Abstract
Despite previous efforts, the fabrication of superhydrophobic substrate via an environment friendly and easy approach remains a great challenge. In this study, a low cost, simple, and green procedure was developed to prepare a superhydrophobic paper surface that is acceptable for the papermaking industry. First, a wax mixture (beeswax & carnauba wax) was emulsified and coated on the filter paper surface. Then, the coated paper was annealed at different temperatures. The further heat-treatment-rendered wax-coated paper hydrophobic or superhydrophobic because submicrometer or micrometer wax structures were present on the paper surface. The water contact angle of the annealed filter paper sample reached 151.5° at 60 °C, and the sliding angle was under 10°. Further, the relationship between surface composition and the hydrophobic properties of the coated paper samples was discussed. The obtained paper samples showed great potential in water/oil separation, as they had an efficiency over 99%. This work proposed a new simple and mild approach to fabricate superhydrophobic filter papers and explored the hydrophobicity and water/oil separation properties.
Download PDF
Full Article
Fabrication of Super-hydrophobic Filter Paper via Mixed Wax Phase Separation for Efficient Oil/Water Separation
Yating Wang, Xiaochun Chen, Yaqi Liang, and Chenghua Yu *
Despite previous efforts, the fabrication of superhydrophobic substrate via an environment friendly and easy approach remains a great challenge. In this study, a low cost, simple, and green procedure was developed to prepare a superhydrophobic paper surface that is acceptable for the papermaking industry. First, a wax mixture (beeswax & carnauba wax) was emulsified and coated on the filter paper surface. Then, the coated paper was annealed at different temperatures. The further heat-treatment-rendered wax-coated paper hydrophobic or superhydrophobic because submicrometer or micrometer wax structures were present on the paper surface. The water contact angle of the annealed filter paper sample reached 151.5° at 60 °C, and the sliding angle was under 10°. Further, the relationship between surface composition and the hydrophobic properties of the coated paper samples was discussed. The obtained paper samples showed great potential in water/oil separation, as they had an efficiency over 99%. This work proposed a new simple and mild approach to fabricate superhydrophobic filter papers and explored the hydrophobicity and water/oil separation properties.
Keywords: Superhydrophobic; Wax emulsion; Phase separation; Water/oil separation
Contact information: College of Chemistry, Guangdong University of Petrochemical Technology, Guandu Road, Maoming 525000, PR China; *Corresponding author: ych@gdupt.edu.cn
GRAPHICAL ABSTRACT
INTRODUCTION
Wettability is an important surface property for all solids. Superhydrophobic surfaces are defined as those that have a water-resistant property with a water contact angle over 150° and a water slide angle under 10° (Soliveri et al. 2012). Superhydrophobic surfaces, such as plant leaves and the wings or legs of some insects exist widely in nature. The most representative superhydrophobic surface is the lotus leaf (Leng et al. 2009), which has micro- and nanostructures and wax components on it. Researchers conclude that both low-energy surface composition and fine roughness are essential to form a superhydrophobic surface (Celik et al. 2021; Zhang et al. 2021). Therefore, the fabrication of superhydrophobic surfaces is achieved by either roughening the low-surface-energy material surface or reducing the surface energy of the rough surface via chemical/ physical modification or the combination of both methods (Han et al. 2004; Zhang et al. 2004; Sun et al. 2005).
Superhydrophobic materials are water resistant, antifouling, and self-cleaning, among other properties (Wang et al. 2019; Song et al. 2020). The surface tension of superhydrophobic surfaces is low, so oil can easily infiltrate (Cheng et al. 2017; Upadhyay and Waghmare 2020). Feng et al. (2004) utilized a superhydrophobic metal mesh to separate oil/water mixtures for the first time. The metal mesh was sprayed with a PTFE emulsion and then became highly water-resistant. Diesel oil could quickly pass through the obtained mesh, which achieved the goal of oil-water separation. Since then, there has been an increasing number of studies on the oil-water separation performance of hydrophobic or superhydrophobic materials.
Initially, the substrates of superhydrophobic fibrous materials were mostly hard metals. For example, Li et al. (2007) prepared a superhydrophobic surface with a petal-like structure on the nickel metal substrate via a simple chemical method using mono-alkyl phosphoric acid. In addition, the obtained superhydrophobic nickel mesh could effectively separate the oil-water mixture. Gu et al. (2014) used porous Al2O3 ceramic membrane as the substrate to deposit carbon nanotubes (CNTs) with excellent mechanical, thermal, and conductive properties through filtration. The CNTs provided micro- and nano- rough structure, and they contained a large amount of -OH and -COOH groups, which grafted hydrophobic polystyrene (PS) onto the surface of the CNTs via photoinitiated polymerization. A hydrophobic polymer brush was formed that had an oil removal rate of up to 99.94% from an oil-water emulsion, and the water flux was up to 5000 L / (m2·h). These hard substrate materials have good mechanical properties, but their applications are often limited, as they are easily corroded and do not biodegrade easily under natural conditions.
Researchers have attempted to obtain superhydrophobic materials based on soft substrates, such as paper or fabric, without using harmful reagents such as fluorochemicals. Cheng et al. (2018) developed a dip-coating method to obtain superhydrophobic cotton fabric. They used renewable, biodegradable, nanoscale-sized cellulose nanocrystal as the rough structure component instead of inorganic nanoparticles (TiO2 or SiO2) to fabricate renewable and degradable superhydrophobic cotton fabric. To prevent nanocellulose from falling off, the cured epoxidized oil resin was used as the binder. The prepared superhydrophobic cotton fabric could selectively absorb oil from oily water and could separate various oil/water mixtures efficiently, and it had a separation efficiency of over 98%. Li et al. (2019) successfully fabricated superhydrophobic/superoleophilic papers through the combination of layer-by-layer (LBL) deposition of chitosan (CS) and hexadecyltrimethoxysilane (HMTS-g-TiO2) on the paper surface and subsequent heat treatment. The paper modified with 20 multilayers of (CS/HTMS-g-TiO2) followed by heat treatment showed superhydrophobicity with a water contact angle of 167.4° and superoleophilicity, with an oil contact angle of 0°. Further, the prepared paper effectively separated both immiscible oil-water mixtures and water-in-oil emulsions solely driven by gravity.
This study aimed to develop a low cost, green, and simple procedure to prepare a superhydrophobic paper surface that is acceptable for the papermaking industry. Plant waxes are important materials for preparing superhydrophobic surfaces, firstly because they can impart water repellency to an extent with good hydrophobicity, and secondly, they are renewable. Thus, they do not pose any environmental or health concerns. In relevant studies (Zhang et al. 2014; Naderizadeh et al. 2019), micrometer wax particles were produced via the emulsifying process, which increases the hydrophobicity of paper after coating the surface. However, the roughness of wax-latex-coated paper is not sufficient to render the surface superhydrophobic. A robust water impact resistant and solution-processable all-organic superhydrophobic coating was prepared from natural and renewable carnauba wax and low-cost candle soot nanoparticles by Onses and coworkers. It exhibited superhydrophobicity with a water contact angle of 172◦ and sliding angle of 3°, respectively (Celik et al. 2021). This method was simple, but getting candle soot was time consuming. Another study (Celik et al. 2020) developed an approach to fabricate hierarchically structured superhydrophobic surfaces from dispersions of hydrophobic nanoparticles and wax with a water contact angle of 175° and a sliding angle of 3°. The composite coating was mechanically durable and fluorine-free. The only minor drawback was that inorganic particles were difficult to degrade naturally.
Wax displays a certain degree of hydrophobic properties, and the authors assumed that the waxes with different thermal properties could be separated from the wax mixture at the proper temperature. The wax with a higher melting point would keep its solid state, while the wax with a lower melting point would melt and flow out. This phenomenon would likely lead to a surface morphology change in the materials coated with the wax mixture. In the experiments, a wax mixture composed of beeswax and carnauba wax was first emulsified to form a wax mixture emulsion. Then, the original filter paper was coated with the mixed wax emulsion and dried at room temperature. After further heat treatment for 10 h at different temperatures, filter papers with high water resistance and lipophilicity were obtained. Thus, (super) hydrophobic paper was prepared simply and cost-effectively. The key advantage of this method was that no fluorochemicals and organic solvents were used in the preparation process, which facilitated the industrial application and fit the needs of sustainable development due to the use of green-based materials.
EXPERIMENTAL
Materials
The beeswax (food grade, melting point 62 °C) and carnauba wax (food grade, melting point 80 °C) were purchased from Usolf Chemical Technology Co., Ltd. (Linyi, China). The chitosan was obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The acetic acid used was sourced from Qiangsheng Chemical Industry Co., Ltd. (Changshu, China), and the glycerol was sourced from Damao Chemical Reagent Factory (Tianjin, China). Finally, the filter paper was purchased from Wohua Co., Ltd. (Hangzhou, China), and the diesel was obtained from a gas station.
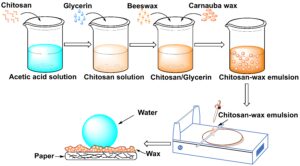
Fig. 1. Illustration of the fabrication of superhydrophobic filter paper
Preparation of the mixed wax emulsion
A 1 wt% acetic acid solution was prepared in a beaker. Then, a certain amount of chitosan powder was slowly added to the acetic acid solution of the same concentration (1 wt% chitosan) and stirred quickly until the solution became clear. Glycerin was added into the chitosan solution as a plasticizer, and the solution was heated in a water bath to approximately 85 °C. The mixture of waxes with different weight ratios of beeswax & carnauba wax (the mass ratios of beeswax to carnauba were 3:7, 5:5, and 7:3) was added to the heated solution in liquid state. The solution was stirred for 20 min after the wax had melted. A high-speed homogenizer (T25, IKA, Staufen, Germany) was used to stir the obtained emulsion for an additional 3 min. Finally, the emulsified chitosan-wax emulsion (weight percentage of the emulsion was 5%) was cooled and stirred until reaching room temperature. An illustration of the fabrication of superhydrophobic filter paper is shown in Fig. 1.
Coating and annealing
Medium-speed qualitative filter paper was coated with the wax mixture latex using a coating instrument (K303, RK Print Coat Instruments Ltd, Royston Hertfordshire, England) at a speed of 3 m/min. A coating weight of 7.5 g/m2 was chosen. After wet coating, the papers were dried at room temperature for 24 h. Then, the coated paper was further annealed (dried) at different temperatures (25 °C, 60 °C, and 75 °C) for 10 h. Thus, a minor structure on the wax coating layer was produced during the phase separation process.
Separation of water/oil mixtures
Deionized water was mixed with diesel (the total amount was 10 g, and the proportion of water was 10 wt%), and the mixture was ultrasonically dispersed to simulate high-water-content diesel. The prepared paper samples were cut to a suitable size and placed into a glass sand funnel (G3) for filtration. During oil/water separation, the mixtures were slowly poured into the separation system. The vacuum circulation pump was used to facilitate filtration. Separation occurred, as water remained in the upper container and oil fell into the lower container.
Methods
Characterization
The thermal properties of mixed wax were determined via differential scanning calorimetry (DSC) (SDT, Q600, TA Instruments, New Castle, DE, USA). The wax mixture samples were first heated above their melting point, and they were then cooled to room temperature to achieve uniform initialization conditions. Thermograms of the re-heated process were recorded at temperatures ranging from room temperature to 90 °C with a heating rate of 1 °C/min.
The contact angle measurements were performed using OCA20 (Dataphysics, Filderstadt, Germany). An automatic single liquid dispenser was used to dispense a precise volume of deionized water (5 μL), which descended until the drop reached the paper samples. Then, it was raised again so that the water drop stayed on the sample surface. Each paper sample was measured at least 5 times to determine average values in the different positions on the surface. The maximum angle of inclination of the stage at which the water drop could not roll off was marked as the water sliding angle.
The surface topography was characterized using scanning electron microscopy (SEM) (Shimadzu SS-550, Kyoto, Japan) at an accelerating voltage of 5.0 kV. Samples were mounted on aluminium stubs using conducting resin and then coated with approximately 10 nm of gold in an ion sputtering coating instrument (1080anto, Cressington, Watford, England).
The moisture content of the oil-water mixture after filtration was determined using an accurate tester (870, Metrohm, Herisau, Switzerland). The pore size and permeability of each paper sample was measured via a capillary pore size analyzer (Porolux, 100FM, Gent, Belgium) five times to obtain an average value.
RESULTS AND DISCUSSION
Thermal Properties of Beeswax and Carnauba Wax
Plant-derived wax has excellent hydrophobicity because its main components are esters of long-chain fatty alcohols, acids, and long-chain alkanes (Saji 2020). Therefore, such wax is a natural medium for the preparation of hydrophobic materials. In this study, beeswax and carnauba wax, which were selected to improve the hydrophobicity of filter paper, could be mixed at any weight ratio because they had similar ingredients. The thermal properties of the two wax types are shown in Fig. 2.
Fig. 2. The differential scanning calorimetry (DSC) thermograms of carnauba wax (A) and beeswax (B)
The complete melting points of beeswax and carnauba wax were 62 and 80 °C, respectively. Figure 2 shows that before reaching the melting point, beeswax had an obvious heat absorption peak at 52 °C, and the carnauba wax had an absorption peak at 74 °C. The heat absorption peaks were generated due to the polymorphic phase transitions of the pure wax. In the process of the phase transitions, wax existed in a semi-solid and semi-liquid form. During the cooling process of the melted wax, the exothermic peaks corresponded to the endothermic peaks, but they were not at exactly the same temperature. The temperature of the exothermic peak was generally lower than that of the corresponding endothermic peak, which might have been caused by the hysteresis of the waxy crystal transition. When the two waxes were evenly mixed, the heat absorption and heat release behaviors of the mixture reflected the combined behaviors of the two component waxes. Beeswax could likely be separated from the wax mixture at a certain temperature and duration at which beeswax is in liquid form and carnauba wax remains in a solid state. The solid-liquid phase separation phenomenon would occur, which facilitates the generation of the micro-/nano-scale rough structure.
Hydrophobic Properties of the Emulsion Coated Paper
The surface wettability of the emulsion coated filter paper was characterized by the water contact angle and the sliding angle. Water quickly wetted and penetrated the untreated filter paper with a water contact angle of 0°. After coating (the coating amount was 7.5 g/m2) and annealing, the filter papers became very hydrophobic, as shown in Fig. 3. The mass ratios of beeswax to carnauba were 3:7, 5:5, and 7:3.
The hydrophobicity of the coated filter papers improved notably, and all the contact angles were above 130°. During the drying process at 25 °C, the moisture in the wax emulsion evaporated, and the waxy particles stacked on the filter paper to form a micro coarse structure. Without further heating treatment, the wax particles would have retained their granular shape on the filter papers. When the coated paper samples were annealed at 60 °C for 12 h, all the WCAs increased dramatically to approximately 150°. Figure 3 showed that when the mass ratio of wax was 7:3, the WCA of the filter paper sample was 151.5°, and the sliding angle was less than 10°. The filter paper surface began to provide self-cleaning properties, as water drops could easily roll off to remove dust particles, which is a property also exhibited by lotus leaves.
Fig. 3. The hydrophobicity of filter papers with different treatment temperature
When the annealing temperature reached 75 °C, the WCA values of the paper samples were lower than that at 25 °C or 60 °C. As carnauba wax had an endothermic peak at 74 °C, both the two waxes melted on the paper surface at temperatures below 75 °C. The melting process weakened the surface roughness of the coated filter papers. Notably, most the WCA values of the coated filter paper samples did not differ substantially at the same annealing temperature. This finding suggested that the ratio of the two waxes did not affect much on the hydrophobicity of coated papers (our previous experiments showed that the surface energy of different proportions of mixed wax varies little; they were 34.2 mN/m (3:7), 36.2 mN/m (5:5), 32.1 mN/m (7:3), respectively). The hydrophobicity of a material depended on the surface energy and surface morphology, therefore, the surface morphology transformation during heat treatment may be the key reason for the change in surface hydrophobicity.
Cassie and Baxter (1944) developed a model to describe a wetting state on a rough, solid surface, in which the liquid droplet is situated on a solid-air-liquid composite surface rather than penetrating into the rough grooves, and the apparent contact angle (θc) is the sum of two contributions of a liquid-solid interface and a liquid-vapor interface as described in Eq. 1,
cosθc = f1cosθ1 – f2 (1)
where cosθc is the apparent contact angle (°), which was the measured WAC of the paper sample, f1 is the fractional area (%) of the solid surface with an intrinsic contact angle of θ1 (°), θ1 is the intrinsic contact angle (°) of the solid surface, f2 is the fractional area (%) of the air, and f1+f2=1. In the experiment, θ was the WCA of the smooth mix wax surface, which was 114.2°. When the WCA of the paper sample was 151.5°, f was 19.73%. When the cosθc was 134.7°, f was 39.44%. These results demonstrated that any hydrophobic material can become superhydrophobic by reducing the solid-liquid contact area. Further, it was shown that surface roughness is more important in superhydrophobicity at low f levels.
Surface Morphology of the Coated Paper
Through analysis of the WCA, the annealing temperatures were predicted to have a great influence on surface morphology. This was determined by the SEM images in Fig. 4.
Figures 4 A-1 and A-2 show that the filter paper fibers were covered by wax coating and many pores were partially filled in, but some pores remained unfilled. In Figs. 4 B-1 and 4 B-2, the wax beads were smooth and adhered to the surface of filter paper, and some of them aggregated. The randomly overlapping wax beads might have fallen off the papers without further annealing. When the drying temperature was 60 °C, the beeswax on the paper surface melted and flowed out, whereas the carnauba wax remained in a solid state. Compared to Fig. 4-B, Fig. C-2 showed that several wrinkles on the wax particles dried at 60 °C, which increased the roughness of the filter paper. Upon increasing the temperature to 75 °C, the spherical wax structure was destroyed, and the folds at wax beads disappeared (Fig. 4 D-1 and Fig. 4 D-2) because the beeswax and carnauba both completely melted. The transformation of the wax mixture beads was largely consistent with the DSC results. Only the appropriate temperature would lead to the phase separation between the two waxes and produce a complex microstructure, which was beneficial for the high contact angle.
Annealing treatment for the wax-latex-coated papers had another advantage, which was that the wax particles combined more strongly amongst each other. The wax particles melted out and then cooled like an adhesive, which made them combine and strengthen the connection with the filter paper and increase the mechanical strength of the coated papers.
Fig. 4. The surface morphology of filter paper coated with the mixed wax emulsion (Beeswax: Carnauba wax = 7 : 3). Images A-1, A-2, B-1, B-2, were at 25 °C; images C-1 and C-2 were at 60 °C; images D-1 and D-2 were at 75 °C
Oil-water Separation Performance of the Coated Paper
The pristine filter paper was easily wetted by both water and oil, which made it impossible to accomplish oil/water separation. The wetting behavior of the filter paper exhibited superoleophilicity and (super)hydrophobicity after surface modification via wax emulsion. Thus, the modified filter paper exhibited good efficiency in oil/water separation.
Table 1. The Oil/Water Separation Ability of Wax Emulsion Coated Filter Papers (B:C = 7:3)
Because of the immiscibility of oil and water, the mixture quickly permeated through the filter paper, and the water was retained above the filter paper due to the excellent (super)hydrophobic-superoleophilic ability of the modified filter paper. Table 1 showed that the oil/water separation effect of the filter paper dried at 25 °C was the best. The water content in the water/diesel mixture decreased from 10% to 0.0207% (207 ppm) after filtration separation, the water-repelling efficiency reached 99.79%, and the permeation efficiency of diesel was 94.97%. Similarly, the oil/water separation performance of the filter paper sample dried at 60 °C was also good, its separation efficiency reached 99.47%. However, that of the sample dried at 75 °C was less than satisfactory, in Table 1, it showed that the water content after separation was 4190 ppm (0.419%). Thus, the water-repelling efficiency was only 95.81%, which meant that more water passed through the filter paper than others. As Fig. 3 and Table 1 showed, the oil-water separation efficiency and water-repelling ability were not only determined by the filter paper’s hydrophobicity.
Fig. 5. The air permeability of the filter papers with different drying temperatures (B : C = 7 : 3)
Fig. 6. The average pore diameter of the filter papers with different drying temperatures (B : C = 7 : 3)
Figures 5 and 6 show the pore structures of the filter paper samples with different annealing (drying) temperatures. Air permeability represented the porosity of the filter paper. The pristine filter paper had air permeability of nearly 700 mL/min. Through the process of coating and drying, the porosity of the filter papers decreased notably because the wax beads enfolded the fibers and filled parts of the paper pores. Figure 5 shows that the filter paper samples dried at 25 and 60 °C both had air permeability below 50 mL/min, whereas that of the filter paper dried at 75 °C was higher. This explained why the oil-water separation efficiency and water-repelling ability of the paper samples differed so substantially. In addition, the pore size of the filter paper affected the separation results. Figure 6 shows that the average pore diameter of the filter paper treated at 60 °C was twice that of the filter paper treated at 25 °C. Further, the average pore size of the filter paper dried at 75 °C was the largest.
As the SEM pictures show, with the coated filter paper annealed at 75 °C, the mixed wax melted, and the wax globular structure disappeared. Instead, the two kinds of wax formed a layer structure with pores on the paper fibers, and some of the fibers that had been covered by the globular wax were exposed. The results of air permeability and average pore size analysis were consistent with the morphological observations. This showed that the larger pores and higher porosity led to more water passing through the filter paper.
Reusability of the Coated Paper
The performance of the coated paper (dried at 60 °C) had been evaluated for water/oil separation cycles without cleaning/washing treatments. The unwashed coated paper showed a water/oil mixture separation efficiency all over 95.00% up to 5 cycles, as shown in Fig. 7. The coated paper maintained its high separation efficiency after repeated usage.
Fig. 7. The performance for separation of water/diesel mixture for 5 times
CONCLUSIONS
- Superhydrophobic filter paper was successfully created via a mild mix-wax phase separation method. The major advantage of this novel approach was to eliminate the use of organic solvents or fluorochemicals that have been conventionally applied for superhydrophobic modification.
- A micrometer spherical structure of mixed wax particles was produced in the emulsifying process, and the annealing process produced a submicrometer structure, which made the surface of the coated paper highly hydrophobic or superhydrophobic.
- The obtained composite filter paper could resist water strongly with a contact angle of up to 151.5° and could separate oil/water mixture with 87.1% water-repelling and 94.7% oil-permeation. The 25 °C samples had the best water/oil separation efficiency.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (Grant No. 22005065), the Scientific Research Fund of Guangdong University of Petrochemical Technology (Grant No. 2019rc050, Grant No. 2019rc051), and the Ordinary University Youth Innovation Project of Guangdong Province (Grant 2020KQNCX050).
REFERENCES CITED
Cassie, A. B. D., and Baxter, S. (1944). “Wettability of porous surfaces,” Transactions of the Faraday Society 40, 546-551.
Celik, N., Kiremitler, N. B., Ruzi, M., and Onses, M. S. (2021). “Waxing the soot: Practical fabrication of all-organic superhydrophobic coatings from candle soot and carnauba wax,” Progress in Organic Coatings 153, article ID 106169. DOI: 10.1016/j.porgcoat.2021.106169
Celik, N., Torun, I., Ruzi, M., Esidir, A., and Onses, M. S. (2020). “Fabrication of robust superhydrophobic surfaces by one-step spray coating: Evaporation driven self-assembly of wax and nanoparticles into hierarchical structures,” Chemical Engineering Journal 396, article ID 125230. DOI: 10.1016/j.cej.2020.125230
Cheng, Q., An, X., Li, Y., Huang, C., and Zeng, J. (2017). “Sustainable and biodegradable superhydrophobic coating from epoxidized soybean oil and ZnO nanoparticles on cellulosic substrates for efficient oil/water separation,” ACS Sustain. Chem. Eng. 5(12), 11440-11450. DOI: 10.1021/acssuschemeng.7b02549
Cheng, Q.-Y., Guan, C.-S., Wang, M., Li, Y.-D., and Zeng, J.-B. (2018). “Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation,” Carbohydrate Polym. 199, 390-396. DOI: 10.1016/j.carbpol.2018.07.046
Feng, L., Zhang, Z., Mai, Z., Ma, Y., Liu, B., Jiang, L., and Zhu, D. (2004). “A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water,” Angewandte Chemie International Edition 43(15), 2012-2014. DOI: 10.1002/anie.200353381
Gu, J., Xiao, P., Chen, J., Liu, F., Huang, Y., Li, G., Zhang, J., and Chen, T. (2014). “Robust preparation of superhydrophobic polymer/carbon nanotube hybrid membranes for highly effective removal of oils and separation of water-in-oil emulsions,” J. Mater. Chem. A 2(37), article ID 15268. DOI: 10.1039/C4TA01603C
Han, J. T., Lee, D. H., Ryu, C. Y., and Cho, K. (2004). “Fabrication of superhydrophobic surface from a supramolecular organosilane with quadruple hydrogen bonding,” Journal of the American Chemical Society 126(15), 4796-4797. DOI: 10.1021/ja0499400
Leng, B., Shao, Z., de With, G., and Ming, W. (2009). “Superoleophobic cotton textiles,” Langmuir 25(4), 2456-2460. DOI: 10.1021/la8031144
Li, H., Wang, X., He, Y., and Peng, L. (2019). “Facile preparation of fluorine-free superhydrophobic/superoleophilic paper via layer-by-layer deposition for self-cleaning and oil/water separation,” Cellulose 26(3), 2055-2074. DOI: 10.1007/s10570-018-2187-3
Li, M., Xu, J., and Lu, Q. (2007). “Creating superhydrophobic surfaces with flowery structures on nickel substrates through a wet-chemical-process,” Journal of Materials Chemistry 17(45), article no. 4772. DOI: 10.1039/B709665H
Naderizadeh, S., Heredia Guerrero, J. A., Caputo, G., Grasselli, S., Malchiodi, A., Athanassiou, A., and Bayer, I. S. (2019). “Superhydrophobic coatings from beeswax-in-water emulsions with latent heat storage capability,” Advanced Materials Interfaces 6(5), article ID 1801782. DOI: 10.1002/admi.201801782
Saji, V. S. (2020). “Wax-based artificial superhydrophobic surfaces and coatings,” Colloids and Surfaces A: Physicochemical and Engineering Aspects 602, article ID 125132. DOI: 10.1016/j.colsurfa.2020.125132
Soliveri, G., Annunziata, R., Ardizzone, S., Cappelletti, G., and Meroni, D. (2012). “Multiscale rough titania films with patterned hydrophobic/oleophobic features,” The Journal of Physical Chemistry C 116(50), 26405-26413. DOI: 10.1021/jp309397c
Song, L., Hu, J., Huang, X., Zhong, L., Pei, Y., Wu, L., and Zhang, X. (2020). “Superhydrophobic self-healing coatings comprised of hemispherical particles arrays decorated by fluorocarbon-coated nanoscale Fe2O3 rods and SiO2 particles,” ACS Applied Nano Materials 3(10), 10342-10348. DOI: 10.1021/acsanm.0c02259
Sun, T., Tan, H., Han, D., Fu, Q., and Jiang, L. (2005). “No platelet can adhere—Largely improved blood compatibility on nanostructured superhydrophobic surfaces,” Small 1(10), 959-963. DOI: 10.1002/smll.200500095
Upadhyay, R. K., and Waghmare, P. R. (2020). “Eco-friendly preparation of superhydrophobic copper surfaces for oil/water separation,” Environmental Chemistry Letters 18(2), 505-510. DOI: 10.1007/s10311-019-00952-3
Wang, H., He, M., Liu, H., and Guan, Y. (2019). “One-step fabrication of robust superhydrophobic steel surfaces with mechanical durability, thermal stability, and anti-icing function,” ACS Applied Materials & Interfaces 11(28), 25586-25594. DOI: 10.1021/acsami.9b06865
Zhang, X., Shi, F., Yu, X., Liu, H., Fu, Y., Wang, Z., Jiang, L., and Li, X. (2004). “Poly-electrolyte multilayer as matrix for electrochemical deposition of gold clusters: Toward super-hydrophobic surface,” Journal of the American Chemical Society 126(10), 3064-3065. DOI: 10.1021/ja0398722
Zhang, W., Lu, P., Qian, L., and Xiao, H. (2014). “Fabrication of superhydrophobic paper surface via wax mixture coating,” Chemical Engineering Journal 250, 431-436. DOI: 10.1016/j.cej.2014.04.050
Zhang, Z., Xue, F., Bai, W., Shi, X., Liu, Y., and Feng, L. (2021). “Superhydrophobic surface on Al alloy with robust durability and excellent self-healing performance,” Surface and Coatings Technology 410, article ID 126952. DOI: 10.1016/j.surfcoat.2021.126952
Article submitted: April 1, 2021; Peer review completed: May 23, 2021; Revised version received and accepted: June 24, 2021; Published: July 2, 2021.
DOI: 10.15376/biores.16.3.5794-5805
