Abstract
Thermal flow molding of wood powder was carried out with a natural binder consisting of sucrose and citric acid (SC binder) for fabricating wood products without synthetic resins. The testing of thermal flow and molding of the wood powder with SC binder was done to analyze the effect of added SC binder, as well as to optimize the molding conditions, such as temperature, binder content, and mixture ratio of the sucrose and citric acid. The wood powder with SC binder flowed successfully at an optimum temperature of 180 °C in the thermal flow test. In addition, it was possible to form a composite wood plate by molding with thermal flow, and the moldability was improved by increasing the sucrose ratio in the SC binder. Density, bending strength, and thickness swelling after water immersion of the plate molded under the optimum molding conditions were 1.4 g/cm3 to 1.5 g/cm3, 28 MPa to 37 MPa, and 7% to 10%, respectively. These results indicated a possibility
Download PDF
Full Article
Fabrication of Naturally Derived Wood Products by Thermal Flow Molding of Wood Powder with Sucrose and Citric Acid
Shohei Kajikawa,a,* Masaya Horikoshi,a Takashi Kuboki,a Soichi Tanaka,b Kenji Umemura,b and Kozo Kanayama b
Thermal flow molding of wood powder was carried out with a natural binder consisting of sucrose and citric acid (SC binder) for fabricating wood products without synthetic resins. The testing of thermal flow and molding of the wood powder with SC binder was done to analyze the effect of added SC binder, as well as to optimize the molding conditions, such as temperature, binder content, and mixture ratio of the sucrose and citric acid. The wood powder with SC binder flowed successfully at an optimum temperature of 180 °C in the thermal flow test. In addition, it was possible to form a composite wood plate by molding with thermal flow, and the moldability was improved by increasing the sucrose ratio in the SC binder. Density, bending strength, and thickness swelling after water immersion of the plate molded under the optimum molding conditions were 1.4 g/cm3 to 1.5 g/cm3, 28 MPa to 37 MPa, and 7% to 10%, respectively. These results indicated a possibility of effective production of wood products with a natural binder.
Keywords: Molding; Thermal flow; Wood; Powder; Composite; Cedar; Natural binder; Sucrose; Citric acid
Contact information: a: Department of Mechanical and Intelligent Systems Engineering, The University of Electro-Communications, 1-5-1 Chofu Gaoka, Chofu, Tokyo 182-8585, Japan; b: Research Institute for Sustainable Humanosphere, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan;
* Corresponding author: s.kajikawa@uec.ac.jp
INTRODUCTION
Mass disposal of petroleum-derived plastic, such as disposable bags, plates, and drinking straws, has become a serious environmental problem, causing ocean pollution and depletion of fossil resources. A new technology1715 is needed to increase the use of environmentally friendly resources. Wood is an environmentally friendly resource because of its biodegradable and carbon-neutral nature. In addition, it is possible to sustain wood production by appropriate tree planting and trimming. Therefore, it is effective to use wood as an alternative material to petroleum-derived plastic for environmental protection.
An effective wood processing method that makes products with various shapes is needed for the use of wood as an alternative material to petroleum-derived plastic. Wood products are mainly manufactured by cutting processes, as the deformability of the wood is poor. However, the productivity of wood products by cutting processes is low compared to that of conventional plastic products, which are produced by press molding, extrusion, and injection molding. This low productivity increases the production cost.
It is possible to use wood as a feedstock for wood–plastic composites (WPCs), which are used in automotive components, architectural materials, and household electronics (Eder and Carus 2013). The WPC is produced by processing a mixture of wood powder and a thermoplastic binder, such as polypropylene (Wolcott 2001), and the processing method for WPC is the same as that for conventional plastics. Therefore, various shapes can be produced effectively. In particular, the productivity of injection molding is very high because it allows continuous production. Figure 1 is a schematic diagram of injection molding using WPC. In this method, the binder between the wood particles plasticizes by heating, and then the material, which is composed of the binder and particles, flows into the mold by pressure. When the material is cooled in the mold, the wood particles are bonded by solidification of the binder.
Fig. 1. Schematic diagram of conventional injection molding of wood powder
Unfortunately, the binder content in WPC is about 40% to 50% at minimum (Stark et al. 2004; Nagaya 2014) because the thermal fluidity of the material decreases when the binder content decreases. If the thermal fluidity of the material is low, the material cannot flow smoothly into the mold. Therefore, the problem of high plastic content, which drives the disposal of petroleum-derived plastic, remains, and the synthetic binder content in WPC needs to be decreased. The use of bioplastic, such as polylactic acid (PLA), as the binder was proposed (Csikós et al. 2015), but a large amount of energy is needed for producing PLA from bioresources. Miki et al. (2004) developed a technology for fabricating products composed solely of wood powder by injection molding. In this process, the wood powder flows with water under high temperature and pressure and becomes self-bonded when the powder is cooled. This is because the binder consists of low-molecular-weight sugars, which are generated by hydrolysis of wood components under high temperature and pressure with water (Kajikawa and Iizuka 2015). However, molding defects occur easily when wood powder is injection molded without plastic because the thermal fluidity of the wood powder is lower compared to that of the wood powder with plastic.
It can be effective to add sugar as a wood powder binder to improve its thermal fluidity. Kajikawa et al. (2017) carried out a fundamental investigation on the injection moldability of wood powder with the sugar sucrose, and they confirmed that adding sucrose improves the moldability and thermal fluidity. Further enhancement of the thermal fluidity is needed for stable production without molding defects. In addition, favorable product properties, such as strength and water resistance, are needed for practical use, such as for disposable tableware.
This paper proposes the use of a mixture of sucrose and citric acid (SC) as a binder for wood powder injection molding to enhance its thermal fluidity and the product properties. When a wood powder with SC binder is heated, the sucrose is hydrolyzed by the citric acid to low-molecular-weight components (Bailey and Bailey 1989), and then the fluidity improves compared with the case of using only sucrose as the binder. The fluidized powder is self-bonded in the product by cooling, which should give the product good strength and water resistance, because ester linkages form between the citric acid and the wood and/or the sucrose (Umemura et al. 2015). On this basis, adding SC binder to wood powder for injection molding is considered to be effective, but the effect of adding SC binder on the moldability has not been investigated previously.
In this study, a fundamental exploration of the injection molding of wood powder with a SC binder was carried out to clarify the effect of the binder, as well as to optimize molding conditions such as the temperature T, binder content ratio B, and sucrose and citric acid mixture ratio S:C for fabricating products with good appearance and properties. First, thermal flow testing of the wood powder with SC binder was carried out to investigate the optimum T for good thermal flow of the powder. The results of the thermal flow tests were used to fabricate plates by thermal flow molding under various conditions of B and S:C. Finally, the density, strength, and water resistance of the produced plates were evaluated.
EXPERIMENTAL
Wood Powder and Natural Binder
Wood powder was obtained by milling Japanese cedar chips, passing them through a 500-μm sieve, and dehydrating the powder in a drying oven at 80 °C for 24 h before the experiment. A mixture of sucrose and citric acid (anhydrous), both of which were obtained from Nacalai Tesque, Inc. (Kyoto, Japan), was used as natural binder. Five types of binder with various mixture ratios of sucrose to citric acid, S:C, were prepared. The S:C value was set at 100:0 (sucrose only), 75:25, 50:50, 25:75 (SC binder), and 0:100 (citric acid only) by weight. The wood powder was combined with the binder by stirring it into an aqueous binder solution. A dried mixture of wood powder and binder, called WB (wood:binder) powder in this paper, was obtained by dehydration in a drying oven at 80 °C for 48 h. The concentrations of the binder aqueous solution were 3.8, 5.7, 7.4, and 9.1 wt% to set the binder content B, which is the binder weight ratio in WB powder, to 20, 30, 40, and 50 wt%.
Thermal Flow Test
Figure 2 and Table 1 show a schematic diagram and the conditions for the thermal flow test. In this test, WB powder flowed out from a nozzle by pressing. The fluidity of the WB powder was evaluated by measuring the pressing force during the test. The test procedure was as follows. The dried WB powder was pre-heated at a temperature T for a time th in the container. The punch was moved downward at a constant velocity vp. The WB powder was compressed by the punch to force the powder into and out from the nozzle. The punch press was stopped when the punch position z, which is the distance between the punch and the nozzle, was 5 mm, or the punch surface pressure P reached 200 MPa.
In the case that WB powder flowed, a thermal flow curve, such as that shown in Fig. 2(c), was obtained from the measurement of the pressing force. The punch surface pressure P initially increased with compression of the WB powder prior to flow, and then decreased when the WB powder began to flow out. P was approximately constant during WB powder flow. When the punch approached the nozzle surface, P increased again because the punch met the “dead zone,” where the WB powder does not flow into the nozzle. Flow point pressure Pf, which is the pressure at the beginning of flow, and apparent viscosity η were used as indicators of the fluidity of the WB powder. η was calculated from the following equation according to Newton’s law of viscosity,
where η is apparent viscosity (kPas) and τ and γ are the apparent shear stress (kPa) and apparent shear velocity (/s), respectively, at the nozzle wall. τ and γ are calculated from the following equations,
where P is the punch surface pressure (MPa), dn is the nozzle diameter (mm), ln is the nozzle length (mm), vp is the punch press velocity (mm/s), and dc is the container inner diameter (mm). The values of dn, ln, vp, and dc were constant, as shown in Table 1. η was calculated from P in the range in which WB powder stably flowed, as shown in Fig. 2(c).
In this test, the effects of the temperature T and the sucrose and citric acid mixture ratio S:C on the flow point pressure Pf and the apparent viscosity η of the WB powder were investigated, while the binder content B was kept constant at 50 wt%. The test was carried out three times for each condition.
Fig. 2. Schematic diagram of thermal flow test: (a) Setup, (b) pressing, and (c) typical thermal flow curve
Table 1. Thermal Flow Test Conditions
Molding of Plate with Thermal Flow
A molding test was carried out using a simple injection molding device to investigate the effect of molding parameters in a simple way, as shown in Fig. 3. The WB powder flowed into a rectangular cavity with dimensions of lc×wc×hc (mm) from the container via the nozzle by pressing WB powder in the container, as shown in Figs. 3(a) and 3(b). Successful filling of the cavity produced a plate with dimensions of lc×wc×hc (mm). The molding procedure was almost the same as that of the thermal flow test.
The dried WB powder was pre-heated, and then the punch was moved downward at a constant velocity of vp to inject the WB powder into the cavity. The punch press was stopped when the punch surface pressure P reached 200 MPa. The molded plate, which was composed of WB powder, was cooled in the cavity until the temperature T fell below 80 °C.
Table 2 shows the molding conditions. In this experiment, the effects of the sucrose and citric acid mixture ratio S:C and the binder content B on the moldability of WB powder was investigated, while the temperature T was kept constant at 180 °C. The molding was carried out two times for each condition.
Evaluation of Moldability and Plate Properties
Moldability and the molded plate properties, such as strength, density, and water resistance, were evaluated. Moldability was evaluated by observation of the plate, and a scanning electron microscope (SEM S-4300), which was developed by Hitachi High-Technologies Corp., was used as needed. A bending test was carried out for evaluation of strength according to Japanese Industrial Standard JIS K 7171 (2016). The specimen with dimensions of 80×10×4 mm for the bending test was obtained by cutting the plate as shown in Fig. 3(c). The span of the support points was 64 mm, and the test speed was 10 mm/min. Six specimens with dimensions of 10×10×4 mm were cut from each plate for measurement of ρ, as shown in Fig. 3(c), and the effect of the distance from the nozzle on ρ was investigated. The thickness swelling TS was measured after water immersion for 24 h for evaluation of the water resistance of the plate. The specimen for the measurement of TS was the same as that used to measure ρ, and the TS measurement was carried out after ρ measurement.
Fig. 3. Schematic diagrams of molding test and specimen for evaluation: (a) Setup, (b) pressing, and (c) dimensions of specimen
Table 2. Molding Conditions
RESULTS AND DISCUSSION
Thermal Fluidity of the Wood Powder with Sucrose and Citric Acid
Figure 4 shows variations in the punch surface pressure P and appearance of the material during the thermal flow test under conditions in which the mixture ratio S:C and binder content B were 50:50 and 50 wt%, respectively. The WB powder did not flow out from the nozzle at a temperature T of 100 °C; instead, it was merely compressed between the punch and the nozzle, as shown in Fig. 4(b). In this case, P increased with compression of the WB powder, up to the maximum test pressure of 200 MPa, as shown in Fig. 4(a). The WB powder flowed when T was over 120 °C. Figure 4(c) shows the appearance of the material that flowed out from the nozzle. During WB powder flow, P decreased with increasing T and reached a minimum at T = 180 °C. However, during WB powder flow at T = 200 °C, P increased a little, and it severely fluctuated, because too much gas was generated within the container by thermal decomposition of the binder and wood powder. A large amount of gas inside the container is a hazard in the molding process. Therefore, the optimum temperature was kept at 180 °C for the thermal flow of WB powder.
Fig. 4. Effect of temperature T on thermal flow behavior (S:C=50:50, B=50 wt%): (a) Thermal flow curve, (b) material compressed in container, and (c) material extruded from nozzle
Fig. 5. Effect of temperature T and mixture ratio S:C on (a) flow point pressure Pf and (b) apparent viscosity η (B=50 wt%)
Figure 5 shows the effect of the temperature T and the mixture ratio S:C on the flow point pressure Pf and apparent viscosity η when the binder content B was 50 wt%. Pf showed the same tendency as η. In the case of using sucrose (S:C=100:0) as the binder, the WB powder did not flow out from the nozzle at T=140 °C and 160 °C, and the WB powder flowed at T=180 °C because the sucrose melted around a temperature of 185 °C to 192 °C (Roos 1993). The WB powder flowed at T=140 °C to 180 °C, and Pf and η became low by using SC binder (S:C = 75:25, 50:50, and 25:75) or citric acid (S:C = 0:100), compared with only sucrose. The melting temperature of citric acid is 153 °C, which is lower than that of sucrose. In addition, sucrose is hydrolyzed to low-molecular-weight components, such as fructose and glucose, with an acid (Bailey and Bailey 1989), and the melting temperatures of fructose and glucose are 103 °C and 146 °C, respectively. Therefore, Pf and η of the WB powder with SC binder was lower than those of the WB powder with only citric acid at T = 140 °C. The effect of S:C was small when SC binder was used. The result shows that adding SC binder to wood powder is effective for improving the thermal fluidity.
Thermal Flow Moldability of the Wood Powder with Sucrose and Citric Acid
A molding test was carried out at T = 180 °C, which was the optimum temperature for activating the fluidity of WB powder in the thermal flow test. Table 3 shows the appearance of the molded plates under the conditions of various binder contents B and mixture ratios S:C. The moldability was improved by increasing B in each S:C mixture. In the case of S:C = 50:50, plates with a uniform dark brown color were molded successfully when B was 40 wt% or 50 wt%. However, when B was 30 wt%, a crack appeared on the surface of the plate, and the color of the plate was different between the center and the edge. When B was 20 wt%, the WB powder did not flow into the cavity, although the WB powder was pressed at maximum pressure of 200 MPa in the container. Figure 6 shows micrographs of the surfaces of the plate molded using WB powder of S:C = 50:50. The plate surface was not smooth, and large voids appeared in the light brown portion of the surface, as shown in Fig. 6(a). The surface was smooth in the dark brown area, as shown in Fig. 6(b). That is to say, the color of the plate indicates the degree of WB powder filling, and WB powder filling was incomplete near the edges, as indicated by light brown coloration. Under the condition of a low B value, the plasticized portion is small and the solid portion is large in WB powder. Therefore, the fluidity became poor by reducing the B value. WB powder, which has a low B value, cannot flow into the cavity, or it cannot reach the cavity edges due to the poor fluidity.
The moldability was also improved by increasing the sucrose ratio in the SC binder, as shown in Table 3. The effect of the mixture ratio S:C on the moldability was small under a high binder content B, such as 40 wt% or 50 wt%, but S:C affected the moldability when B was under 30 wt%. In particular, it was possible to fabricate the plate under the condition of B = 20 wt% when S:C = 75:25 because the WB powder with S:C=75:25 had better fluidity than powder with other S:C values. Apparently, the amount of sucrose, which generates low-molecular-weight sugars, such as fructose and glucose, by hydrolysis with acid, in the SC binder was important, because low-molecular-weight sugars have better thermal fluidity. However, in the case that the binder was sucrose without citric acid, the fluidity became low, as shown in Fig. 5, because sucrose hydrolysis was not promoted and an insufficient amount of low-molecular-weight sugars was generated. Therefore, the optimum S:C for WB powder filling was 75:25 in this experiment.
Table 3. Appearance of Molded Plates (T = 180 °C)
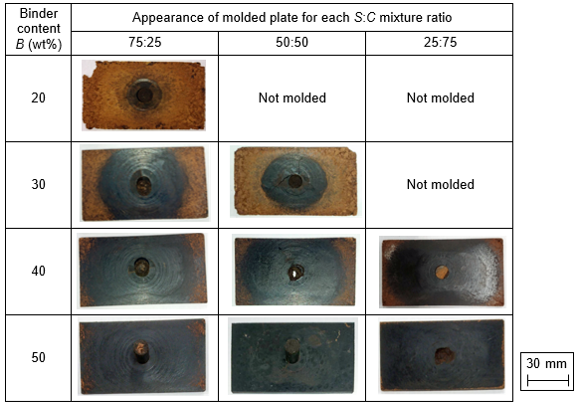
Fig. 6. Scanning electron micrograph of the surface of molded plates (S:C = 50:50): (a) B = 30 wt% and (b) B = 50 wt%
Properties of Molded Plate
Figure 7 shows the average value and the distribution of the density ρ of molded plates. The value of ρ tended to increase with an increase in B, and it rose to 1.4 g/cm3 to 1.5 g/cm3 under the condition of the binder content B = 40 wt% or 50 wt% for each mixture ratio S:C, as shown in Fig. 7(a). This is because the increased B content improved the fluidity of the WB powder, and it completely filled the cavity during molding, as shown in Table 3. This density value of 1.4 g/cm3 to 1.5 g/cm3 is very high compared with that of the original Japanese cedar wood, which is 0.33 g/cm3 (Forestry and Forest Products Research Institute 2004). In addition, the true density of the plate was calculated to be about 1.53 g/cm3 to 1.57 g/cm3, as determined from the densities of the wood cell wall (1.5 g/cm3), sucrose (1.59 g/cm3), and citric acid (1.67 g/cm3). Therefore, the density was almost the same as the true density in the case of the molded plate. That is to say, the plate fabricated under the optimum condition had few voids.
Figure 7(b) shows the density ρ distribution of the plate when the mixture ratio S:C was 75:25. The density was almost uniform when the binder content B was 40 wt% or 50 wt%, but the density decreased with the distance from the nozzle when B was 30 wt%. The decreased density is thought to be caused by an increase in the number or the size of voids inside the molded plate, which is shown in Fig. 6. In the case of B = 30 wt%, the WB powder could not reach the cavity edges completely due to the poor fluidity of WB powder, and then voids remained at the edges of the molded plate, as shown in Fig. 6.
Fig. 7. Density ρ of molded plates (T = 180 °C): (a) Effect of binder content B and mixture ratio S:C on average ρ and (b) ρ distribution in molded plates under condition of S:C = 75:25
Figure 8 shows the effect of the binder content B and the mixture ratio S:C on the bending strength σb of the molded plates, and the relationship between σb and density ρ. As shown in Fig. 8(a), strength σb tended to increase with increasing B from 30 wt% to 40 wt% because the moldability of the plate improved with increasing ρ. As shown in Fig. 8(b), σb showed a linear dependence on ρ. In particular, when ρ was large, σb was large, and its error was small. Therefore, to improve the strength, it is important to increase ρ by more completely filling the mold with WB powder.
The bending strength σb of the plate was 28 MPa to 37 MPa when the binder content B was 40 wt% to 50 wt%. According to JIS A 5741 (2016), a bending strength of 20 MPa or more is required for the use of WPC as an exterior or interior architectural material. The bending strength of conventional plastic, such as polypropylene, is approximately 31 MPa (Karmaker and Youngquist 1996). Therefore, the plate fabricated from wood powder and SC binder has good mechanical properties, comparable to those for conventional WPC or plastic. This is attributed to the formation of ester linkages between the components (Umemura et al. 2015). The citric acid reacts with the components with a hydroxyl group, such as sucrose and the wood components, and then this reaction forms ester linkages between the components.
Figure 9 shows the effects of the binder content B and the mixture ratio S:C on the thickness swelling TS (%) after the molded plates were immersed in water. The TS was 8% to 10%, and the effect of B or S:C was small. According to JIS A 5905 (2014) for fiberboard, a TS of 10% to 17% or less is required for various architectural materials. Therefore, it is considered that the plate fabricated from wood powder and SC binder has the required water resistance for practical use.
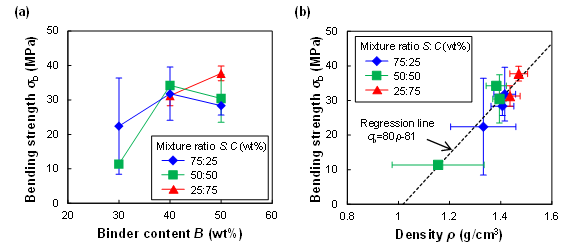
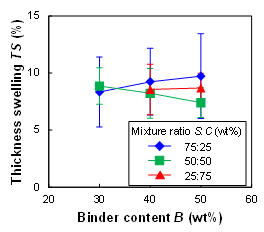
Fig. 8. Bending strength σb of molded plates (T = 180 °C): (a) Effect of binder content B and mixture ratio S:C and (b) relationship between density ρ and bending strength σb

Fig. 9. Effects of binder content B and mixture ratio S:C on thickness swelling TS after water immersion of molded plates (T = 180 °C)
Table 4 summarizes the thermal flow moldability and plate properties of strength and water resistance under the various molding conditions of the binder content B and the mixture ratio S:C. Moldability was most important for fabricating a plate with good properties by this method, because the moldability correlates with the plate properties. In the case that the moldability was good, the density became high and uniform in the plate, as shown in Fig. 7, and then high strength and water resistance were achieved. For improving the moldability, the fluidity of WB powder should be improved. The fluidity improved by increasing the B value and sucrose ratio in the SC binder, while the B value should be decreased for producing good wood-based products. Therefore, the optimum S:C was 75:25 because it produces plates with good properties over a wide range of B values in this study.
However, variations in the plate properties, which are shown in Figs. 8 and 9, exist under the optimum molding conditions in this experiment. This variation could be attributed partially to the variation of SC binder in the plate. Therefore, the method for combining the wood powder and SC binder before molding should be evaluated in the future.
Table 4. Moldability and Plate Properties under Various Conditions
CONCLUSIONS
- This paper described a study of the molding of wood-based products by the thermal flow of wood powder mixed with a natural binder composed of sucrose and citric acid, which is referred to as an SC binder in this paper, for fabricating wood products without synthetic resins. Optimum molding conditions for temperature, binder content, and mixture ratio of sucrose and citric acid were clarified for fabricating products with high strength and water resistance for practical use. The results indicated a possibility for effective production of naturally derived products with various shapes by injection molding.
- In thermal flow tests of the wood powder with SC binder, the powder flowed at over 120 °C. It flowed with the lowest pressure and viscosity at 180 °C under the various sucrose and citric acid mixture ratios. In addition, the pressure and temperature for the thermal flow of wood powder with SC binder were remarkably low compared with the case that the binder was only sucrose.
- In molding tests, the powder filling of the mold became more complete when the binder content was increased. The binder content range for obtaining a plate was enlarged by increasing the sucrose ratio in the SC binder, and the minimum binder content ratio used was 20 wt% when the mixture ratio of the sucrose and citric acid was 75:25 in this experiment. The appropriate binder content was over 40 wt% for obtaining a plate with a uniform density of 1.4 g/cm3 to 1.5 g/cm3. Bending strength and thickness swelling after water immersion of the plate molded under the appropriate conditions were 28 MPa to 37 MPa and 7% to 10%, respectively.
ACKNOWLEDGMENTS
This work was supported by a KAKENHI grant (No. 16K18265) from the Japan Society for the Promotion of Science and by Grants-in-Aid for Humanosphere Mission Research in 2016 (No. 11) from the Research Institute for Sustainable Humanosphere at Kyoto University.
REFERENCES CITED
Bailey, P. S., and Bailey, C. A. (1989). “Carbohydrates,” in: Organic Chemistry – A Brief Survey of Concepts and Applications, Fourth Ed. Allyn and Bacon, Boston, MA.
Csikós, Á., Faludi, G., Domján, A., Renner, K., Móczó, J., and Pukánszky, B. (2015). “Modification of interfacial adhesion with a functionalized polymer in PLA/wood composites,” Euro. Polym. J. 68, 592-600. DOI: 10.1016/j.eurpolymj.2015.03.032
Eder, A., and Carus, M. (2013). “Global trends in wood-plastic composites,” Bioplastic Mag. 8, 16-17.
Forestry and Forest Products Research Institute (2004). “Characteristic of wood,” in: Wood Industry Handbook, (4th Ed.), Maruzen Publishing, Japanese Industrial Standards Committee, Tokyo, Japan (in Japanese).
JIS A 5905 (2014). “Fiberboards,” Japanese Industrial Standards Committee, Tokyo, Japan (in Japanese).
JIS K 7171 (2016). “Plastics – Determination of flexural properties,” Japanese Industrial Standards Committee, Tokyo, Japan (in Japanese).
Kajikawa, S., and Iizuka, T. (2015). “Effect of water-soluble components mass on the fluidity of the steam-treated bamboo powder caused by heating and compression,” J. Soc. Mater. Sci., Jpn. 64, 381-386. DOI: 10.2472/jsms.64.381 (in Japanese)
Kajikawa, S., Horikoshi, M., Tanaka, S., Umemura, K., and Kanayama, K. (2017). “Molding of wood powder with a natural binder,” Procedia Eng. 207, 113-118. DOI: 10.1016/j.proeng.2017.10.747
Karmaker, A. C., and Youngquist, J. A. (1996). “Injection molding of polypropylene reinforced with short jute fibers,” J. Appl. Polym. Sci. 62, 1147-1151. DOI: 10.1002/(SICI)1097-4628(19961121)62:8<1147::AID-APP2>3.0.CO;2-I
Miki, T., Takakura, N., Iizuka, T., Yamaguchi, K., and Kanayama, K. (2004). “Effects of forming conditions on injection moulding of wood powders,” in: Proceedings of 7th Esaform Conference, Trondheim, Norway, pp. 295-298.
Nagaya, T. (2014). “Mass production of automobile interior material using kenaf fiber which is one of the biomass,” J. Jpn. Soc. Technol. Plast. 55(637), 108-111. DOI: 10.9773/sosei.55.108 (in Japanese).
Roos, Y. (1993). “Melting and glass transitions of low molecular weight carbohydrates,” Carbohydr. Res. 238, 39-48. DOI: 10.1016/0008-6215(93)87004-C
Stark, N. M., Matuana, L. M., and Clemons, C. M. (2004), “Effect of processing method on surface and weathering characteristics wood-flour/HDPE composites,” J. Appl. Polym. Sci. 93, 1021-1030. DOI: 10.1002/app.20529
Umemura, K., Sugihara, O., and Kawai, S. (2015). “Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature,” J. Wood. Sci. 61, 40-44. DOI: 10.1007/s10086-014-1437-8
Wolcott, M. P. (2001). “Wood-Plastic Composites,” in: Encyclopedia of Materials: Science and Technology (2nd Ed.), 9759-9763. DOI:10.1016/B0-08-043152-6/01772-1
Article submitted: October 11, 2019; Peer review completed: December 15, 2019; Revised version received and accepted: January 21, 2020; Published: January 22, 2020.
DOI: 10.15376/biores.15.1.1702-1715
