Abstract
The combination of metal organic frameworks (MOFs) with other functional materials is a potential strategy for the preparation of advanced MOF-based materials. In this study, a simple approach is reported for the fabrication of cellulosic paper containing zeolitic imidazolate framework (ZIF-8) through in-situ loading in the papermaking process. The results showed that the ZIF-8 was evenly distributed in the paper substrate owing to the multi-layers structure of the cellulosic fibers, although the loading of the ZIF-8 particles on the single cellulosic fiber was nonuniform. The as-prepared ZIF-8 composites can be used as a highly efficient adsorbent material for anionic dyes in aqueous solution thanks to the positive charge on the surface of the ZIF-8 particles. More than 92% of the methyl orange (MO-) dye in the aqueous solution was rapidly removed through a simple filtration process using the ZIF-8 composite cellulosic paper (hand-sheets made in lab) when the content of ZIF-8 in cellulosic paper was high as 25.1%. In addition, the ZIF-8 composite paper had acceptable flexibility and could be reused at least 4 cycles by washing out the adsorbed dye.
Download PDF
Full Article
Fabrication of Cellulosic Paper Containing Zeolitic Imidazolate Framework and Its Application in Removal of Anionic Dye from Aqueous Solution
Zicheng Chen,a,b Huiwen Zhang,a Xiangyang He,a Guangyuan Fan,a Xiaosong Li,a Zhibin He,b Guanhua Wang,b,c,* and Lanhe Zhang a,*
The combination of metal organic frameworks (MOFs) with other functional materials is a potential strategy for the preparation of advanced MOF-based materials. In this study, a simple approach is reported for the fabrication of cellulosic paper containing zeolitic imidazolate framework (ZIF-8) through in-situ loading in the papermaking process. The results showed that the ZIF-8 was evenly distributed in the paper substrate owing to the multi-layers structure of the cellulosic fibers, although the loading of the ZIF-8 particles on the single cellulosic fiber was nonuniform. The as-prepared ZIF-8 composites can be used as a highly efficient adsorbent material for anionic dyes in aqueous solution thanks to the positive charge on the surface of the ZIF-8 particles. More than 92% of the methyl orange (MO–) dye in the aqueous solution was rapidly removed through a simple filtration process using the ZIF-8 composite cellulosic paper (hand-sheets made in lab) when the content of ZIF-8 in cellulosic paper was high as 25.1%. In addition, the ZIF-8 composite paper had acceptable flexibility and could be reused at least 4 cycles by washing out the adsorbed dye.
Keywords: Zeolitic imidazolate framework; Hand-sheets; Cellulosic paper; Filtration; Methylene orange
Contact information: a: School of Chemical Engineering, Northeast Electric Power University, Jilin, Jilin Province 132012, P. R. China; b: Department of Chemical Engineering, University of New Brunswick, Fredericton, NB E3B 5A3, Canada; c: Tianjin Key Laboratory of Pulp and Paper, College of Light Industry Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China;
* Corresponding authors: ghwang@tust.edu.cn; zhanglanhe@163.com
GRAPHICAL ABSTRACT
INTRODUCTION
Metal-organic frameworks (MOFs) have been studied and applicated extensively in the field of gas storage, separation, sensing, and catalysis due to their abundant nanopores (Kim et al. 2017; Sun et al. 2019; Demakov et al. 2020; Pérez-Cejuela et al. 2020). The composition of MOFs and other materials is a valuable approach to the development of new materials with better applicability considering the recycling and the improved properties of MOFs (Li et al. 2017; Choi et al. 2020). In particular, the growth of MOFs on the surface of different substrates can lead to the formation of membrane MOF materials, which are particularly valuable in separation techniques and catalysis (Guo et al. 2010; Jiang et al. 2013; So et al. 2013; Lange and Obendorf 2015; Wang et al. 2015; Matsumoto and Kitaoka 2016; Yu et al. 2016; Rafti et al. 2017). However, the use of paper as a substrate for the immobilization of MOFs in the preparation of flexible MOF materials is quite rare (Zhuang et al. 2013).
As a cellulosic-lignin film material, paper has received much attention in various fields such as printing and packaging due to its renewable, biodegradable, and low-cost characteristics. Paper is an appropriate carrier in filter technology since it can provide a high degree of flexibility and porosity. Recently, studies on MOF and cellulosic paper composite materials have been published (Wang et al. 2015; Yang et al. 2017; Duan et al. 2018; Qian et al. 2018). Cellulose matrix-supported MOF hybrid aerogel composites also displayed excellent dye removal properties. ZIF-67 coated on the surface of the cellulosic aerogel and the composite showed a high adsorption capacity for MO−, which was up to 617 mg/g (Song et al. 2019). These composite materials combine the merits of MOFs and cellulosic paper to exhibit novel properties such as bacterial inhibition and dye adsorption.
The dyes in effluent have become a global concern because many of them are toxic and nonbiodegradable, such that they pose a serious threat to the environment (Chen et al. 2020; Wang et al. 2020). Numerous technologies have been developed to remove dyes. Among the various methods, adsorption has been preferred on account of its low operation cost and high efficiency (Sui et al. 2020). In terms of dye adsorption, MOFs are an excellent candidate for removal of organic dyes by adsorption. Among the various MOF materials, zeolite imidazole salt frameworks (ZIFs) are a new type class of porous crystalline materials with excellent properties such as uniform pores, a large surface area, and exceptional chemical and thermal stability (Park et al. 2006). In addition, since ZIFs are stable in water; thus, there is increasing interest in using this porous material for the removal of pollutants from aqueous environments (Park et al. 2006). In previous work, a composite material of diatomite and ZIF-8 through in-situ loading method was prepared and used to remove the anionic dye, i.e., methylene orange in aqueous solution. This material has good anionic dye capture ability and excellent reusability, which is mainly attributed to the electrostatic interaction between the surface positive charges of ZIF-8 and the anionic dye; meanwhile, the special hierarchical structure of this composite material increases its adsorption capacity and efficiency (Chen et al. 2020).
Coupling water-stable ZIF materials with flexible paper can provide a quick, simple, and convenient way to produce flexible MOF materials that can be used to separate organic dyes in water. Particularly, the construction of MOF paper through MOFs growth on filter paper has been reported and the approach of cellulosic fiber carboxymethylated modification and MOF growth in methanol solution have been introduced (Park and Oh 2017). However, this process is not suitable for industrial implementation, considering the conventional process of paper making. Its chemical modification towards the cellulosic fibers makes the process more complex and increases the cost of the composite paper and its environmental load.
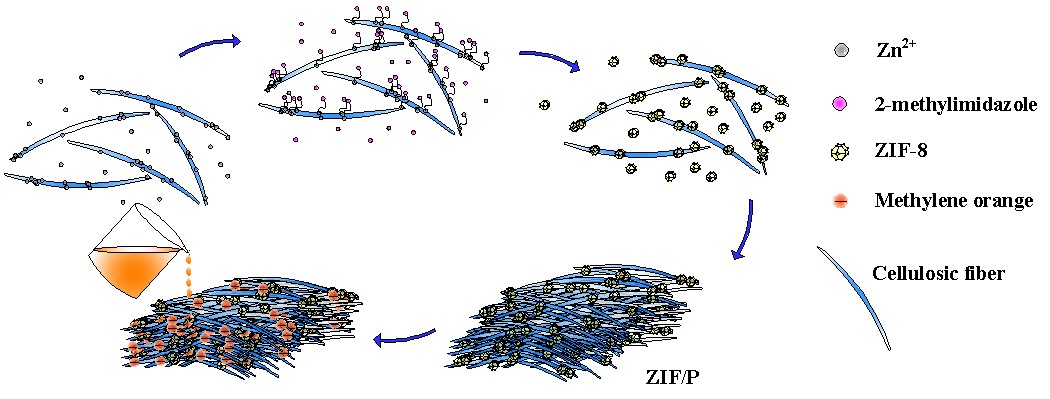
In this work, a simple approach was developed to prepare MOF (ZIF-8) paper without chemical modification of the cellulose fibers, employing a classic paper-making process. Powdered X-ray diffraction (PXRD) patterns, nitrogen adsorption-desorption isotherms, thermo-gravimetric analysis (TGA), and scanning electron microscopy (SEM) analysis were employed to characterize and analyze the MOF composite paper. The prepared MOF paper was used to separate the anionic dye from water by filtration, and its dye-capturing performance was evaluated. The preparation of the MOF paper and the capture of the dye are shown in Fig. 1.
EXPERIMENTAL
Materials
Softwood neutral sulfite semi-chemical (NSSC) pulp was provided generously by Irving Lake Utopia Pulp & Paper Ltd., Utopia (Canada). All the chemicals used in this study were purchased from Sigma-Aldrich Co. (St. Louis, MO), and were used as received.
Methods
Preparation of the MOF-paper
Softwood neutral sulfite semi-chemical (NSSC) pulp with a consistency of 10% was refined with a PFI mill (S40110; Frank PTI GmbH, Birkenau, Germany) to a Canadian Standard Freeness of 750 mL. The pulp was then concentrated to 18% consistency via centrifugation. Then, 1.42 g bone-dry of the centrifuged NSSC pulp and 200 mL of deionized water were dispersed in a 500 mL beaker with a magnetic stirrer for 10 min. Four addition levels of zinc nitrate hexahydrate (H12N2O12Zn) were added to the beaker and stirred for 10 min. Four addition levels of 2-methylimidazole (C4H6N2) were also added to the beaker and stirred for another 30 min to form the ZIF inside the fiber network. The reaction for the synthesis of ZIF-8 is shown below in Eq. 1 (Şahinet et al. 2017),
(1)
The organic ligand molar ratio was 70 with respect to the Zn2+ ions. Although the reaction produced nitric acid and the pH of the solution may be changed, the acidity was not sufficient to hydrolyze and damage the cellulose in the short time frame of the experiment. Lab paper-sheets (hand-sheets) with a basis weight of 80 g/m2 were prepared from the above-treated pulp via a standard hand-sheet former and air-dried without pressing treatment. These flexible MOF paper sheets prepared by the above process were labeled as ZIF/P-1, ZIF/P-2, ZIF/P-3, and control. For comparison, a neat MOF sample (ZIF-8) was also prepared by the following procedures in the absence of cellulose fibers. While agitated by a magnetic stirrer, 22.70 g of C4H6N2 was added into an aqueous solution containing 1.17 g of H12N2O12Zn (Pan et al. 2011). After 30 min of mixing, the mixture was filtered through a cellulose acetate filter paper to retain the ZIF-8 particles. The collected ZIF-8 particles were dried at 105 °C for 4 h in an oven. The composition of the prepared samples can be seen in Table 1.
Table 1. H12N2O12Zn and C4H6N2 Addition Levels for the ZIF Composite Samples
Evaluation of the MOF-paper as a dye capturer
The dye capturing performance of the MOF composite paper was evaluated by a single stage filtration process. The MOF paper was placed in a conical glass funnel and 15 mL of methylene orange (MO−) aqueous solution of 5 ppm concentration was poured into the funnel. The filtrate was collected and analyzed using ultraviolet-visible (UV-Vis) spectroscopy.
The MOF paper adsorbed with the MO− was washed thoroughly with methanol and dried at 90 °C for further filtration or testing of the basis weight, the tensile index, and the folding endurance.
Characterization techniques
The tensile index and the folding endurance of the control paper and the MOF composite paper were evaluated in accordance with the TAPPI standard test methods T494 om-96 (1996) and T423 cm-98 (1998), respectively. All the paper samples were conditioned at an atmosphere of 50.0% ± 2.0% relative humidity and 23.0 ± 1.0 °C in a standard room for 8 h before they were tested. The test results were reported as the mean of 10 tests of each paper sample. Considering relatively low physical strength of handmade paper-sheets in this work, the folding endurance values were expressed as raw data of the folding number (double folds) which was different from their logarithmic values (base 10) that were recommended in the TAPPI standard T423 cm-98. The folding endurance numbers were reported as the average of 10 samples of hand-sheets.
The PXRD patterns were analyzed with a Maxima X XRD-7000 S/L unit (Shimadzu, Kyoto, Japan). The patterns of the samples were characterized with Cu Kα radiation (λ = 1.5418 Å) at 30 mA, 40 kV, and a scan rate of 2°/min with a step size of 0.02°. The nitrogen adsorption-desorption isotherms were measured at 77 K using an ASAP-2020M volumetric measured analyzer (Micromeritics, Norcross, GA). The TGA was carried out with a Mettler Toledo TGA2 unit (Greifensee, Switzerland) under air flow at a heating rate of 10 °C/ min up to 800 °C. The SEM analysis was conducted with a Hitachi XE-100 unit equipped with EDAX energy (Hitachi, Tokyo, Japan) in order to characterize and analyze the morphology of the MOF composite paper and the control paper. The samples were subjected to gold spray-treatment before they were analyzed.
Fig. 1. Preparation of the MOF composite paper and the capturing of the dye
RESULTS AND DISCUSSION
The PXRD spectra of the control paper (CP), the MOF composite paper (ZIF/P-3), and the neat MOF (ZIF-8) (Pan et al. 2011) can be seen in Fig. 2. The characteristic peaks of the neat MOF are present in the spectrum of the ZIF/P-3 sample, which indicates the formation of the MOF in the fiber network of the paper. The PXRD pattern of the ZIF-8 sample agreed with the reported crystal structure of a cubic space group (I3m) (Cravillon et al. 2009).
Fig. 2. a) XRD of the CP, ZIF/P-3, and ZIF-8 samples; b) TGA curves of the ZIF/-1, ZIF/P-2, ZIF/P-3, CP, and ZIF-8 samples
Figure 2b shows the TGA results of the MOF composite paper, the ZIF-8, and the control paper. The residual mass, primarily composed of zinc oxide (ZnO), increased after the thermal treatment with higher dosages of the H12N2O12Zn and C4H6N2 used in the fabrication process. The heat-treated ZIF-8 sample produced ZnO. The TGA diagram (Fig. 2b) illustrates that the loading mass of the ZIF-8 in the composite materials was proportional to the dosage of the ZnO. The TGA curve for the CP shows that there was almost no residual material after the thermal treatment. Meanwhile, the weight of the ZIF-8 sample remained stable as the temperature reached 600 °C. The ZIF-8 content in the ZIF/P-1, ZIF/P-2, and ZIF/P-3 samples was 3.7%, 14.2%, and 25.1%, respectively. The ZIF-8 content was calculated from the weight difference between the control paper and paper samples which loaded with the ZIF-8 (Lange and Obendorf 2015).
Fig. 3. SEM images of the a, b) ZIF/P-3 and c) Control paper sample. Figure 3b is the magnified SEM image of the red circle in Fig. 3a.
The SEM images in Figs. 3a and 3b show that the ZIF-8 particles were ununiformly distributed in clusters on the surface of the cellulosic fibers. Compared to the control paper sample (Fig. 3c), the MOF composite paper samples had clear crystalline structures that were loaded on the surface of the cellulosic fibers, while the control paper sample fibers were clear. The element Zn mapping of the ZIF/P-3 sample (Figs. 4a and 4b) indicate that the distribution of the ZIF-8 in the paper substrate was relatively uniform, owing to the multi-layered structure of the hand-sheets. The uniform distribution of the MOF in the paper is critical for the high-efficiency removal of anionic dye through a simple filtration process (Fig. 1). The nitrogen adsorption isotherm results in Figs. 5a and 5b show that the ZIF-8 sample displays a typical type I isotherm, indicating the co-existence of micropores and mesopores in the ZIF-8 (Hu et al. 2018). The appearance of hysteresis loop in ZIF/P-3 sample exhibits the characteristics of both nanoporous and mesoporous structures, which could derive from the special layered structure of ZIF-8 loaded on the cellulosic fibers (Xu et al. 2018).
Fig. 4. Elemental mapping of the Zn for the a) top and b) bottom sides of the ZIF/P-3 sample
Fig. 5. N2 adsorption-desorption isotherms of the a) ZIF-8 and b) ZIF/P-3 samples
The ZIF/P-3 sample could be expected to act as a highly efficient adsorbent since it possesses nano-porous and mesoporous structures. Because of the electrostatic interaction of the positive surface charges of the ZIF-8 with the negative charges of the MO− dye molecules in the water, the prepared MOF paper could be used as convenient filter paper to selectively capture the MO− dye (Lange and Obendorf 2015). In addition, π-π interaction between ZIF-8 and MO− dye were also reported as an absorption mechanism for ZIF-8 (Haque et al. 2011). Moreover, based on the adsorption mechanism of the electrostatic interactions and the ion exchange, the MOF composite paper could have potential applications for other anionic dyes (Du et al. 2017). In a simple procedure, the MOF composite paper was placed in a conical glass funnel and an aqueous MO− solution was poured into the funnel (Fig. 6c). The filtrate was collected and tested by UV-Vis spectroscopy to ascertain the MO− concentration. The results in Fig. 6a show that the MO− dye was successfully captured via a single stage filtration operation of the aqueous MO− solution with the ZIF/P-3. Figure 6b shows that the MO− removal efficiencies of the control sample, ZIF/P-1, ZIF/P-2, and ZIF/P-3 were 6.03%, 23.27%, 51.35% and 92.35%, respectively. The increased dye removal efficiency of the MOF composite paper was proportional to the ZIF-8 content in the paper. The recyclability of the ZIF/P-3 paper was also investigated in the capturing of the MO− dye. The used ZIF/P-3 paper was regenerated by washing it with methanol and then reused for the MO− filtration. It was found that the ZIF/P-3 paper could be recycled after regeneration, and its excellent MO− capture ability was preserved even after 4 cycles (Fig. 6d).
Fig. 6. a) The UV-Vis spectra of the MO− dye solution and the filtrates, b) the percentage of the removed MO− dye, c) a photograph showing the filtration (from left to right: the MO− aqueous solution before filtration, the MO− aqueous solution after the filtration by ZIF/P-3, the de-ion water as the control), and d) percentage of the removed MO−after several reuse cycles for the ZIF/P-3
Cellulosic paper is a flexible material due to its special interwoven structure of plant fibers and abundant hydrogen bonding. The flexibility of cellulosic paper is a unique quality that enables it to be used in various applications. Generally, the hydrophobic substance in paper, such as inorganic nonmetallic fillers and sizing agents, will lower its flexibility because these hydrophobic substances can interfere with the hydrogen bonding of the cellulose fibers (Shen et al. 2009). Contrary to the cellulosic fibers, the MOF materials in this study were hydrophobic. As shown in Table 2, the tensile index and the folding endurance of the ZIF/P-3 sample decreased remarkably compared to the control paper since it contained hydrophobic ZIF-8. These physical properties indexes of the ZIF/P-3 sample decreased further after it was reused after four cycles, owing to fiber hornification caused by multiple wet and dry conversions (Hubbe et al. 2007). However, most of the tensile index strength and more than half of the folding endurance of the ZIF/P-3 was preserved even though it endured four cycles. This reusability illustrated that the flexible properties of the ZIF/P-3 sample were acceptable.
Table 2. Physical Strength Properties of the Control Paper, the ZIF/P-3, and the ZIF/P-3 after Four Uses
CONCLUSIONS
- A simple process was developed in this study to fabricate flexible composite paper through in-situ loading of zeolite-based ZIF-8 using a papermaking process. The uniform presence of ZIF-8 particles in the fiber network promotes the adsorption performance of the metal organic framework (MOF) composite paper.
- The prepared composite paper was used to selectively remove methyl orange (MO−) dye from water through a simple filtration process. It has a good adsorption capacity because of the electrostatic interaction of the positive surface charges of the ZIF-8 with the negative charges of the MO− dye in solution. It was found that the MO− dye removal efficiency reached more than 92% in a single stage filtration process with a MOF (ZIF-8) loading rate of 25%.
- It was also found that the composite paper could be regenerated and reused for the MO− dye filtration process, and the MO− dye capturing ability was largely preserved even after four cycles of use.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Science and Technology Development Plan of Jilin Provence of the People’s Republic of China. (Grant No. 20190303065SF) and the Science and Research Programs of Education Department of Jilin Province of the People’s Republic of China (Grant No. JJKH 20200125KJ).
REFERENCES CITED
Chen, S., Wang, G., Sui, W., Parvez, A. M., Dai, L., and Si, C. (2020). “Novel lignin-based phenolic nanosphere supported palladium nanoparticles with highly efficient catalytic performance and good reusability,” Industrial Crops and Products 145, 112164. DOI: 10.1016/j.indcrop.2020.112164
Choi, J. W., Park, Y. J., and Choi, S. J. (2020). “Synthesis of metal-organic framework ZnOx-MOF@MnO2 composites for selective removal of strontium ions from aqueous solutions,” ACS Omega 5(15), 8721-8729. DOI: 10.1021/acsomega.0c00228
Cravillon, J., Munzer, S., Lohmeier, S. J., Feldhoff, A., Huber, K., and Wiebcke, M. (2009). “Rapid room-temperature synthesis and characterization of nanocrystals of a prototypical zeolitic imidazolate framework,” Chemistry of Materials 21(8), 1410-1412. DOI: 10.1021/cm900166h
Demakov, P. A., Volynkin, S. S., Samsonenko, D. G., Fedin, V. P., and Dybtsev, D. N. (2020). “A selenophene-incorporated metal-organic framework for enhanced CO2 uptake and adsorption selectivity,” Molecules 25(19), 4396-4408. DOI: 10.3390/molecules25194396
Du, X., Wang, C., Liu, J., Zhao, X., Zhong, J., Li, Y., Li, J., and Wang, P. (2017). “Extensive and selective adsorption of ZIF-67 towards organic dyes: Performance and mechanism,” Journal of Colloid and Interface Science 506, 437-441. DOI: 10.1016/j.jcis.2017.07.073
Duan, C., Meng, J., Wang, X., Meng, X., Sun, X., Xu, Y., Zhao, W., and Ni, Y. (2018). “Synthesis of novel cellulose-based antibacterial composites of Ag nanoparticles@ metal-organic frameworks@ carboxymethylated fibers,” Carbohydrate Polymers 193, 82-88. DOI: 10.1016/j.carbpol.2018.03.089
Guo, H., Zhu, Y., Qiu, S., Lercher, J. A., and Zhang, H. (2010). “Coordination modulation induced synthesis of nanoscale Eu1-xTbx-metal-organic frameworks for luminescent thin films,” Advanced Materials 22(37), 4190-4192. DOI: 10.1002/adma.201000844
Haque, E., Jun, J. W., and Jhung, S. H. (2011). “Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235),” Journal of Hazardous Materials 185(1), 507-511. DOI: 10.1016/j.jhazmat.2010.09.035
Hu, L., Chen, L., Fang, Y., Wang, A., Chen, C., and Yan, Z. (2018). “Facile synthesis of zeolitic imidazolate framework-8 (ZIF-8) by forming imidazole-based deep eutectic solvent,” Microporous and Mesoporous Materials 268(15), 207-215. DOI: 10.1016/j.micromeso.2018.04.039
Hubbe, M. A., Venditti, R. A., and Rojas, O. J. (2007). “What happens to cellulosic fibers during papermaking and recycling? A review,” Bioresources 2(4), 739-788. DOI: 10.15376/BIORES.2.4.739-788
Jiang, D., Burrows, A. D., Xiong, Y., and Edler, K. J. (2013). “Facile synthesis of crack-free metal-organic framework films on alumina by a dip-coating route in the presence of polyethylenimine,” Journal of Materials Chemistry A 18, 5497-5500. DOI: 10.1039/C3TA10766C
Kim, H., Yang, S., Rao, S. R., Narayanan, S., Kapustin, E. A., Furukawa, H., Umans, A. S., Yaghi, O. M., and Wang, E. N. (2017). “Water harvesting from air with metal-organic frameworks powered by natural sunlight,” Science 356(6336), 430-434. DOI: 10.1126/science.aam8743
Lange, L. E., and Obendorf, S. K. (2015). “Functionalization of cotton fiber by partial etherification and self-assembly of polyoxometalate encapsulated in Cu3(BTC)2 metal-organic framework,” ACS Applied Materials & Interfaces 7(7), 3974-3980. DOI: 10.1021/am506510q
Li, X., Yuan, H., Quan, X., Chen, S., and You, S. (2017). “Effective adsorption of sulfamethoxazole, bisphenol A and methyl orange on nanoporous carbon derived from metal-organic frameworks,” Journal of Environmental Sciences 63, 250-259. DOI: 10.1016/j.jes.2017.10.019
Matsumoto, M., and Kitaoka, T. (2016). “Ultraselective gas separation by nanoporous metal-organic frameworks embedded in gas-barrier nanocellulose films,” Advanced Materials 28(9), 1765-1769. DOI: 10.1002/adma.201504784
Pan, Y., Liu, Y., Zeng, G., Zhao, L., and Lai, Z. (2011). “Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system,” Chemical Communications 47(7), 2071-2073. DOI: 10.1039/c0cc05002d
Park, J., and Oh, M. (2017). “Construction of flexible metal-organic framework (MOF) papers through MOF growth on filter paper and their selective dye capture,” Nanoscale 9(35), 12850-12854. DOI: 10.1039/C7NR04113F
Park, K. S., Ni, Z., Côté, A. P., Choi, J. Y., Huang, R., Uribe-Romo, F. J., Chae, H. K., O’Keeffe, M., and Yaghi, O. M. (2006). “Exceptional chemical and thermal stability of zeolitic imidazolate frameworks,” Proceedings of the National Academy of Sciences 103(27), 10186-10191. DOI: 10.1073/pnas.0602439103
Pérez-Cejuela, H. M., Herrero-Martínez, J. M., and Simó-Alfonso, E. F. (2020). “Recent advances in affinity MOF-based sorbents with sample preparation purposes,” Molecules 25(18), 4216-4237. DOI: 10.3390/molecules25184216
Qian, L., Lei, D., Duan, X., Zhang, S., Song, W., Hou, C., and Tang, R. (2018). “Design and preparation of metal-organic framework papers with enhanced mechanical properties and good antibacterial capacity,” Carbohydrate Polymers 192, 44-51. DOI: 10.1016/j.carbpol.2018.03.049
Rafti, M., Allegretto, J. A., Segovia, G. M., Tuninetti, J. S., Giussi, J. M., Bindini, E., and Azzaroni, O. (2017). “Metal-organic frameworks meet polymer brushes: Enhanced crystalline film growth induced by macromolecular primers,” Materials Chemistry Frontiers 11, 2256-2260. DOI: 10.1039/C7QM00235A
Şahin, F., Topuz, B., and Kalıpçılar, H. (2017). “Synthesis of ZIF-7, ZIF-8, ZIF-67 and ZIF-L from recycled mother liquors,” Microporous and Mesoporous Materials 261, 259-267. DOI: 10.1016/j.micromeso.2017.11.020
Shen, J., Song, Z., Qian, X., and Liu, W. (2009). “Modification of papermaking grade fillers: A brief review,” Bioresources 4(3), 1190-1209.
So, M. C., Jin, S., Son, H. J., Wiederrecht, G. P., Farha, O. K., and Hupp, J. T. (2013). “Layer-by-layer fabrication of oriented porous thin films based on porphyrin-containing metal-organic frameworks,” Journal of the American Chemical Society 135(42), 15698-15701. DOI: 10.1021/ja4078705
Song, W., Zhu, M., Zhu, Y., Zhao, Y., Yang, M., Miao, Z., Ren, H., Ma, Q., and Qian, L. (2019). “Zeolitic imidazolate framework-67 functionalized cellulose hybrid aerogel: An environmentally friendly candidate for dye removal,” Cellulose 27(4), 2161-2172. DOI: 10.1007/s10570-019-02883-2
Sui, W., Pang, T., Wang, G., Liu, C., Parvez, A. M., Si, C., and Li, C. (2020). “Stepwise ethanol-water fractionation of enzymatic hydrolysis lignin to improve its performance as a cationic dye adsorbent,” Molecules 25(11), 2603-2618. DOI: 10.3390/molecules25112603
Sun, J., Zhang, X., Zhang, A., and Liao, C. (2019). “Preparation of Fe-Co based MOF-74 and its effective adsorption of arsenic from aqueous solution,” Journal of Environmental Sciences 80, 197-207. DOI: 10.1016/j.jes.2018.12.013
TAPPI T 423 cm-98 (1998). “Folding endurance of paper (Schopper type tester),” TAPPI Press, Atlanta, GA, USA.
TAPPI T494 om-96 (1996). “Tensile properties of paper and paperboard (using constant rate of elongation apparatus),” TAPPI Press, Atlanta, GA, USA.
Wang, C., Qian, X., and An, X. (2015). “In situ green preparation and antibacterial activity of copper-based metal-organic frameworks/cellulose fibers (HKUST-1/CF) composite,” Cellulose 22(6), 3789-3797. DOI: 10.1007/s10570-015-0754-4
Wang, J., Yang, X., Zheng, D., Yao, A., Hua, D., Srinivasapriyan, V., and Zhan, G. (2020). “Fabrication of bioinspired gallic acid-grafted chitosan/polysulfone composite membranes for dye removal via nanofiltration,” ACS Omega 5(22), 13077-13086. DOI: 10.1021/acsomega.0c01013
Xu, X., Qi, C., Hao, Z., Wang, Hao., Jiu, J., Liu, J., Yan, H., and Suganuma K. (2018). “The surface coating of commercial LiFePO4 by utilizing ZIF-8 for high electrochemical performance lithium ion battery,” Nano-Micro Letters 10(1), 1-9. DOI:10.1007/s40820-017-0154-4
Yang, Q., Zhang, M., Song, S., and Yang, B. (2017). “Surface modification of PCC filled cellulose paper by MOF-5 (Zn3(BDC)2) metal-organic frameworks for use as soft gas adsorption composite materials,” Cellulose 24(7), 3051-3060. DOI: 10.1007/s10570-017-1331-9
Yu, M., Li, W., Wang, Z., Zhang, B., Ma, H., Li, L., and Li, J. (2016). “Covalent immobilization of metal-organic frameworks onto the surface of nylon − A new approach to the functionalization and coloration of textiles,” Scientific Reports 6, 22796-22796. DOI: 10.1038/srep22796
Zhuang, J. L., Ar, D., Yu, X. J., Liu, J. X., and Terfort, A. (2013). “Patterned deposition of metal-organic frameworks onto plastic, paper, and textile substrates by inkjet printing of a precursor solution,” Advanced Materials 25(33), 4631-4635. DOI: 10.1002/adma.201301626
Article submitted: December 5, 2020; Peer review completed: January 16, 2021; Revised version received; February 1, 2021; Accepted: February 14, 2021; Published: February 19, 2021.
DOI: 10.15376/biores.16.2.2644-2654
