction water. A S:F ratio of ca. 24% gave an excellent yield of FGP. Chips between 1.8 and 3.8 mm gave the highest mass yields. Pre-extracting the chips with methanol and C-18 filtration of the water extract both yielded a lighter product. The EAI for the FGP was higher than that for GA; however, its ESI was lower. Ultrafiltration of the crude extract separated glucose, fructose, and sucrose from the FGP. FGP with glucose, fructose, and sucrose adsorbed water and became darker.
DOI: 10.15376/biores.18.3.4610-4635
Keywords: Frost grape; Pressure swing extraction; Arabinogalactan; Ultrafiltration; Emulsification; Viscosity; Resveratrol; Titanium; ε-Viniferin
Contact information: a: USDA, Agricultural Research Service, National Center for Agricultural Utilization Research, Functional Foods Research Unit, 1815 N University, Peoria, IL 61604, USA; b: USDA, Agricultural Research Service, National Center for Agricultural Utilization Research, Renewable Product Technology Research Unit, 1815 N University, Peoria, IL 61604, USA; c: USDA, Agricultural Research Service, National Center for Agricultural Utilization Research, Plant Polymer Research Unit, 1815 N University, Peoria, IL 61604, USA; *Corresponding author: Fred.Eller@USDA.gov
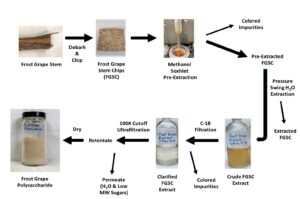
GRAPHICAL ABSTRACT

Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
INTRODUCTION
Polysaccharides are used in many commercial non-food and food processing applications (Free et al. 1976; Phillips et al. 1994; Ray et al. 1995; Phillips 1998). Gum arabic (GA) is one such polysaccharide important in printing, paint production, glue, cosmetics, viscosity control in inks and in textile industries, and as a thickening agent and emulsifier in the food industry (Saeed et al. 2011). GA is a structurally complex branched polysaccharide arabinogalactan (AG) from wound-induced exudate from two species of acacia, for which the product comes almost exclusively from trees in the Sahel region of Africa (Churms et al. 1983: Defaye and Wong 1986; Osman et al. 1993; Eltohami 2018). GA is not produced in the U.S., and GA can vary widely in its quality and availability (Thevenet 1988; Dickinson 2003). GA is also known to contain an antigenic hydroxyproline-rich protein (Connolly et al. 1988; Dickinson et al. 1988). Therefore, identifying a stable, high quality domestic substitute for GA without antigenic proteins is desirable.
Price et al. (2015) identified a novel AG from Frost (Riverbank) Grape (Vitis riparia Michx), and this AG was termed frost grape polysaccharide (FGP). Other similar polysaccharides rich in arabinose and galactose (PRAG) have been identified from grapes (Canalejo et al. 2021; Jones-Moore et al. 2022). In addition to arabinose and galactose, FGP also contains lesser amounts of xylose, mannose, and gluconic acid. FGP does not contain any antigenic glycoproteins as found in GA and it has excellent emulsification, viscosity properties as well as film forming properties. Although a similar AG is found in the roots and trunks of western larch (Adams 1967), the range of western larch is limited to eastern Oregon, eastern Washington, northern Idaho, and western Montana (Arno and Hammerly 2020). Because grapes are much more widely distributed, FGP might be a better potential domestic replacement for GA than larch AG.
Earlier research has described the isolation, identification, physical and rheological (emulsification and viscosity), film forming and stability properties of FGP (Price et al. 2015; Hay et al. 2017; Leathers et al. 2017). However, the yields of FGP obtained have not previously been described nor have methods been reported for improving the extraction yields and maximizing the purification of FGP from frost grape stems (FGS).
The objective of this study was to develop an effective method for extracting and purifying FGP, determine its yield from frost grape stems and further chemically characterize the FGP and its physical properties.
EXPERIMENTAL
Collection and Comminution of Frost Grape Stems (FGS)
Frost grape stems (FGS) were collected from wild plants growing in Woodford County, IL, on March 5, 2021. The cut stems were transported to the laboratory and the shaggy loose bark peeled off by hand to reveal the tan inner bark. This tan inner bark was scraped off with a sharp knife to give a light-colored wood (Fig. 1). A close-up photo of the cut end of a frost grape stem showing the exudate is provided in Fig. 2. Initially, for the pressure swing extraction study, sawdust was made from the fresh undried FGS using a compound miter saw as described by Eller et al. (2014). Later, the FGS were milled into chips.
Collection of Frost Grape Stem Exudates (FGSExu)
Exudates from living frost grape stems were collected on March 31, 2021 (210331), in Tazewell County, IL. A 4-mm hole was drilled into the center of the stem and a 35-mL plastic syringe (Monoject, 10-000-885, Fisher Scientific, Chicago, IL) was placed into the drilled hole to seal the hole and collect the mucilaginous exudates. The syringe was used to help keep debris from the exudate and help prevent microbial contamination. The syringe plunger was retracted ca. 15 mL to allow the exudate to flow easily into the syringe barrel.
Fig. 1. Photograph of frost grape stems showing shaggy loose outer bark (A & B), tan inner bark (B & C), and partially removed inner bark (D).
Fig. 2. Close-up photograph of cut end of frost grape stem showing polysaccharide exuding from around stem pith
Figure 3 shows a syringe placed in a frost grape stem. The syringes were collected the next day and percent solids determined after dying in a vacuum oven at 105 °C and minus 0.088 MPa. The dried exudates were analyzed for their sugar compositions as described below. There were 10 replications performed.
Fig. 3. Field collection of frost grape stem exudate
A second set of collections were made on May 7 (210507) and May 17, 2021 (210517), as described above. At this time of year, the exudate was a watery clear sap requiring less than two minutes to fill the 35-mL syringe. The collected sap was dried by lyophilization and percent solids and sugar composition were determined.
Pressure Swing Aqueous Extraction
FGS have air pockets that decrease the contact between the extraction water and FGP, leading to lower extraction efficiency. Vacuum/pressure impregnation was used to increase the retention of wood preservatives by removing air within wood and subsequently replacing it with a pressurized preservative solution (Eller et al. 2010, 2018, 2021). In an analogous manner, it was hypothesized that vacuum could be used to remove air inside FGS increasing the contact of water with FGP within the FGS leading to higher extraction efficiency. Similarly, pressurizing the extraction water would also reduce the volume of air within the FGS and increase water contact with the FGP, thereby possibly providing a more efficient extraction of the FGP.
A schematic of the apparatus used for performing the pressure swing aqueous extraction of FGP is shown in Fig. 4. It consisted of three tubes connected using PVC unions (Thick-Wall Clear Threaded PVC Pipe Nipples, 4.8 cm OD, 3.8 cm ID X 15 cm long, part # 4677T44 and Thick-Wall Plastic Pipe Fitting for Water-Union Straight Connector, part # 4596K66, McMaster-Carr Supply Co., Elmhurst, IL 60126). There was a ball-valve (Easy-to-Install Double-Union On/Off Valve, part # 4852T55, McMaster-Carr Supply Co., Elmhurst, IL 60126) between the extraction tube and collection tube for draining extracts. The top cap was made from a PVC fitting (Thick-Wall Plastic Pipe Fitting for Water, part # 4596K46, McMaster-Carr Supply Co., Elmhurst, IL 60126). The bottom cap was made from a PVC coupling and a PVC plug (Thick-Wall Plastic Pipe Fitting for Water-Straight Connector, part # 4596K56 and Thick-Wall Plastic Pipe Fitting for Water-Plug with External Hex Drive, part # 4596K77, McMaster-Carr Supply Co., Elmhurst, IL 60126). Stainless Steel Wire Cloth (20 X 20 mesh, part # 9481T16, McMaster-Carr Supply Co., Elmhurst, IL 60126) was used to make screens to contain the FGC within the extraction tube.
Fig. 4. Schematic of frost grape stem chip pressure swing extraction apparatus
Water was chosen as the extraction solvent because FGP is a polar compound previously shown to be soluble in water but not ethanol (Price et al. 2015). The FGP was not expected to be soluble in less polar solvents such as acetone or hexane.
The middle extraction tube held the FGC (22.5 g) and had a volume of ca. 200 mL. The upper overflow tube was used to assure the FGC were completely covered by the extraction water. The lower collection tube was used to separate the extraction water after exposure to the FGS. The extraction apparatus could be switched between atmospheric pressure, vacuum (i.e., -0.088 MPa), and pressure (i.e., 0.69 MPa nitrogen) by changing valve positions. The apparatus was held inside an environmental chamber maintained at 25 °C.
For the vacuum extractions, 250 mL deionized water was drawn into the extraction tube using house vacuum to completely cover the FGC, as visualized in the overflow tube. Vacuum -0.088 MPa (ca. 26 inches Hg) was applied to remove air from within the FGC and held for 15 min. The vacuum was released back to atmospheric pressure, and water was allowed 15 min to move into the FGC and solubilize the FGP. The first vacuum extraction was performed by re-applying vacuum for 15 min to pull the water back out of the FGC. While under vacuum, the water extract was drained by gravity from the extraction tube into the collection tube by rotating the ball valve. After releasing the vacuum, the water extract was removed from the collection tube into a glass bottle. Subsequent extractions (i.e., second, third, fourth, etc.) were performed by drawing additional water (i.e., 167 mL) into the extraction tube and extracting in an identical manner.
For the pressure extractions, 250 mL deionized water was drawn up into the extraction tube using house vacuum to completely cover the FGC, as visualized in the overflow tube. Pressure (15 psi = 0.1 MPa nitrogen) was applied to force water into the FGC, and then it was held for 15 min. After releasing the pressure back to atmospheric pressure, water was allowed 15 min to move back out of the FGC. The water extract was then drained by gravity from the extraction tube into the collection tube without vacuum by rotating the ball valve. The water extract was removed from the collection tube into a glass bottle. Subsequent extractions (i.e., second, third, or fourth, etc.) were performed in an identical manner by drawing additional water (i.e., 167 mL) into the extraction tube and extracting in an identical manner.
The collected aqueous extracts were poured into glass baking dishes (ca. 20 cm X 20 cm) and frozen at ca. -80 °C. The frozen extracts were subsequently freeze dried (Labconco Bulk Tray Dryer, catalog number 7960046, Labconco, Corp., Kansas City, KS 64132) and FGP yields gravimetrically determined based on the mass of the freeze-dried residue.
The vacuum/atmospheric pressure cycle treatment was compared to the pressure/ atmospheric pressure cycle treatment for percent residue yield. Four replications were performed for each treatment.
Solvent to Feed Ratio (S:F)
The effect of solvent to feed ratio (S:F) on yield was examined by doing five sequential extractions of the frost grape stem chips (FGSC) and determining the mass yields, as described above. A vacuum-atmospheric pressure cycle was used to perform the extraction. The mass of FGSC was 22.5 g, and the volumes of water used for the five sequential extractions were: 200, 167, 167, 167, and 167 mL with cumulative solvent:feed (S:F) ratios of 8.9, 16.3, 23.7, 31.2, and 38.6, respectively. The Trendline function of Excel (Microsoft 365, Version 2022, Microsoft Corp., Redmond, WA) was used to fit a line to the data.
Frost Grape Stem Chip (FGSC) Size
The frost grape stems (FGS) were first coarsely chopped using an electric woodchipper/leaf shredder (model CSV-2515, Patriot Products Inc., Pewaukee, WI). A Fritsch Rotor-Speed Mill (model VDE 0530, Fritsch Milling and Sizing, Inc., Pittsboro, NC 27312) was then used to give a range of smaller sized chips by passing the coarse frost grape chips (FGC) through the mill in three successive steps. The 4th milling step included a 6-mm screen for final reduction. These chips were then classified by size into five fractions using four sieves. The sieve numbers (opening in mm) were 4 (4.76), 7 (2.83), 20 (0.84), and 40 (0.42). Five fractions (A-E) were collected; retained by 4 (A), passed 4 retained by 7 (B), passed 7 retained by 20 (C), passed 20 retained by 40 (D), and passed 40 (E). The estimated FGSC sizes for these fractions were 4.76, 3.80, 1.84, 0.63 and 0.42 mm, respectively. Figure 5 shows the five fractions of the frost grape stem chips (A-E) and scrapings of inner bark (F). Each fraction was weighed, and its percentage of the total mass was calculated. Samples of each fraction were subsequently extracted three times and fraction yields determined. Three samples of fraction C were dried overnight in a vacuum oven (105°C and -0.088Mpa) to determine its moisture content. The average (±SEM) moisture content was 9.5% (±0.1).
Fig. 5. Frost grape stem chips after milling and fractionating with sieves (A-E) and scraped frost grape stem inner bark (F). For scale, plastic weigh trays are 8.7 cm wide.
Purification and Separation of FGP from Aqueous Extract
C-18 filtration
Aqueous frost grape extracts were passed through a 10-gram HyperSep C-18 filter cartridge (Thermo Scientific, Rockwood, TN, USA, part # 60108-703) to remove amber colored material from the FGS extract. The aqueous extract was drawn through the cartridge using a vacuum flask. After collecting the amber colored material on the C-18 filter, it was subsequently extracted from the filter using methanol. The methanol was evaporated under a gentle stream of nitrogen and the mass of the extracted amber colored material determined.
Ultrafiltration
FGP has a reported molecular weight of between 1 – 10 MDa (Price et al. 2015) and 16 MDa (Leathers et al. 2017). Size exclusion chromatography has been used previously to separate grape polysaccharides of lower molecular weight (i.e., 195 kDa) (Guadalupe et al. 2012; Manjon et al. 2022). It was hypothesized that ultrafiltration could be used to separate the FGP from the low molecular weight sugars such as glucose, fructose, and sucrose with molecular weights of 180, 180 and 342, respectively. Ultrafiltration was performed using a 100,000 molecular weight (Da) cutoff membrane Millipore Pellicon Cassette System (Millipore Corp., Bedford, MA).
Freeze drying (lyophilization)
Extract solutions were freeze dried using a Labconco Freezone 6 L freeze dryer (Labconco, Kansas City, MO).
Spray drying
The frost grape polysaccharide was spray-dried using a Niro atomizer spray dryer (Niro, Columbia, MD, USA) equipped with a co-current two-fluid atomization nozzle. Nozzle flow through the atomizer was 1.3 bar (50 % of maximum flow). The FGP solution (2.0 wt%) was fed at 20 mL/min using the following conditions: inlet temperature of 139.0 ± 1.7 °C, outlet temperature of 73.0 ± 5.0 °C, air pressure of 2.6 bar. Percentage yield was determined by measuring the mass of the material recovered divided by the mass of solution (corrected for percent solids) passed through the spray dryer. Recovered material was stored as a powder in a desiccator with Drierite desiccant (W. A. Hammond Drierite Co. LTD, Xenia, OH, USA) until analyses were performed.
Chemical Properties of FGP
Carbohydrate analysis
Sugar compositional analyses of the samples were performed as described by Price (2004). Briefly, mixtures of component sugars were converted to Per-O-acetylated aldonitrile (PAAN) derivatives and then analyzed by gas chromatography/mass spectrometry (GC/MS) and identified using monosaccharide PAAN standards (Price et al.2015). The FGP was hydrolyzed with trifluoroacetic acid (2.0 M, 120 °C, 1 h) and the neutral sugars released were converted to aldonitrile acetates (PAANs) and analyzed as above.
Elemental analyses
Elemental composition of the FG components was determined by X-ray fluorescence measurements using a Malvern Panalytical Epsilon 1 (Malvern Panalytical, Westborough, MA, USA). Samples were measured as dry powders in analytical cups using Mylar (3.6 µm thickness) as the base. Composition was quantified using the Omnian Standardless analysis package. The empirical formula used to represent the matrix was CH2O.
Dietary fiber analysis
Dietary fiber is a class of compounds that are typically nondigestible. The class includes a variety of carbohydrate oligosaccharides and polymers that have beneficial physiological effects in humans (Elleuch et al. 2011). Dietary fiber from natural sources is appealing for use as a food ingredient to improve their nutritional value and functional properties. FGP represents a potentially novel fiber source that may contain high levels of soluble fiber and, therefore, the dietary fiber content was determined.
Insoluble dietary fiber (IDF), soluble dietary fiber (SDF), and total dietary fiber (TDF) of the frost grape polysaccharide samples were measured by enzymatic-gravimetric methods according to AOAC methods 985.29 and 991.43 using a Megazyme total dietary fiber assay kit (K-TDFR). The procedure was conducted following the manufacturer’s instructions, as previously described (Felker et al. 2018). Total dietary fiber was performed in duplicate and calculated as the sum of IDF and SDF.
Physical Properties of FGP
Emulsifying activity index (EAI) and emulsion stability index (ESI)
The emulsifying activity index (EAI) and emulsion stability index (ESI) were determined as described by Hay et al. (2017). Briefly, solutions of FGP and GA were prepared at 0.1% solids solutions (1 mg/mL), solutions were centrifuged at 4000 g for 20 min. Six milliliters of the supernatant were transferred to a beaker containing 2 mL of corn oil, and the mixture was homogenized for 1 min at 20,000 rpm. Fifty microliters of the homogenized solution were added to 5 mL of 0.1% SDS, to prevent any flocculation or adherence to the sides of the cuvette, and the light absorbance of the solution was measured at 500 nm using a background of 0.1% SDS with a UV−vis spectrophotometer. After 10 min, an additional 50 microliter aliquot was treated in the same fashion and the light absorbance of the solution was measured as above. The procedure was repeated in triplicate. The EAI was calculated: EAI (m2/g) = 2T(A0 × dilution/C × Φ × 10 000), where T = 2.303; A0 = absorbance measured immediately after homogenization; dilution = 100, C = mass of emulsifier/unit volume (g/mL) of aqueous phase prior to emulsion formation, and Φ is the oil volume fraction of the emulsion. The ESI was calculated as,
ESI (min) = A0 × Δt/ΔA
where Δt = 10 min and ΔA is the change in absorbance from A0 to A10, with the absorbance measured at 10 min.
Viscosity
Viscosity measurements were made using an ARES G2 controlled-strain rheometer (TA Instruments New Castle, DE) equipped with a Peltier plate and 50 mm stainless steel parallel upper geometry. Viscosity measurements were determined using a steady rate sweep from 0.1 to 100 sec-1 at 25+/-0.1 °C and from these points a zero-shear viscosity was calculated using TRIOS software version 5.1.1.46572. Best fit analysis determined the Cross Model to be the most accurate (Eq. 1). This model is used to fit many types of fluids including dispersions, polymer melts, and polymeric solutions,
(1)
where γ (dotted) is the shear strain rate; η0 is infinite-shear viscosity; η∞ is infinite-shear viscosity; k is the consistency index; and n is the flow behavior index.
Methanol Pre-Extraction of FGSC
Soxhlet methanol extraction
FGSC were extracted using Soxhlet methanol extraction to remove amber-colored material prior to aqueous extraction to prevent it from co-extracting with the FGP. Individual Soxhlet extraction thimbles held ca. 75 g of FGSC and several individual Soxhlet extractors were run simultaneously to obtain required amounts of pre-extracted FGSC for subsequent aqueous extractions. The Soxhlet condenser was set at 5 °C, and 20 cycles were run before removing the FGSC from the thimble. The extracted FGSC were dried in a hood to evaporate residual methanol from the FGSC. The methanol extract containing the amber-colored material was concentrated by rotary evaporation and the mass of amber residue determined.
Methanol extract analysis
LC-ESI-MS analysis was used for compound identification in the amber residue from the methanol extraction of FGSC. Samples were run on an Thermo Scientific (San Jose, CA) Orbitrap IDX Mass Spectrometer – a “tribrid” MS system with a quadrupole high energy collision (HCD) cell, a linear ion trap MS, coupled to a high precision electrostatic ion trap (Orbitrap) MS – with an Ion Max heated electrospray ionization (HESI) source, and a Thermo Scientific Vanquish series HPLC system (1250 UHPLC pump, HTC cool stack autoinjector, and a 80 Hz PDA detector) all running under Thermo Scientific Xcalibur 4.4.16.14 LC-MS software. The MS was calibrated weekly with a standard calibration mixture recommended by Thermo Scientific, and the signal detection was optimized by running the autotune software feature as needed. The MS was run with the HESI probe in the switching polarity mode with the vaporizer temperature set at 400 ˚C. The source inlet temperature was 350 ˚C, the sheath gas rate was typically set at 70 arbitrary units, the auxiliary gas rate was set at 20 arbitrary units, and the sweep gas rate was set at 10 arbitrary units. The maximal mass resolution was set at 60,000. The spray voltage was set at 2.3 kV for negative mode and 3.5 kV for positive mode. The software package was set up to collect MS-MS fragmentation data from the HCD cell for the major ion found at any given time interval, and the collision energy was set in a stepped mode for 15, 30, 45 CE units. Other parameters were determined and set by the calibration and tuning process. For phenolic UPLC separation, the initial solvent system was 95% aqueous phase (0.1% formic acid in nanopure water) 5% organic phase (methanol:acetonitrile (1:1) with 0.1% formic acid) at a flow rate of 0.55 mL/min. After injection (5 µL or less) the column was developed with a linear gradient to 100% methanol over 24 min. The column used was an Inertsil ODS-3 reverse phase C-18 column (3 µm, 150 x 3.0 mm, GL Sciences, Torrance, CA). The column effluent was monitored at 280 nm and 340 nm in the PDA detector. The software package was set to collect mass data between 100-2000 AMUs. Generally, the most significant sample ions generated under these conditions were [M+H]+, [M-H]- or [M+HCOO].
Compound concentrations were determined using HPLC analysis. Weighed samples (typically 0.25 g) were placed in a vial and extracted with 3 mL of a methanol:DMSO (1:1) solvent. The samples were typically sonicated for 30 min and allowed to stand overnight at room temperature. The extract was filtered through a 0.45 µm nylon 66 filter for HPLC analysis. HPLC analysis was conducted on a Shimadzu (Columbia, MD) LC-20 HPLC system (LC-20AT quaternary pump, DGU-20A5 degasser, SIL-20A HT autosampler, and an SPD M20A photodiode array detector, running under Shimadzu LCSolutions version 1.22 chromatography software, Columbia, MD, USA). The column used was an Inertsil ODS-3 reverse phase C-18 column (5 µm, 250 x 4.6 mm, GL Sciences, Torrance, CA). For phenolic compound analysis, the initial conditions were 10% methanol (or acetonitrile) with 0.25% trifluoracetic acid and 90% water with 0.25% trifluoracetic acid, at a flow rate of 1 mL/min. The effluent was monitored at 280 and 340 nm on the variable wave detector. After injection (typically 25 µL), the column was held at the initial conditions for 2 minutes, then developed to 100% methanol with 0.25% trifluoracetic acid in a linear gradient over 50 additional minutes.
Scaled-Up Extraction
A larger-scale apparatus was developed to obtain increased amounts of FGSC extracts. An 18.9-L (5-gal) vacuum chamber (Smooth-On Corp., Macungie, PA) was used to hold a 4.7-L (1.25-gal) plastic pail with 300g of FGSC. The FGSC were covered with 3 liters of de-ionized water (S:F = 10:1). The pail placed in the vacuum chamber, and the chamber sealed with a thick Plexiglas cover. Vacuum (-0.088 MPa) was applied to the chamber and the air allowed to escape from the FGSC for 30 min. The vacuum was then released, and the extract water separated from the FGSC. The FGSC were covered with 2.5 liters of de-ionized water (S:F = 8.3:1) and the vacuum/extraction process repeated in this manner twice for a total S:F of 26.6. The extracts were filtered through a coarse sintered glass frit and purified by ultrafiltration as described above. The 100 K cut-off retentate was freeze dried and the yield determined by weighing the freeze-dried residue. The sugar compositions of the extracts were determined by PAAN and TFA/PAAN analysis. There were three replications performed.
Water Adsorption
Hay et al. (2017) reported that FGP is a hygroscopic polysaccharide that adsorbs atmospheric water vapor, reaching a moisture content of 38% at 90% relative humidity. Freeze dried samples of unfractionated FGSC extracts were hygroscopic and prone to darkening and a decrease in volume or syrup formation (Fig. 6). The unfractionated FGSC aqueous extracts were found to contain approximately equal amounts of FGP and low molecular weight sugars (i.e., glucose, fructose, and sucrose). After noting that a highly purified (by ethanol precipitation) FGP extract did not appear to adsorb moisture even after being left exposed to humid air in the lab, it was hypothesized that the low molecular weight sugars were responsible for the water adsorption.
A sample of the ethanol precipitated FGP was dissolved in water and then freeze dried. A second sample of the ethanol precipitated FGP was mixed with glucose, fructose, and sucrose in a ratio of 0.5:0.2:0.2:0.1, respectively. Approximately 400 mg of the ethanol precipitated FGP was placed in a 60-mL uncapped bottle. Approximately 400 mg of the FGP:glucose:fructose:sucrose mixture was placed in another uncapped 60-mL bottle. Both bottles were left on a lab bench overnight and re-weighed after 24 h. The saccharide mixtures were also observed for changes in appearance.
Fig. 6. Two examples of freeze-dried frost grape stem chip extract samples inadequately protected against humid laboratory conditions showing varying degrees of darkening and decreased volume
Statistical Analyses
Statistix 8.1 software (Analytical Software, Tallahassee, FL, USA) was used to perform statistical analyses of the data using analysis of variance (ANOVA). Treatment means were compared using least significant difference (LSD) after obtaining a significant F-test (P ≤ 0.05).
RESULTS AND DISCUSSION
Collection of Frost Grape Stem Exudates (FGSExu)
Figure 3 shows a photograph of the field syringe after collecting frost grape stem exudate. The mean (±SEM) wet mass exudate collected on March 31, 2021 was 1.05 g (± 0.42) with a range of 0.05 to 3.5 g. The mean (±SEM) percentage solids was 15.1% (± 5.1) with a range of 0.36 to 40.67%. The PAAN analysis of the unhydrolyzed stem exudate did not indicate the presence of any free arabinose or galactose, but it did detect glucose (57.1%) and fructose (30.1%). However, the TFA/PAAN hydrolysis analysis indicated that the stem exudate contained mostly arabinose (28.0%) and galactose (51.0%), with mannose (9.7%) and xylose (5.3%) as FGP with some glucose (6.0%) present as well.
During collections made on May 7, 2021, only one of the five replications had sufficient flow to measure immediately, and it was determined to be 16.2 mL/min. The remaining four replications were re-checked after 24 h, and all had some collected material with an average (n=5) of 13.2 g collected. On May 17, sap immediately began flowing in all five replications after drilling the hole, and the average flowrate on this date was 10.9 mL/min. The mean percent solids for the May 7 and May 17 collections were both very low at 0.38% and 0.43%, respectively and these means were not significantly different (F1,8 = 0. 0.36, P = 0.56). The PAAN analyses indicated that both sap collections contained very high amounts of glucose (90.1 and 75.3%, respectively). The PAAN analyses revealed that free arabinose and galactose were present in the 210507 collection but were not detected in the 210517 collection. The TFA/PAAN hydrolysis analyses also indicated that both 2100507 and 210517 contained some FGP, but that its contribution to the total sugar content was relatively low (ca. 10% and 5%, respectively).
Lebon et al. (2008) noted that glucose, fructose, and sucrose are the major sugars present in the grape xylem and phloem sap, which feed the developing inflorescence. The present sugar analyses also indicate the presence of large amounts of glucose and fructose in the grape sap.
Over the range of time collections were made, there were clear changes in the composition of the exudates from living FGS, including appearance, percent solids, sugar composition, and FGP percentage. The collections made in March were thick and mucilaginous, with very high percentage solids, and a high proportion of the FGP. However, the collections made in May were very thin and watery, with very low percentage solids, composed of mostly glucose and fructose with only low amounts of the FGP.
Although the function of the FGP in frost grapes is uncertain, it is hypothesized that it is related to wound healing in an analogous manner to that of GA in acacia. Although the FGP is soluble in water, interestingly, Price et al. 2015 reported that FGP forms stable gels at 15%, the exact same percentage solids that were measured for the stem exudates in the present work.
In addition, the lower portion of the trunk of western larch, referred to as the butt cut, contains so much AG (i.e., ca. 15 to 25% of the dry log), that the trunk is neither good for lumber nor for pulping purposes (Dewitt 1990). Butt cuts discarded 40 years ago are exceedingly well preserved. The arabinogalactans might confer resistance to decay (e.g., termites and/or wood-decay fungi). Leather et al. (2017) reported that the FGP was resistant to degradation by fungal enzymes.
To enhance the feasibility of FGP being used commercially, new methods will be needed to collect the grape sap. It might be possible to collect exudates from frost grape stems using a vacuum system in a manner analogous to that used for sap from maple trees (Pitcoff 2014; Perkins et al. 2016). Perkins and van den Berg (2016) describe a method to collect maple sap from small diameter maple trees. In a similar fashion, frost grape exudates might be collected from small diameter vines planted for this purpose.
As current production of grapes in the U.S. is approximately 5.5 million metric tons on over 360,000 hectares (USDA, NASS, 2022), it would likely only require a small percentage of this area devoted to grape production for FGP processing to obtain sufficient FGP for use as a food emulsifier.
The mean percent residue yields for the vacuum/atmospheric pressure cycle and pressure/atmospheric pressure cycle treatments were 9.0 and 8.2%, respectively, and these means were statistically equivalent (F1,10 = 0.53, P = 0.48). Because the vacuum/ atmospheric pressure cycle treatment was numerically higher, subsequent extractions were performed using this method.
The pressure swing extraction method did force water into the FGSC, and it is believed that this assisted in the subsequent extraction of the FGP. Although sonication was considered as an alternative means to improve extraction efficiency, Leathers et al. (2017) reported that the average molecular weight of the FGP decreased during sonication and was associated with a decrease in viscosity.
Solvent to Feed (S:F) Ratio
The percentage yields for the five sequential extractions are shown in Fig. 7.
Fig. 7. Frost grape stem chip extract (FGSCExt) yield as a function of number of extractions
The yields decreased exponentially with extraction number and a power function fit the data extremely well (R2 = 0.9938) with the equation: Y=4.7357 X-1.871, where X= extraction number and Y = extraction yield (%). The first three extractions (i.e., total S:F = 23.7) gave ca. 92% of what was obtained after five extractions (i.e., S:F = 38.6). Therefore, subsequent extractions were limited to three extractions.
Frost Grape Stem Chip (FGSC) Size
The percentages of the total chip mass for the five chip size fractions were as follows: A (15.4%); B (42.0%); C (36.6%); D (3.9%); and E (2.0%). The majority (i.e., over 78%) of the chips were in fractions B and C. The extraction yields for the chip size fractions are shown in Fig. 8.
Fig. 8. Mean (n=3) (±SEM) extract mass yields of frost grape stem chip extracts after milling and fractionating with sieves. Means without letters in common differ significantly using Least Significant Difference (P≤0.05).
Fractions C and B gave the highest yields and were statistically equivalent at 12.2% and 11.6%, respectively. Fraction A yielded 11.1% followed by fraction D at 9.1%, and lastly fraction E, which yielded only 1.7%. Chip size had a significant effect on mass yield, as well and the highest yields were obtained from chips between 1.84 and 3.80 mm. Coincidentally, these two fractions also accounted for 78% of the total chip mass.
Purification and Separation of FGP from Aqueous Extract (FGSCExt)
C-18 filtration
The aqueous extracts of the FGSC were initially light yellow in color (Fig. 9). However, after filtration through the C-18 filter, the extract was clear and nearly colorless. The colored material removed was clearly retained on the C-18 and could subsequently be removed by extraction with methanol. The quantity of this amber-colored material adsorbed by the C-18 filter was determined to be 0.278 g from 22.5 g FGSC (i.e., 1.2% of the original mass). It is hypothesized that this amber-colored material contains phenolic lignans and/or tannins. An aqueous extraction of the scaped inner bark gave a very dark extract demonstrating the inner bark contained high amounts of the amber-colored material. In addition, PAAN and TFA PAAN analyses of this methanol extract did not indicate the presence of any arabinose, galactose, xylose, or mannose (i.e., FGP sugar components). Instead, it only revealed the presence of glucose. Figure 10 shows the FGSCExt after freeze drying and the extracts that were filtered with the C-18 are much lighter in color than the unfiltered FGSCExt.
Fig. 9. Frost grape stem extracts before C-18 filtering (A), after C-18 filtering (B) and bark extract without C-18 filtering (C)
The TFA/PAAN hydrolysis analyses of the FGSCExt before and after C-18 filtration were nearly identical. The relative percentages of arabinose, xylose, mannose, glucose, and galactose were 32.8%, 6.9%, 9.2%, 3.7%, and 47.5% (before C-18) and 35.8%, 6.8%, 8.5%, 3.3% and 45.7% (after C-18 filtration), respectively. The C-18 filtration does not appear to influence the FGP composition.
The C-18 solid phase filtration removed co-extracted colored material from the aqueous frost grape stem chip extract (FGSCExt) efficiently and provided a significantly lighter solid product. Because it was somewhat tedious to perform, it might not always be necessary, particularly applications where light-colored FGP is not required.
Fig. 10. Freeze-dried frost grape stem chip extracts without C-18 filtering (left) and with C-18 filtering (right)
Spray drying
The spray-dried FGP was recovered in 49% yield as a light tan powder. The EAI for freeze dried and spray-dried FGSCExt were 126.6 and 120.0, respectively, which were statistically equivalent to each other as well as to gum arabic (116.8). The ESI for freeze dried and spray dried FGSCExt were 26.0 and 24.6, respectively, which were also statistically equivalent to each other as well as gum arabic (23.9). Although both freeze drying and spray drying effectively removed water from the FGP solution, freeze drying is slow and likely a more costly method than spray drying. The two methods gave essentially equivalent products. Therefore, the quicker, less costly method is preferred.
Ultrafiltration
The results of the 100K cut-off ultrafiltration of the FGSCExt are shown in Table 1. The PAAN analysis indicated that the unfractionated FGSCExt contained ca. 40% glucose, 45% fructose, and 15% sucrose. Arabinose, galactose, mannose, and xylose (i.e., FGP components) were not detected in the FGSCExt. Likewise, the PAAN analysis indicated only the presence of glucose, fructose, and sucrose with no free arabinose, galactose, xylose, or mannose. The 100K cut-off ultrafiltration was very effective at separating the FGP into the retentate and the low molecular weight sugars (i.e., glucose, fructose, and sucrose) into the permeate. These co-extracted low molecular weight sugars could be fermented into ethanol, providing an additional revenue stream from FGP processing. In addition, the ultrafiltration concentrated the FGP solution by removing water from the solution.
Table 1. GC-MS Analysis after Per-O-acetylated Aldonitrile Acetate (PAAN) Derivatization
Unfractionated frost grape stem chip extract (FGSCExt), retentate of 100K cutoff ultrafiltration of FGSCExt and permeate of 100K cutoff ultrafiltration of FGSCExt. na stands for not applicable due to the degradation of fructose and sucrose by the TFA hydrolysis.
Table 2. GC-MS analysis after Trifluoroacetic Acid (TFA) Hydrolysis Followed by Per-O-acetylated Aldonitrile Acetate (PAAN) Derivatization
na stands for not applicable due to the degradation of fructose and sucrose by the TFA hydrolysis.
Table 3. Elemental Analyses Before and After 100K Cut-off Ultrafiltration of Frost Grape Stem Chip Extract (FGSCExt)
Within a column, means (n=3) without letters in common differ significantly using Least Significant Difference (P ≤ 0.05).
The TFA/PAAN hydrolysis analysis indicated that although the unfractionated FGSCExt contained ca. 47% glucose, it also contained the FGP components arabinose, galactose, xylose, and mannose (ca. 53%) (Table 2). The TFA/PAAN hydrolysis analysis indicated that the 100K permeate contained over 90% glucose and only very small amounts of galactose and arabinose. Conversely, the 100K retentate contained less than 2% glucose with the remainder FGP components, arabinose, galactose, xylose, and mannose. The 100K cut-off membrane concentrated the FGP into the retentate by partitioning the low molecular weight sugars into the permeate. The sugar analyses (i.e., PAAN and TFA/PAAN hydrolysis) of the unfractionated FGSCExt indicated that ca. 50% was low molecular weight sugars, including glucose, fructose, and sucrose, and the other 50% was the FGP.
Price et al. (2015) used ethanol precipitation to purify FGP. In that case, the ethanol both separated the FGP from water as well as from low molecular weight sugars. EtOH precipitation partitions the low MW sugars into the supernatant and the FGP into the precipitate.
Chemical Properties of FGP
Elemental analysis
The results of the elemental analyses of the unfractionated, 100K cut-off retentate and permeate are shown in Table 3. The unfractionated FGSCExt contained many different elements with very high levels of potassium (K) and calcium (Ca). Le et al. (2017) also reported high levels of these two compounds from the “bleeding sap of grapes”.
The elemental analyses performed by X-ray fluorescence after 100K cut-off ultrafiltration of the FGSCExt indicated that most elements (i.e., K, Ca, phosphorus (P), silicon (Si), sulfur (S), chlorine (Cl), manganese (Mn), copper (Cu), zinc (Zn), and nickel (Ni)) were concentrated into the permeate. Most of the elements identified likely occur as small ionic compounds and would be expected to move into the permeate. Only titanium (Ti) and iron (Fe) had significantly higher concentrations in the retentate than the unfractionated extract. Interestingly, only Ti was both significantly lower in the permeate than in the unfractionated extract and significantly higher in the retentate than in the unfractionated extract. Similarly, the XRF results suggest that the polysaccharide was both significantly lower in the permeate than in the unfractionated extract and significantly higher in the retentate than in the unfractionated extract. Although this method only calculates the polysaccharide matrix as the difference from the other elements, this is expected from the ultrafiltration with the MW cut-off that was used. Ti might be associated with the large molecular weight FGP that constitutes the polysaccharide in the retentate.
Carbohydrate analyses indicated that the purified FGP in this study were essentially identical to that reported by Price et al. (2015). The elemental analyses indicated that although most of the elements that were measured partitioned into the permeate, Ti and Fe partitioned into the retentate with the FGP. This suggests that these two compounds are somehow associated with the FGP and partition with it during the ultrafiltration. It has been reported that most plants have about 1 to 2 ppm Ti, although horsetail and nettles contain up to 80 ppm and plants may use Ti to stimulate production of carbohydrates (Emsley 2001). The ultrafiltration separation of Ca into the permeate while the FGP remains in the retentate could influence the subsequent viscosity of FGP if the Ca has a significant interaction with the glucuronic acid portion off the FGP (Price et al. 2015).
Dietary fiber analysis
The isolated FGP had 50.45 ± 0.69 g/100 g of total fiber that was composed of 44.36 ± 0.53 g/100 g of soluble fiber and 6.09 ± 1.21 g/100 g of insoluble fiber. These initial findings show FGP to be fiber rich and contain a high level of soluble dietary fiber due to its arabinogalactan polymeric structure.
Dietary fiber is a class of compounds that are typically nondigestible and includes a variety of carbohydrate oligosaccharides and polymers that have beneficial physiological effects in humans (Elleuch et al. 2011). Dietary fiber from natural sources is appealing for use as a food ingredient to improve their nutritional value and functional properties. FGP represents a potentially novel fiber source that may contain high levels of soluble fiber and, therefore, the dietary fiber content was determined. The fiber analysis of the FGP showed that it contained ca. 50% fiber, which was mostly soluble fiber. It is possible that the FGP could be used as a fiber source in food applications after further testing to demonstrate its safety and physiological functionalities.
Physical Properties of FGP
Emulsifying activity index (EAI) and emulsion stability index (ESI)
The EAIs for unfractionated FGSCExt, 100K permeate, and 100K retentate were 120.1, 94.0, and 176.9, respectively. The EAI for the 100K retentate was statistically higher than the unfractionated FGSCExt, and the unfractionated FGSCExt was statistically higher than the 100K permeate. The EAI for the 100K retentate was also statistically higher than the gum arabic (116.8). The ESI for unfractionated FGSCExt, 100K permeate and 100K retentate were 22.5, 57.1 and 14.9, respectively. The ESI for the 100K permeate was statistically higher than both the unfractionated FGSCExt and 100K retentates, which were statistically equivalent to each other. The ESI for gum arabic (23.9) was statistically lower than the100K permeate, equivalent to the unfractionated FGSCExt and higher than the 100K retentate.
The emulsification analyses indicated that EAI for the FGP in the retentate was higher than for the unfractionated. Somewhat unexpectedly, the EAI for the permeate was also quite high and the ESI for the permeate was higher than both the unfractionated material as well as the retentate. Apparently, the low molecular weight sugars have significant emulsification activity.
Fig. 11. Viscosity of frost grape stem chip extract (FGSCExt) as a function of shear rate
Viscosity
The viscosity of FGSCx as a function of shear rate is shown in Fig. 11. It was very similar to that reported by Hay et al. (2017).
Methanol Pre-Extraction of FGSC
Soxhlet methanol extraction
The Soxhlet methanol pre-extraction of the FGSC yielded a dark amber material with an average (±SEM) mass of 14.0g (±0.37), which was 4.7% (±0.12) of the original 300 g of FGSC extracted. The methanol pre-extraction of the FGSC removed a significant amount of colored material from the FGSC (i.e., ca 5%). This methanol extract was subsequently shown to contain significant amounts of resveratrol and ε-viniferin. Both compounds are reported to have antioxidant activity and have reputed beneficial human health effects. The methanol extract of FGSC could be a source of these compounds and a potential additional revenue source from a by-product of FGP production.
Methanol extract analysis
Two major UV absorbing peaks were seen in the HPLC chromatograms of the methanol extract of FGSC. When the extract was run on the LC-MS the initial eluting peak gave ions of m/z 229.0855(+) and 227.0712(-), which corresponds to MW of 228.0777/228.0790 and matches an empirical formula of C14H12O3. This was confirmed as being resveratrol, as the retention time matched that of a standard from Chromadex (Irvine, CA). The second eluting peak gave ions of m/z 455.1482(+) and 453.1342(-), which corresponds to MW of 454.1404/454.1420 and matches an empirical formula of C28H22O6. This matches the empirical formula for ε-viniferin a dimer of resveratrol that is found in a number of grape species.
A five-point standard curve was prepared from a standard of resveratrol for the evaluation of the concentration of the identified phenolics for the determination of extinction coefficients at 280 and 340 nm, which was used to quantitate the compounds in the frost grape stem methanol extracts at 30.2 mg/g resveratrol and 58.7 mg/g ε-viniferin. Trace amounts of piceatannol, another commonly found phenolic in grape species tissues were also detected.
Scaled-Up Extraction
The mean (±SEM) mass of FGP obtained after aqueous extraction, C-18 filtration, retentate collection of 100K cut-off ultrafiltration, and freeze drying was 8.9 g (±0.50), which was 3.0% (±0.17) of the original 300 g of FGSC extracted. The PAAN analyses of the FGP revealed that it contained almost no glucose. The TFA/PAAN hydrolysis analyses indicated the FGP had the following mean relative percentages of arabinose, xylose, mannose, glucose, and galactose: 37.6%, 8.0%, 5.8%, 0.7% and 47.9%, respectively.
Table 4 compares several variables associated with the collection methods tested (i.e., Exudate, Sap, or Scaled-Up Pressure-Swing Aqueous Extraction). Percent solids varied widely for three methods, with a low of only 0.22 percent for the pressure swing aqueous extraction and a high of 15 percent for the exudate collection. Percentage FGP also varied widely for the three methods, with a low of only 5 percent for the sap and a high of 94 percent for exudate. When the data are extrapolated to higher numbers of syringes (e.g., 25) or more pressure swing extractions (i.e., 4 per day), the sap collection gave the highest rate of FGP mass per day. However, the sap collection can only be performed during the limited time of year (i.e., early spring) when the grape sap is running. In addition, the sap contains a very high percentage of water (i.e., over 99%) that must be removed to obtain the solids, which in turn, are only 5 percent FGP. The exudate had a very high percentage FGP (i.e., 94%), and its percent solids was ca. 35 times more concentrated than the sap. However, this collection method is tedious and inconvenient (i.e., required trip to the field, finding dispersed vines, drilling holes, inserting syringes, waiting 24 hours prior to collecting syringes, and removing exudate from the syringes). The scaled-up extraction of FGSC can conveniently be performed any time after harvest and chipping the FGS, the extract has a relatively high percent FGP (i.e., 50%), the water can be removed by spray drying or ultrafiltration, and the low molecular weight sugars can be separated from the FGP by ultrafiltration or ethanol precipitation.
Table 4. Comparison of FGP Collection Methods: Exudate, Sap and Scaled-Up Pressure-Swing Aqueous Extraction
aExtrapolations are based on 25 syringes per day for Exudate and Sap Collections and 4-90 minute extractions of 300 g FGSC.
b % Solids based on 17.8 g solids from 300 g FGSC using 8 liters water.
This material had a mean (±SEM) EAI of 157.0 (± 1.8) and a mean ESI (±SEM) of 14.9 (±0.3). Although the FGP had a higher EAI than gum arabic, its ESI was slightly lower. It might be desirable to increase the ESI of the FGP by some means.
Water Adsorption
Figure 12 shows the purified FGP with added glucose, fructose, and sucrose and FGP without added glucose, fructose, and sucrose after 24 h on the lab bench. In this time, there was no apparent change in the appearance of sample containing only purified FGP, while the FGP with glucose, fructose and sucrose had visibly decreased in volume and had darkened slightly in color.
Fig. 12. Purified frost grape polysaccharide (by ethanol precipitation) with added glucose, fructose, and sucrose (left) and purified frost grape polysaccharide without added glucose, fructose, or sucrose (right) after air exposure on bench top in laboratory.
After 24 h, the purified FGP had lost 0.0269 g (-6.5%), while the FGP with glucose, fructose and sucrose had gained 0.0792 g (19.2%). The presence of the glucose, fructose, and sucrose with the FGP appear responsible for much of the adsorption of water and corresponding change in appearance of the FGP mixture.
Hay et al. (2017) stated that proper storage of FGP must consider the relative humidity of the storage environment to prevent processing issues. Separation of low molecular weight sugars such as glucose, fructose, and sucrose from the FGP does reduce its water adsorption and should increase its stability. The presence of these low molecular weight sugars and their absorption of water appears analogous to the absorption of water from air by the confection cotton candy in humid conditions.
In addition, the storage problems seen with the dried samples of FGP, FGSCExt held in a refrigerator at ca. 5 °C for several months showed definite signs of microbial contamination. Bottles of FGSC extracts left in the refrigerator (ca. 5°C) were found to contain masses of an unidentified microbe, presumably a fungus or multiple species of fungi that were growing in the extract. The extractions were not done aseptically, and although autoclaving the extracts was considered to kill microbes present in the extracts, Leathers et al. (2017) reported a decrease in the MW of FGP after autoclaving. Therefore, it is probably prudent to process FGP extracts to dryness as quickly as possible to prevent the growth of any microbes that might be present.
CONCLUSIONS
- Pressure swing water extraction of frost grape stem chips (FGSC) using a solved-to feed ratio (S:F) of 23.7 effectively removes frost grape polysaccharide (FGP) from FGSC.
- Methanol pre-extraction of FGSC removes interfering colored material and gives a lighter extract.
- C-18 filtration of the water extract removes co-extracted colored material from the water extract and yields lighter FGP.
- Ultrafiltration (100K cut-off ) of the water extract separates FGP from low molecular weight sugars (i.e., glucose, fructose, and sucrose) and water in the FGSC extract.
- Separation of the low molecular weight sugars increases the stability of the FGP.
- Both emulsifying activity index (EAI) and emulsion stability index (ESI) were higher for FGP than gum arabic.
- Titanium appeared to be tightly associated with the FGP.
- Resveratrol and ε-viniferin were present in the methanol extract of the FGSC.
ACKNOWLEDGMENTS
The authors wish to thank Jefferey Teel, Jackson Edwards, Trina Hartman, Loren Iten, Steven Lyle, Grace Nieukirk, Karen Ray, Andrew Thomas, Suzanne Unser, Kelly Utt, and Robert Bartelt for their assistance. This research was supported in part by the U.S. Department of Agriculture, Agricultural Research Service.
REFERENCES CITED
Adams, M. F. (1967). “Process for preparing arabinogalactan,” U.S. Patent 333,752,6.
Arno, S. F., and Hammerly, R. P. (2020). Northwest Trees: Identifying & Understanding the Region’s Native Trees (field guide Ed.), Seattle, Mountaineers Books.
Canalejo, D., Guadalupe, Z., Martínez-Lapuente, L., Ayestarán, B., and Perez-Magari, S. (2021). “Optimization of a method to extract polysaccharides from white grape pomace by-products,” Food Chemistry 365, article 130445. DOI: 10.1016/j.foodchem.2021.13044
Churms, S. C., Merrifield, E. H., and Stephen, A. M. (1983). “Some new aspects of the molecular structure of Acacia senegal gum (gum arabic),” Carbohydr. Res. 23, 267-279. DOI: 10.1016/0008-6215(83)88483-3
Connolly, S., Fenyo, J.-C., and Vandevelde, M.-C. (1988). Effect of a proteinase on the macromolecular distribution of Acacia senegal gum,” Carbohydr. Polym. 8, 23-32. DOI: 10.1016/0144-8617(88)90033-1
Defaye, J., and Wong, E. (1986). “Structural studies of gum arabic, the exudate polysaccharide from Acacia senegal,” Carbohydr. Res. 150, 221-231. DOI: 10.1016/0008-6215(86)80018-0
DeWitt, J. E. (1990). “Method of isolating arabinogalactan from larch,” U.S. Patent 4,950,751.
Dickinson, E. (2003). “Hydrocolloids at interfaces and the influence on the properties of dispersed systems,” Food Hydrocolloids 17, 25-39. DOI: 10.1016/S0268-005X(01)00120-5
Dickinson, E., Murray, B. S., Stainsby, G., and Anderson, D. M. (1988). “Surface activity and emulsifying behaviour of some Acacia gums,” Food Hydrocolloids 2, 477-490. DOI: 10.1016/S0268-005X(88)80047-X
Eller, F. J., Clausen, C. A., Green, F., and Taylor, S. L. (2010). “Critical fluid extraction of Juniperus virginiana L. and bioactivity of extracts against subterranean termites and wood-rot fungi,” Indus. Crops Prod. 32, 481-485. DOI: 10.1016/j.indcrop.2010.06.018
Eller, F. J., Vander Meer, R. K., Behle, R. W., Flor-Weiler, L. B., and Palmquist, D. E. (2014). “Bioactivity of cedarwood oil and cedrol against arthropod pests,” Environ. Entomol. 43, 762-766. DOI: 10.1603/EN13270
Eller, F. J., Mankowski, M. E., Kirker, G. T., and Selling, G. W. (2021). “Effects of loblolly pine extract, primary and quaternary alkyl ammonium chlorides combined with burgundy oil from eastern red cedar against subterranean termites and wood-decay fungi,” BioResources 16, 893-910. DOI: 10.15376/biores.16.1.893-910
Elleuch, M., Bedigian, D., Roiseux, O., Besbes, S., Blecker, C., and Attia, H. (2011). “Dietary fibre and fibre-rich by-products of food processing: Characterization, technological functionality and commercial applications: A review,” Food Chemistry 124, 411-421. DOI: 10.1016/j.foodchem.2010.06.077
Emsley, J. (2001). “Titanium,” in: Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, Oxford, UK.
Eltohami, A. A. (2018). “Threats to green gum Arabic production in Sudan,” Biomed. J. Sci. & Tech. Res. 3, 3526-3530. DOI: 10.26717/BJSTR.2018.03.000951
Felker, F. C., Kenar, J. A., Byars, J. A., Singh, M., and Liu, S. X. (2018). “Comparison of properties of raw pulse flours with those of jet-cooked, drum-dried flours,” Food Science and Technology 96, 648-656. DOI: 10.1016/j.lwt.2018.06.022
Free, D. L., Krumel, K. L., and Snyder, T. C. (1976). “Self-breaking viscous aqueous solutions and the use thereof in fracturing subterranean formations,” U.S. Patent 3,960,736.
Guadalupe, Z., Martínez-Pinilla, O., Garrido, A., Carrillo, J. D., and Ayestarán, B. (2012). “Quantitative determination of wine polysaccharides by gas chromatography-mass spectrometry (GC-MS) and size exclusion chromatography (SEC),” Food Chemistry 131, 367-74. DOI: 10.1016/j.foodchem.2011.08.049
Hay, W. T., Vaughn, S. F., Byars, J. A., Selling, G. W., Holthaus, D. M., and Price, N. P. J. (2017). “Physical, rheological, functional, and film properties of a novel emulsifier: Frost grape polysaccharide from Vitis riparia Michx,” J. Agric. Food Chem. 65, 8754-8762. DOI: 10.1021/acs.jafc.7b03318
Jones-Moore, H. R., Jelley, R. E., Marangon, M., and Fedrizzi, B. (2022). “The interactions of wine polysaccharides with aroma compounds, tannins, and proteins, and their importance to winemaking,” Food Hydrocolloids 123, article 107150. DOI: 10.1016/j.foodhyd.2021.107150
Leathers, T. D., Price, N. P. J., Vaughn, S. F., and Nunnally, M. S. (2017). “Reduced-molecular-weight derivatives of frost grape polysaccharide,” Internat. J. Biolog. Macromolecules 105, 1166-1170. DOI: 10.1016/j.ijbiomac.2017.07.143
Le, L., Umar, A., Iburaim, A., and Moore, N. (2017). “Constituents and antioxidant activity of bleeding sap from various Xinjiang grapes,” Pharmacognosy Mag. 13, 5728-5730. DOI: 10.4103/pm.pm_358_16
Lebon, G., Wojonarowiez, G., Holzapfel, B., Fontaine, F., Vallant-Gaveau, N, and Clement, C. (2008). “Sugars and flowering in the grapevine (Vinis vinifera L.),” J. Exper. Bot. 59, 2566-2578. DOI: 10.1093/jxb/ern135
Manjón, E., Li, S., Dueñas, M.,García-Estévez, I., and Escribano-Bailón, M. T. (2022). “Effect of the addition of soluble polysaccharides from red and white grape skins on the polyphenolic composition and sensory properties of Tempranillo red wines,” Food Chemistry 400, article 134110. DOI: 10.1016/j.foodchem.2022.134110
Osman, M. E., Menzies, A. R., Williams, P. A., Phillips, G. O., and Baldwin, T. C. (1993). “The molecular characterization of the polysaccharide gum from Acacia senegal,” Carbohydr. Res. 246, 303-318. DOI: 10.1016/0008-6215(93)84042-5
Perkins, T. D., Isselhardt, M. L., Wilmot, T. R., and Stowe, B. (2016). “A summary of research to improve vacuum in maple tubing systems,” Maple Syrup Digest February, 11-19.
Perkins, T., and van den Berg, A. (2016). “Sap collection from small-diameter trees,” Maple Syrup Digest October, 29-36.
Phillips, G. O. (1998). “Acacia gum: A nutritional fibre, metabolism and calorific value,” Food Addit. Contam. 15, 251-264. DOI: 10.1080/02652039809374639
Phillips, G. O., Williams, P. A., and Wedlock, D. J. (eds.) (1994). Gums and Stabilizers for the Food Industry 7, IRL Press, Oxford, UK.
Pitcoff, W. (2014). “Boiling it down: A new take on sap collection,” Farming: Journal of Northeast Agriculture June, 62-63.
Price, N. P. J. (2004). “Acyclic sugar derivatives for GC/MS analysis of 13C-enrichment during carbohydrate metabolism,” Anal. Chem. 76, 6566-6574. DOI: 10.1021/ac049198m
Price, N. P. J., Vermillion, K. E., Eller, F. J., and Vaughn, S. F. (2015). “Frost grape polysaccharide (FGP), an emulsion-forming arabinogalactan gum from the stems of native North American grape species Vitis riparia Michx,” J. Agric. Food Chem. 63, 7286-7293. DOI: 10.1021/acs.jafc.5b02316
Ray, A. K., Bird, P. B., Iacobucci, G. A., and Clark, B. C., Jr. (1995). “Functionality of gum arabic: fractionation characterization and evaluation of gum fractions in citrus oil emulsions and model beverages,” Food Hydrocolloids 9, 123-131. DOI: 10.1016/S0268-005X(09)80274-9
Saeed, F., Pasha, I., Anjum, F. M., and Sultan, M. T. (2011). “Arabinoxylans and arabinogalactans: A comprehensive treatise,” Crit. Rev. Food Sci. Nutr. 51, 467-476. DOI: 10.1080/10408391003681418
Thevenet, F. (1988). “Acacia gums: Stabilizers for flavor encapsulation,” in: Flavor Encapsulation, ACS Symposium Series (Vol. 370), American Chemical Society, Washington, DC. DOI: 10.1021/bk-1988-0370.ch005
USDA, NASS (2022). “Grapes,” (https://www.nass.usda.gov/Statistics by Subject-Grapes), Accessed 09-18-2022.
Article submitted: March 21, 2023; Peer review completed: May 6, 2023; Revised version received: May 11, 2023; Accepted: May 12, 203; Published: May 17, 2023.
DOI: 10.15376/biores.18.3.4610-4635
