Abstract
Download PDF
Full Article
Facile Fabrication of Superhydrophobic/Superoleophilic Cotton for Highly Efficient Oil/Water Separation
Qing Wang,a Mingguang Yu,b Guangxue Chen,a,* Qifeng Chen,a,c and Jinglei Tai a
This paper presents a facile and versatile strategy to fabricate robust and superhydrophobic/superoleophilic cotton for the removal of oils and organic solvents from polluted water. The superwettability cotton was prepared via in-situ hydrolysis of tetraethoxysilane (TEOS) on the cotton fiber surface using polyvinylpyrrolidone (PVP) as a coupling agent and subsequent hexadecyltrimethoxysilane modification. By simply adjusting the molecular weight of PVP and the concentration of NH3•H2O, the surface roughness of SiO2-modified cotton fibers could be well controlled to generate cotton fibers with excellent superhydrophobicity. The prepared cotton fibers were used as superabsorbents for oil/water separation. It absorbed up to 35 times and 50 times its own weight of n-hexane and chloroform, respectively, while repelling water completely. After collecting the absorbed oils via a simple squeezing method, the cotton could be reused for at least 5 cycles. Moreover, the whole procedure was carried out in a mild environment, with no intricate instruments or toxic reagents.
Keywords: Cotton fiber; Silica nanoparticles; Polyvinylpyrrolidone; Oil/water separation
Contact information: a: State Key Laboratory of Pulp & Paper Engineering, South China University of Technology, Guangzhou 510640, China; b: School of Materials Science and Energy Engineering, Foshan University, Foshan 528000, China; c: Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China, Qilu University of Technology;
* Corresponding author: chengx@scut.edu.cn
INTRODUCTION
Oil spills, especially marine oil and industrial wastewater, have disastrous consequences for society. Hence, oil spill accidents have attracted intense attention. Methods have been developed for the removal of oils and organic solvents from polluted water such as gravity separation (Kwon et al. 2012), filtration (Tirmizi et al. 1996), centrifuge, flotation, and electrochemical methods (Ichikawa and Nakajima 2004). However, the relatively complicated production equipment or operation procedures limit the actual applications for oil/water separation.
With the rapid development of colloid and interface science, superhydrophobic materials have gained attention for their potential application in oil/water separation (Xue et al. 2014; Chang et al. 2015; Wang et al. 2015 a; Lu and Hu 2016). Various substrate materials have been used to develop superhydrophobic oil sorbents. Among the numerous oil absorbents, porous materials such as metallic mesh films (Feng et al. 2004; Zang et al. 2013), textiles (Zhou et al. 2013; Cao et al. 2016), and polymer-based sponges and foams (Jiang et al. 2014; Li et al. 2016; Xu et al. 2016) are mostly used to separate oil from water. Although these absorption materials have been widely applied in practical applications, there still exist some limitations such as low absorption capacities and environmental incompatibility.
Cotton absorbents are attractive because of their high absorption capacities as well as renewability and biodegradability. These materials have received increasing attention as an oil-absorbing material in oil/water separation for their microscale roughness in the integral fabric and their native porosity (Bi et al. 2013; Wang et al. 2013). Liu et al. (2014) prepared superhydrophobic and superoleophilic SiO2-modified cotton for oil/water separation via a sol-gel process, which was initially pretreated by NaOH aqueous solution. Wang et al. (2015 b) fabricated SiO2-modified superhydrophobic cotton fiber for absorbing oil using marine mussels for bioadhesion. However, the above methods either reduced the mechanical property of the cotton or used expensive materials, which hinders their application in large-scale oil/water separation.
This report describes a facile and environmentally friendly strategy for the fabrication of superhydrophobic/superoleophilic cotton for oil/water separation. The superhydrophobic surfaces of cotton fibers were prepared via in-situ hydrolysis of TEOS using PVP as coupling agent to construct micro/nano hierarchical rough surface structures, which were subsequently modified with a low surface energy material (hexadecyltrimethoxysilane (HDTMS)). The surface roughness of the silica-cotton fibers was controlled by adjusting the molecular weight of PVP and the concentration of NH3•H2O. The prepared cotton fibers exhibited excellent water-repellence with a water contact angle of 162° ± 1.1°. They also showed efficient and rapid oil/water separation ability and were used as superabsorbents for oil/water separation. Moreover, the prepared cotton fibers absorbed various organic solvents and common oils, and the adsorption capacity reached more than 50 times its own weight. After collecting the absorbed oils via squeezing, the cotton was reused for at least 5 cycles.
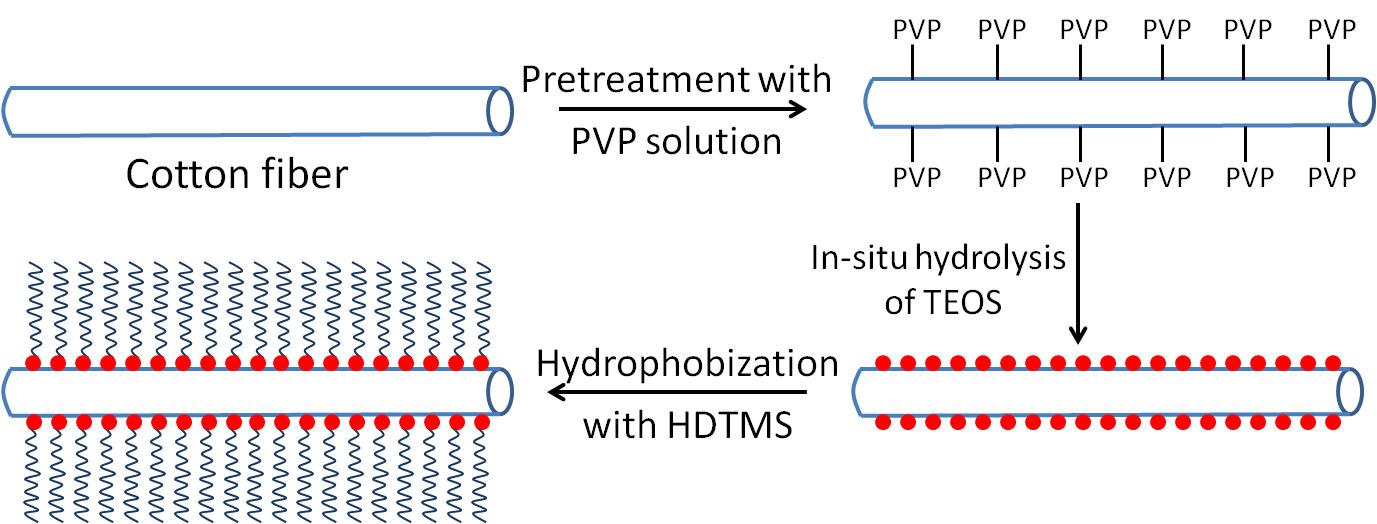
Fig. 1. Schematic illustration for fabrication superhydrophobic/superoleophilic cotton
EXPERIMENTAL
Materials
Cotton was obtained locally. Polyvinylpyrrolidone with different average molecular weights of 10 kg/mol (PVP-10), 58kg /mol (PVP-58), and 130 kg/mol (PVP-130) were purchased from Sigma-Aldrich (Shanghai, China). Hexadecyltrimethoxysilane (HDTMS, > 99%) was purchased from Sigma-Aldrich. Tetraethoxysilane (TEOS), anhydrous ethanol, and aqueous ammonia solution (25 wt.%) were purchased from Guangzhou Chemical Reagent Co. (Guangzhou, China). Deionized water was made in-house.
Preparation of PVP Modified Cotton
Raw cotton was washed several times with deionized water to remove dust and then dried under vacuum at 60 °C for 12 h. Next, 1 g of dry cotton was transferred into 50 mL deionized water containing a certain amount of PVP. The solution was stirred mechanically at 100 rpm for 1 h and then dried under vacuum at 60 °C for 12 h.
Preparation of Silica-Coated Cotton Fabric
Silica-coated cotton fabrics were prepared by a modified Stöber method (Jia et al. 2014). Under gentle magnetic stirring, 0.5 g of PVP-modified cotton and ammonium hydroxide (25 wt.%) were added to 100 mL of ethanol and 20 mL of deionized water in a 250 mL three-neck round bottom flask at room temperature. A total of 1 mL of TEOS was slowly added to the solution.
The mixture was stirred vigorously for 6 h at room temperature. After being washed in sequence with EtOH and water, the silica-coated cotton was dried under vacuum at 60 °C for 12 h.
Fabrication of Superhydrophobic/Superoleophilic Cotton
The solution containing 1.00 mL of hexadecyltrimethoxysilane (HDTMS) and 99 g of absolute ethanol was mechanically stirred for 2 h. After pre-hydrolysis, the silica-coated cotton was added to the solution and stirred mechanically at 100 rpm for 4 h. The stable water-repellent cotton fabrics were dried under vacuum at 60 °C for 12 h.
Characterization
The raw and silica-coated cotton structures were analyzed by Fourier transform infrared (FT-IR) spectroscopy on a Spectrum 2000 FT-IR spectrometer (Perkin Elmer, Waltham, MA, USA).
The elemental composition of samples was determined using X-Ray photoelectron spectroscopy (XPS) on a Thermo Electron Escalab 250 spectrometer (Waltham, MA, USA) with Li Ka radiation (20 eV) as the exciting source. Geometric microstructures were observed on a thermal field-emission scanning electron microscope (FESEM: Quanta 400F, FEI, Hillsboro, OR, USA) at 15 kV. Thermogravimetric analysis (TGA) was performed on a Q600 instrument (TA Instruments, New Castle, DE, USA). All samples were heated from 40 °C to 600 °C at a heating rate of 20 °C/min in a nitrogen flow at a flow rate of 25 mL/min.
Contact angles (CAs) were measured on a Dataphysics OCA20 Contract-Angle System with liquid droplets of 5 μL. The reported values are the averages of measurements in least 5 different positions.
The oil absorption capacity of the prepared cotton fibers was determined in pure oils and organic solvents. The mass of the cotton before and after immersed in pure oil were denoted as m1 and m2. The oil absorption capacity of the specimens were obtained by the following equation,
![]() (1)
(1)
where S is the oil absorption capacity of the prepared cotton fibers (%).
RESULTS AND DISCUSSION
Fabrication and Characterization of Silica Coated Cotton
Superhydrophobic surfaces are governed by a specific micro-nano structured surface topography and low surface energy (Wang et al. 2015b). To form a rough micro/nano surface, TEOS treatment was carried out by in-situ hydrolysis on raw cotton fiber surface using PVP as coupling agent (Fig. 1). The procedure consisted of two steps: adsorption of PVP and hydrolysis of the TEOS on the cotton fiber surface. PVP was first dissolved in water and adsorbed onto the raw cotton surface. The PVP-stabilized cotton fiber was transferred into an ammonia/ethanol/H2O mixture where homogeneous silica particles of varies diameters were formed by the consecutive addition of TEOS. PVP stabilizes the particles during the hydrolysis process. It forms strong hydrogen bonds with cotton fabric and adsorbs TEOS on the cotton surface during the subsequent in-situ hydrolysis into silica nanoparticles (Graf et al. 2003).
The surface morphologies of raw cotton and the prepared silica-coated cotton are shown in Fig. 2. SEM images show that the surface of raw cotton was smooth. The modified cotton was very similar to raw cotton at low magnification (Fig. 2d). Higher magnification SEM images (Figs. 2e and 2f) showed that the cotton fiber was densely and uniformly covered by the silica nanoparticles, which roughen the cotton surface. The cotton fiber modified with special micro/nano rough structures was expected to improve hydrophobicity and oil adsorption ability.
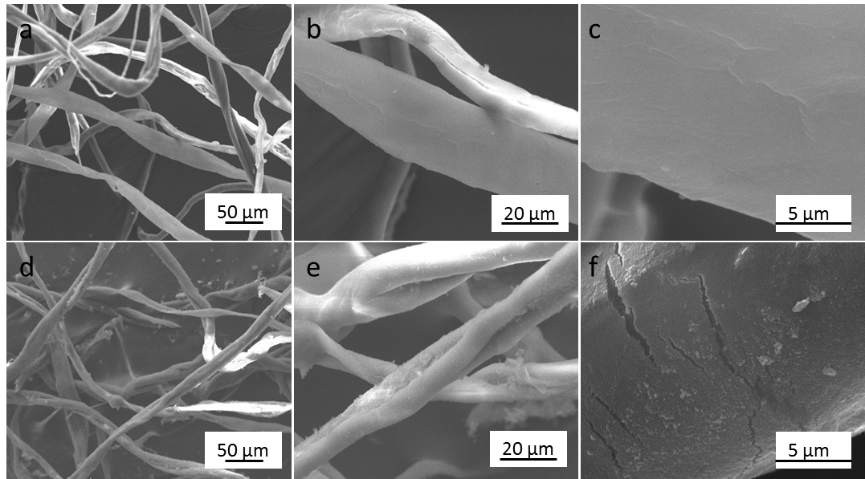
Fig. 2. SEM images of raw cotton (a, b, c) silica-coated cotton (d, e, f) at different magnifications
Figure 3 shows the FT-IR spectra of unmodified, PVP modified cotton and silica-coated cotton. In Fig. 3b, comparing with the spectra with raw cotton in Fig. 3a, the absorption peaks at 1655 cm-1, 1495cm-1, and 1423cm-1 were the stretching vibration absorption and the absorption peaks of –C=O in amide carbonyl. The peak in 1290 cm-1 was the stretching vibration absorption peak of –C-N-, which means that PVP was modified onto the surface of cotton fiber. In Fig. 3c, the characteristic peaks at 463 cm-1 belonged to bending vibration peak of Si-O-Si; 797cm-1 was the antisymmetric stretching vibration peak of Si-O-Si. The absorption peaks at 2923 and 2849 cm-1 can be attributed to the methylene and methenyl groups, implying that the surface of cotton fiber has been successfully modified by SiO2. These results indicated that nano-SiO2 was successfully prepared on the surfaces of the cotton fiber to obtain micro/nano hierarchical rough surface structure.
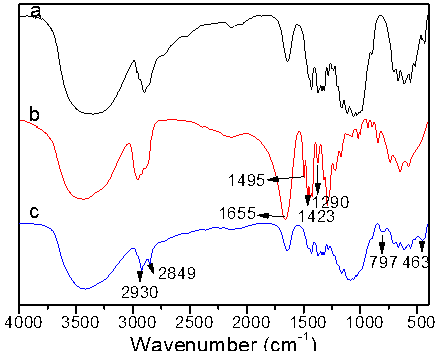
Fig. 3. FT-IR spectra of raw cotton (a), PVP modified cotton (b), and SiO2 modified cotton (c)
XPS spectra were employed to determine the chemical composition of raw and silica-coated cotton fiber. The spectra of raw cotton revealed only carbon (282.1 eV) and oxygen (533.6 eV) (Fig. 4a). In the silica-coated cotton fiber, the relative atomic concentration of carbon decreased from 74.58% to 69.37%. Two peaks with binding energies of 154.54 eV (Si 2s) and 102.78 eV (Si 2p) appeared, which strongly confirmed the presence of silica particles. Figure 4b presents the Si 2p region of silica-modified cotton fiber compared with raw cotton. A single characteristic peak at 103.5 eV was assigned to silica on the modified cotton, which indicated the presence of silica.
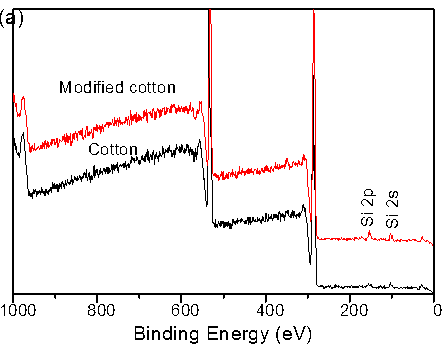
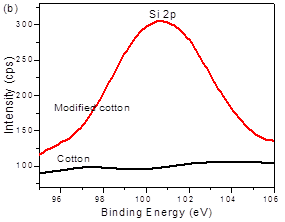
Fig. 4. XPS full-scan spectra of raw cotton and silica modified cotton fiber (a) and high-resolution XPS spectra of Si 2p of raw cotton and silica modified cotton fiber (b)
Effect of the Molecular Weight of PVP
The molecular weight of the PVP played an important role in the stability of the particles during the hydrolysis process, since it made the affinity of raw cotton to silica nanoparticles sufficiently high. So PVP with a different average molecular weight of 10 kg/mol (PVP-10), of 58 kg/mol (PVP-58), and of 130 kg/mol (PVP-130) were used as coupling agent to coat silica on the surface of cotton fibers to examine how the molecular weight of PVP affects the surface silica nanoparticles hydrolyzed on cotton fiber.
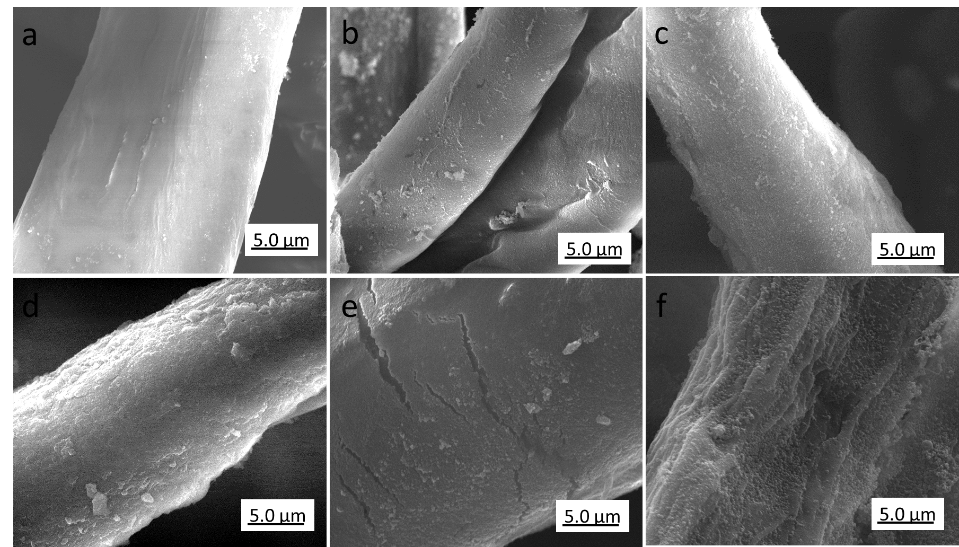
Fig. 5. Scanning electron microscopy images of silica-coated cotton modified (a) without PVP, NH3·H2O = 1 mL; or with (b) PVP-10, NH3·H2O = 1 mL; (c) PVP-58, NH3·H2O = 1 mL; (d) PVP-130, NH3·H2O = 1 mL; (e) PVP-130, NH3·H2O = 2 mL; and (f) PVP-130, NH3·H2O = 3 mL
Figure 5a shows that almost no silica nanoparticles adhered to the surface of cotton fibers when only ammonia was used without PVP, as previously noted (Liu et al. 2014). Due to the natural plant wax layer, the hydroxyl groups exposed on the surface of raw cotton fiber were not sufficient for silica nanoparticle attachment. Compared with raw cotton, the silica-modified cotton pretreated with PVP (Fig. 5b-d) exhibited relatively rough surfaces. When the cotton fiber was pretreated with PVP-10 (Fig. 5b), a thin layer of silica nanoparticles were uniformly adhered to the surface, which was significantly roughened.
With higher molecular weight PVP, the surface roughness became more pronounced, and small humps with a broad size distribution were present (Fig. 5c). For the cotton fiber stabilized with the longer PVP chains (Fig. 5d), the surface had a much more heterogeneous coating thickness and a multilayer silica shell. Thus, the molecular weight of PVP had a noticeable impact on the surface roughness of cotton fibers. The short molecular chains of PVP-10 did not sufficiently stabilize the silica particles during the hydrolysis of the TEOS due to the lack of sufficient van der Waals forces between cotton fiber and silica nanoparticles. With increased chain length of PVP, the SiO2 nanoparticles were easily adsorbed on the surface to create rough micro/nanostructures.
Thermogravimetric analysis was used to quantitate the amount of silica nanoparticles deposited on the cotton surface (Fig. 6). The residue of raw cotton was 11.45 wt.%, due to the inorganic ash content present (Fig. 6a). The residue content slightly increased to 14.12 wt.% after silica hydrolysis (Fig. 6b).
In the silica modified cotton pretreated with PVP-10 (Fig. 6c), the residue content increased to 25.15 wt.%, which illustrated the incorporation of silica nanoparticles. As shown in Figs. 6d and e, with increasing molecular weight of PVP employed from 58 kg/mol to 130 kg/mol, the final residue weight ratio of the cotton increased from 32.38 wt.% to 34.49 wt.%, demonstrating that more silica was incorporated into cotton fiber. The PVP with a molecular weight of 130 kg/mol was the optimum treatment of cotton fiber because it provided sufficient van der Waals forces between cotton fiber and silica nanoparticles to construct hierarchical, rough micro/nanostructures on the surface.
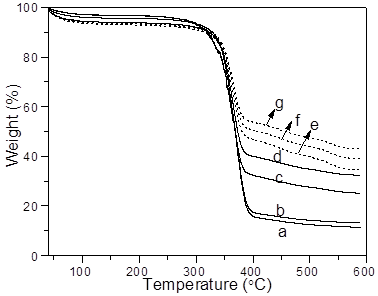
Fig. 6. TGA curves of raw cotton (a) and silica coated cotton modified (b) without pretreated PVP, NH3·H2O = 1 mL; or with (c) PVP-10, NH3·H2O = 1 mL; (d) PVP-58, NH3·H2O = 1 mL; (e) PVP-130, NH3·H2O = 1 mL; (f) PVP-130, NH3·H2O = 2 mL; and (g) PVP-130, NH3·H2O = 3 mL
Effect of the Concentrations of Ammonia
Basically, the surface morphologies and the surface roughness of SiO2-modified cotton fibers could be well controlled by varying the reaction conditions, such as the temperature, medium, and time. In this study, the influence of the amount of ammonia on the surface chemistry and morphology of the silica-modified cotton was studied.
The SEM images of silica nanoparticles prepared at different concentrations of ammonia were shown in Figs. 4d to f. As shown in Fig. 4d, though the surface of as-coated cotton was heterogeneous and multilayered, the silica particles were still at the nanoscale. When 2 mL of ammonia was added, the roughness was more pronounced because of the growth of the nodules. With increasing amounts of ammonia (3 mL), small silica particles with diameters of approximately 200 nm were present on the cotton fiber surface (Fig. 4f). Thus, the incremental addition of ammonia not only increased the size of the silica particles, but also roughened the cotton surface structure, forming a stable solid/air composite surface and a non-wetting Cassie-Baxter surface.
Thermogravimetric analysis was also used to quantitatively determine the effects of the amounts of ammonia on the silica nanoparticles deposited onto cotton surface. As shown in Figs. 5e to g, with increasing ammonia from 1.0 mL, 2.0 mL, to 3.0 mL, the final residue weight ratio of the cotton was 34.49 wt.%, 39.07 wt.%, and 42.35 wt.%, respectively. This result demonstrated that more silica was incorporated into the cotton fiber. When the amount of ammonia further increased, the silica size became large, which was not suitable for the PVP to stabilize the particles on the cotton surface.
Construction of Superhydrophobic Cotton for Oil/Water Separation
Superhydrophobic silica-modified cotton fiber was made for oil/water separation. Although silica-modified cotton was hydrophilic due to the hydroxyl groups of SiO2 shell with a water contact angle of nearly 0° (Fig. 7a), it was easily functionalized by HDTMS to achieve excellent superhydrophobicity with a water contact angle of over 160° (Fig. 7b).
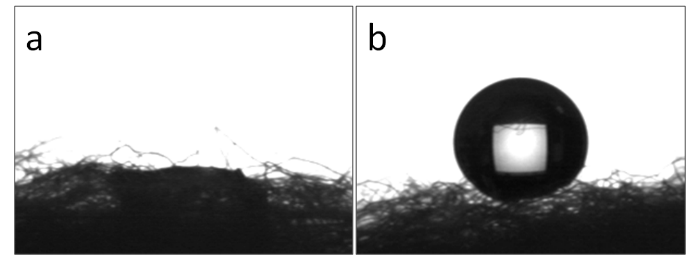
Fig. 7. Shapes of water droplets (5 µL) on (a) raw cotton fibers and (b) superhydrophobic silica coated cotton fibers (prepared with PVP-130, 2 mL NH3·H2O)
To reflect the hydrophobic and oleophilic effect of the prepared cotton, the surface wettability of the prepared cotton was investigated, as shown in Fig. 8a. The blue-colored water droplet beaded up on the prepared cotton, while the red-colored oil droplet submerged completely, suggesting excellent superhydrophobic and superolephilic property. Moreover, the prepared cotton appeared as silver mirror like surfaces when it was immersed into water by an external force (Fig. 8b). This non-wetting Cassie-Baxter behavior was due to a continuous air layer between the cotton surface and water.
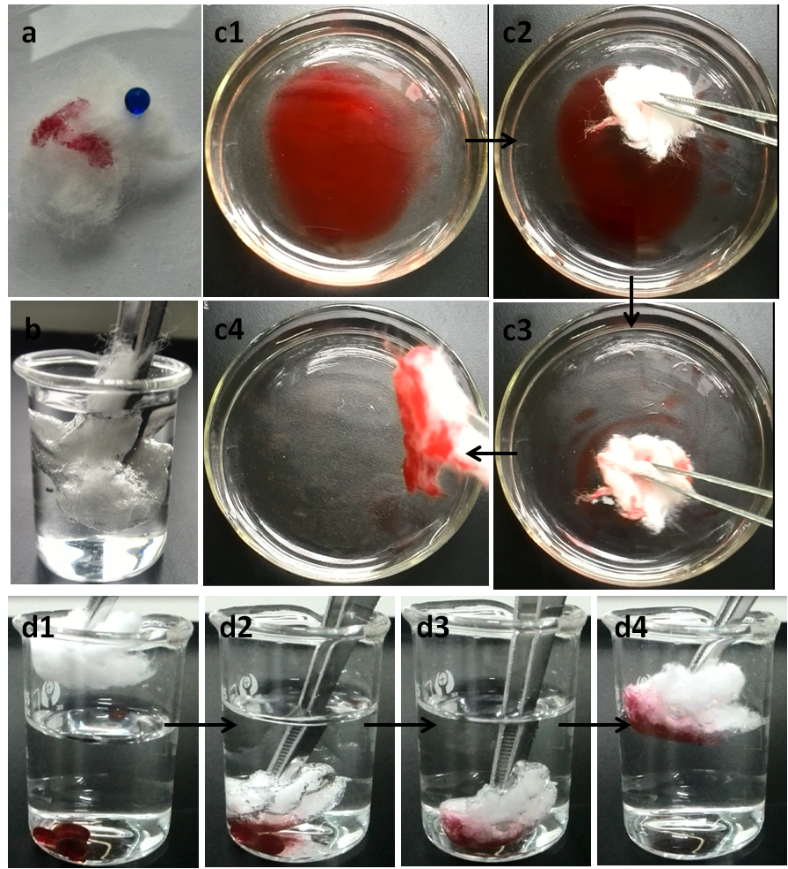
Fig. 8. Optical pictures demonstrating the wettability of the cotton samples: (a) photograph of a water droplet (dyed with methylene blue) on top and chloroform droplets (dyed with red oil) wetting completely; (b) superhydrophobic cotton immersed into water by an external force; (c) sequential snapshots of removing a layer of red oil dyed n-hexane on top of water and (d) sequential snapshots of removing red oil dyed chloroform at the bottom of water
The prepared cotton fibers showed excellent superhydrophobicity and superolephilicity, which made it very promising as a material for removal of oil from water. As illustrated in Fig. 8c, the silane-modified cotton fibers rapidly absorbed red oil dyed n-hexane spread on water, completely removing it within several seconds (Movie S1) and leaving a transparent region on the water surface. The prepared cotton also showed excellent absorption selectivity toward organic solvents with higher densities than water by selectively absorbing the red oil dyed chloroform from the bottom of water (Fig. 8d, Movie S2). The rapid and complete absorption of organic solvents from water demonstrated the excellent oil/water separation capability of silica-modified cotton fibers.
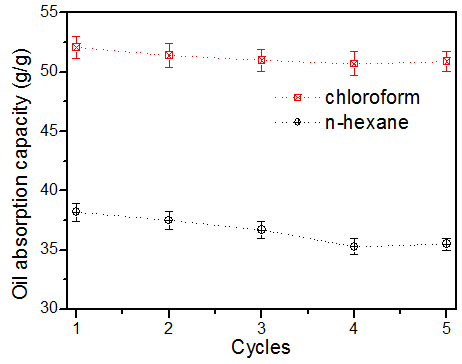
Fig. 9. Reusability of superhydrophobic/superoleophilic cotton in chloroform and n-hexane
As important criteria for practical applications, the oil-absorption capacities and recyclability of the obtained superhydrophobic cotton fibers were also evaluated. To investigate the maximum oil absorption capacities of the prepared cotton, the absorption experiment was carried out in n-hexane and chloroform. As displayed in Fig. 9, the oil absorption capacity of prepared cotton for n-hexane and chloroform were 38.2 and 52.1 g/g, respectively. The oil-loaded cotton was squeezed to collect the absorbed oil, and the cotton was oven-dried at 70 °C for 30 min before being reused for next experiment. As shown in Fig. 9, there was a slight decrease of the maximal oil absorption capacity after 5 separation cycles, and the maximal oil absorption capacity for n-hexane and chloroform were maintained above 35 and 50 g/g, respectively. The decreased oil absorption capacity was caused by the residual oils in the cotton fiber pores. The results demonstrated that the prepared cotton fiber performed excellent oil/water separation with high adsorption efficiency, and it could be reused for at least 5 cycles.
CONCLUSIONS
- A convenient and environmentally friendly approach to prepare superhydrophobic/ superoleophilic cotton for oil/water separation was demonstrated. The cotton was fabricated via in-situ hydrolysis of tetraethoxysilane (TEOS) using polyvinylpyrrolidone (PVP) as a coupling agent and subsequent hexadecyl-trimethoxysilane modification.
- The surface morphology of the silica-modified cotton could be controlled by adjusting the molecular weight of PVP or adjusting the NH3H2O concentration. The procedure was carried out in a mild environment, with no intricate instruments or toxic reagents.
- The superhydrophobic fiber exhibited excellent oil absorption capacity and recyclability for at least 5 times. The results suggest that these cotton fibers are promising sustainable and highly effective materials for large-scale oil spill cleanups.
ACKNOWLEDGMENTS
This work was supported by the Guangdong Provincial Science and Technology Project (No. 2013B010401007), Guangzhou Science and Technology Project, the State Key Laboratory of Pulp and Paper Engineering of South China University of Technology (D7150660), the independent research project of Pulp and Paper Engineering State Key Laboratory, South China University of Technology (No. 2016C01, No. 2016C02), the Fundamental Research Funds for the Central Universities” (No. D2154620), and the Development Fund of Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China, Qilu University of Technology (No. KF201502).
REFERENCES CITED
Bi, H., Yin, Z., Cao, X., Xie, X., Tan, C., Huang, X., Chen, B., Chen, F., Yang, Q., Bu, X., Lu, X., Sun, L., and Zhang, H. (2013). “Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents,” Adv. Mater. 25(41), 5916-5921. DOI: 10.1002/adma.201302435
Cao, C., Ge, M., Huang, J., Li, S., Deng, S., Zhang, S., Chen, Z., Zhang, K., and Al-Deyab, S. S. (2016). “Robust fluorine-free superhydrophobic PDMS-ormosil@fabrics for highly effective self-cleaning and efficient oil-water separation,” J. Mater. Chem. A 4(31), 12179-12187. DOI: 10.1039/C6TA04420D
Chang, H., Tu, K., Wang, X., and Liu, J. (2015). “Facile preparation of stable superhydrophobic coatings on wood surfaces using silica-polymer nanocomposites,” BioResources 10(2), 2585-2596. DOI: 10.15376/biores.10.2.2585-2596
Feng, L., Zhang, Z., Mai, Z., Ma, Y., Liu, B., Jiang, L., and Zhu, D. (2004). “A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water,” Angew. Chem. Int. Edit. 43(15), 2012-2014. DOI: 10.1002/ange.200353381
Graf, C., Vossen, D. L. J., Imhof, A., and Blaaderen, A. V. (2003). “A general method to coat colloidal particles with silica,” Langmuir 19(17), 6693-6700. DOI: 10.1021/la0347859
Ichikawa, T., and Nakajima, Y. (2004). “Rapid demulsification of dense oil-in-water emulsion by low external electric field,” Colloids Surf. A 242(1-3), 27-37. DOI: 10.1016/j.colsurfa.2004.04.042
Jia, L., Tong, L., Liang, Y., Petretic, A., Guerin, G., Manners, I., and Winnik, M. A. (2014). “Templated fabrication of fiber-basket polymersomes via crystallization-driven block copolymer self-assembly,” J. Am. Chem. Soc. 136, 16676-16682. DOI: org/10.1021/ja510019s
Kwon, G., Kota, A. K., Li, Y., Sohani, A., Mabry, J. M., and Tuteja, A. (2012). “On-demand separation of oil-water mixtures,” Adv. Mater. 24(27), 3666-3671. DOI: 10.1002/adma.201201364
Li, L., Liu, L., Lei, J., He, J., Li, N., and Pan, F. (2016). “Intelligent sponge with reversibly tunable super-wettability: Robust for effective oil-water separation as both the absorber and filter tolerate fouling and harsh environments,” J. Mater. Chem. A 4(31), 12334-12340. DOI: 10.1039/C6TA03581G
Liu, F., Ma, M., Zang, D., Gao, Z., and Wang, C. (2014). “Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation,” Carbohyd. Polym. 103, 480-487. DOI: 10.1016/j.carbpol.2013.12.022
Lu, X., and Hu, Y. (2016). “Layer-by-layer deposition of TiO2 nanoparticles in the wood surface and its superhydrophobic performance,” BioResources 11(2), 4605-4620. DOI: 10.15376/biores.112.4605-4602
Tirmizi, N. P., Raghuraman, B., and Wiencek, J. (1996). “Demulsification of water/oil/solid emulsions by hollow-fiber membranes,” AICHE 42(5), 1263-1275. DOI: 10.1002/aic.690420508
Wang, J., Geng, G., Wang, A., Liu, X., Du, J., Zou, Z., Zhang, S., and Han, F. (2015a). “Double biomimetic fabrication of robustly superhydrophobic cotton fiber and its application in oil spill cleanup,” Ind. Crop. Prod. 77, 36-43. DOI: 10.1016/j.indcrop.2015.08.044
Wang, B., Liang, W., Guo, Z., and Liu, W. (2015b). “Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature,” Chem. Soc. Rev. 44, 336-361. DOI: 10.1039/c4cs00220b
Wang, B., Li, J., Wang, G., Liang, W., Zhang, Y., Shi, L., Guo, Z., and Liu, W. (2013). “Methodology for robust superhydrophobic fabrics and sponges from in situ growth of transition metal/metal oxide nanocrystals with thiol modification and their applications in oil/water separation,” ACS Appl. Mater. Inter. 5(5), 1827-1839. DOI: 10.1021/am303176a
Xu, M., Bao, W., Xu, S., Wang, X., and Sun, R. (2016). “Porous cellulose aerogels with high mechanical performance and their absorption behaviors,” BioResources 11(1), 8-20. DOI: 10.15376/biores.11.1.8-20
Xue, Z., Cao, Y., Liu, N., Feng, L., and Jiang, L. (2014). “Special wettable materials for oil/water separation,” J. Mater. Chem. A 2, 2445-2460. DOI: 10.1039/c3ta13397d
Zang, D., Wu, C., Zhu, R., Zhang, W., Yu, X., and Zhang, Y. (2013). “Porous copper surfaces with improved superhydrophobicity under oil and their application in oil separation and capture from water,” Chem. Commun. 49(75), 8410-8412. DOI: 10.1039/C3CC43536A
Zhou, X., Zhang, Z., Xu, X., Guo, F., Zhu, X., Men, X., and Ge, B. (2013). “Robust and durable superhydrophobic cotton fabrics for oil/water separation,” ACS Appl. Mater. Inter. 5(15), 7208-7214. DOI: 10.1021/am4015346
Article submitted: October 4, 2016; Peer review completed: November 9, 2016; Revised version received: November 16, 2016; Accepted: November 18, 2016; Published: November 29, 2016.
DOI: 10.15376/biores.12.1.643-654
