Abstract
Download PDF
Full Article
Flame Retardant Efficiency of Melamine Pyrophosphate with Added Mg-Al-Layered Double Hydroxide in Medium Density Fiberboards
Shanqing Liang,a,b,* Lifang Zhang,b Zhilin Chen,b and Feng Fu b
The main objective of this study was to investigate the flame retardant (FR) efficiency of melamine pyrophosphate (MP) with added layered double hydroxide (LDH) in medium density fiberboards (MDFs) to be used as building decorative materials. The thermal degradation, FR efficiency, and combustion properties of MDF were analyzed by Fourier transform infrared spectra (FTIR), thermogravimetric analysis (TGA), limiting oxygen index (LOI) test, and cone calorimetric test. The TGA results showed that the combination of MP and LDH led to a lower decomposition temperature than the pure MP and pure LDH. The LOI results showed that the LOI values increased with the addition of the FRs. The cone calorimetric results showed that the addition of four proportions (1:1, 2:1, 1:2, and 1:3) of the compounds into MDF can clearly decrease the peak heat release rate (PHRR) and mean heat release rate (MHRR). The results indicated that the MP and LDH compounds have a synergistic effect to improve the flame retardancy and smoke suppression in the MDFs. The combination of MP and LDH in a 1:1 ratio under 10 wt% FR addition has the best flame retardant efficiency in the MDFs.
Keywords: Flame retardant; Layered double hydroxide; Melamine pyrophosphate; Medium density fiberboard
Contact information: a: Research Institute of Forestry New Technology, Chinese Academy of Forestry, Beijing 100091, China; b: Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100091, China; *Corresponding author: liangsq@caf.ac.cn
INTRODUCTION
The use of environmentally friendly flame retardant (FR) composites for building decorative materials has attracted considerable attention because of rising environmental concerns. Global restrictions on the use of polybrominated diphenyl ethers in engineering plastic, electrical products, electric wire, etc. have resulted in the increased use of alternate FR chemicals (Leroux and Besse 2001; Van den Eede et al. 2011; Wang et al. 2011). The FR chemicals are usually compounds containing nitrogen, phosphorus, and boron elements (Seefeldt and Braun 2012; Bai et al. 2014; Tondi et al. 2014). These FRs can drastically reduce the flammability of composites, but they can exhibit disadvantages because of their high smoke production and lack of environmentally friendly performance.
Metal hydroxides, especially magnesium hydroxide and alumina trihydrate, have been widely employed in FR systems to suppress smoke during combustion. Other metal hydroxides that have shown a positive influence on the flame retardation of polymers are layered double hydroxides (LDHs), such as hydrotalcite-like compounds or anionic clays, which have a positive charge on brucite-like sheets compensated by interlayer anions. An LDH compound possesses a similar structure and composition as magnesium hydroxide and alumina trihydrate, which makes it promising for applications such as thermal stabilizers and flame retardants (Ye et al. 2008; Wang et al. 2009; Chen and Wang 2009).
At present, LDHs are mostly used as polymer FRs and have been used as single compounds or in combination with various fire retardant compounds. The combined effect of LDHs and other FRs (e.g., intumescent flame retardant (IFR) carbon nanotubes, and graphite) in fire retarding performance has already been reported to be excellent, and such a combination can also effectively suppress smoke and gases during the combustion process (Wang et al. 2005). The addition of LDH and ammonium polyphosphate (APP) at a weight percent of no more than 10% results in a substantial reduction in the peak heat release rate (PHRR) in FR polystyrene. Similar reductions were also observed for the average mass loss rate, suggesting that the reductions in PHRR were due to the slower rate at which combustible volatiles were produced (Nyambo et al. 2008). Furthermore, the quantity of nanoscale LDH or LDH/IFR (APP) added to composites is less than that for traditional inorganic materials; thus, the effects of the addition on the mechanical and physical properties of the materials are minor (Zhao et al. 2008; Huang et al. 2012).
It is well known that one of the main drawbacks of using wood for industrial and institutional applications is its flammability, as it is an organic material. Thus, it is becoming increasingly important to improve the flame retardancy of composite materials to comply with the safety requirements associated with the use of wood materials. Various additive-type FRs, such as ammonium polyphosphate (APP), melamine phosphate (MP), melamine polyphosphate (MPP), and aluminum hydroxide, have been added to composites for this purpose (Liodakis et al. 2013; Arao et al. 2014). Among such FRs, MP is important for wood cellulose and is an essential component of phosphorus–nitrogen FRs, which can considerably enhance the flame retardancy of wood materials (Khalil et al. 2013). However, MP also has the drawback of a lack of effective suppression of smoke during the burning process. Therefore, the effects of phosphorus–nitrogen FRs are further improved by combining them with inorganic retardants, but more research is needed on the selection of optimal combination formulations.
In this study, flame-retarding interactions between MP and LDH were investigated in prepared FR medium density fiberboards (FR-MDFs) by incorporating MP and LDH. The thermal property was investigated by thermogravimetric analysis (TGA), and the structure was characterized after calcination by Fourier transform infrared spectra (FTIR). The flammability of the FR-MDFs was investigated by the limiting oxygen index (LOI) and cone calorimetric tests. The residual char after combustion was also examined by scanning electronic microscopy (SEM). Thus, the combination of LDH and MP to improve the flame retardancy of FR-MDFs was studied.
EXPERIMENTAL
Materials
Melamine pyrophosphate (C3H9N6PO4) was purchased from Anqing Yuetong Molybdenum Co., Ltd. (Anhui province, China). Magnesium aluminum-layered double hydroxide (MgAlLDH) carbonate with the formula Al2Mg6·CO3·16(OH)·4(H2O) was provided by Shaoyang Heaven Assistant Chemical Industry Co., Ltd. (Hunan, China). MgO (33.1%) and Al2O3 (20.5%) were used with a MgO/Al2O3 molar ratio of 2.6 and an average diameter of 260 nm, as determined by testing with a Malvern Zetasizer Nano ZS90 (Malvern Instruments Ltd., UK). Melamine-urea-formaldehyde resin adhesive (MUF, 52 wt%) and poplar fibers (moisture content 10% to 12%) were obtained from Dongying Zhenghe Wood Industry Co., Ltd (Shandong, China).
Preparation of FR-MDFs
Wood fibers were first dried in an oven to maintain the moisture content at approximately 8%. The MUF loading was done at 16 wt%, as calculated from the dry wood fiber weight. Firstly, the fiber and MUF were blended by blender, then added the FRs further blend, finally using dry mechanical collector to blend the materials for three times. The blend of wood fibers, FRs, and MUF was pressed at 180 °C under a pressure of 17 MPa for 5 min. The density of FR-MDF samples was configured at 0.75 g/cm3, and the FR-MDF had dimensions of 360 mm (length) × 340 mm (width) ×9 mm (thickness). The designation and composition of the FR-MDF samples are listed in Table 1.
Table 1. Designation and Composition of FR-MDF Samples
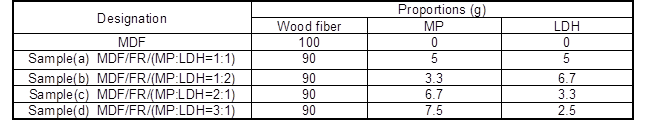
Characterization of Flame Retardant Efficiency
Fourier transform infrared spectra (FTIR)
Pure MP, LDH, and a mixture of LDH and MP (1:1) were heated at 600 °C using a Box Type Resistance Furnace (SX-5-12, Tianjin Taisite Instrument Co., Ltd, China). Then, all powder samples were tested by Fourier transform infrared spectroscopy (FTIR) using a Bruker Vertex 80V spectrometer (Bruker, Germany) over the wavenumber range of 4000 to 400 cm-1. The powdered samples were mixed with KBr and pressed in the form of pellets for FTIR analysis.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) was conducted on a TGA/DSC simultaneous thermal analyzer (Netzsch Sta 449F3, Germany) with MP, LDH, and MP-LDH compounds at various ratios (MP:LDH = 1:1, 1:2, 2:1, and 3:1). A sample of approximately 20 mg was placed in a silica pan to evaluate its thermal stability. The sample was then heated at a rate of 10 °C/min from ambient temperature to 800 °C.
Characterization of FR-MDF Compounds
Limiting oxygen index
Limiting oxygen index (LOI) was measured according to the procedure from GB/T 2406.1(2008). The apparatus used was an HC-2 oxygen index meter (Jiangning Analysis Instrument Company, China). The specimens used for the test had dimensions of 150 mm × 10 mm × 4 mm. The LOI values obtained from this test were the average of 15 tests for each sample.
Cone calorimeter
The cone calorimetric experiment was carried out by FTT Limited of the United Kingdom as per ISO 5660-1(2002) standard procedures. Each specimen with dimensions of 100 mm × 100 mm × 3 mm was wrapped in aluminum foil and then exposed horizontally to an external heat flux of 50 kW/m2. Several results were obtained from the cone calorimetric test, such as time to ignite (TTI), peak heat release rate (PHRR), mean heat release rate (MHRR), total heat release (THR), smoke production rate (SPR), total smoke production (TSP), CO yield, and CO2 yield.
Scanning electron microscopy
An S4800 scanning electron microscope (Hitachi, Japan) was used to observe the morphologies of the char layer after the cone calorimetric testing.
RESULTS AND DISCUSSION
FTIR of MP, LDH, and MP-LDH Compounds
The infrared spectra of MP, LDH, and their compounds are shown in Fig. 1. For MP, the absorptions at 3182 and 1398 cm-1 were assigned to the stretching vibration and deformation vibration of NH4+ ion. The peak at 1249 cm-1 was assigned to the stretching vibration of P=O group. The peaks found at 1068 and 887 cm-1 were due to the symmetric and asymmetric stretching vibrations of P-O-P groups (Yang et al. 2014). For LDH, the absorption peaks at 3429 cm-1 can be assigned to H2O stretching vibration. In addition, the peak at 1631 cm-1 should be from the deformation vibration of OH— ion. The peaks at 1400 and 852 cm-1 represented the symmetric stretching vibration and stretching vibration of CO3-2 ion.
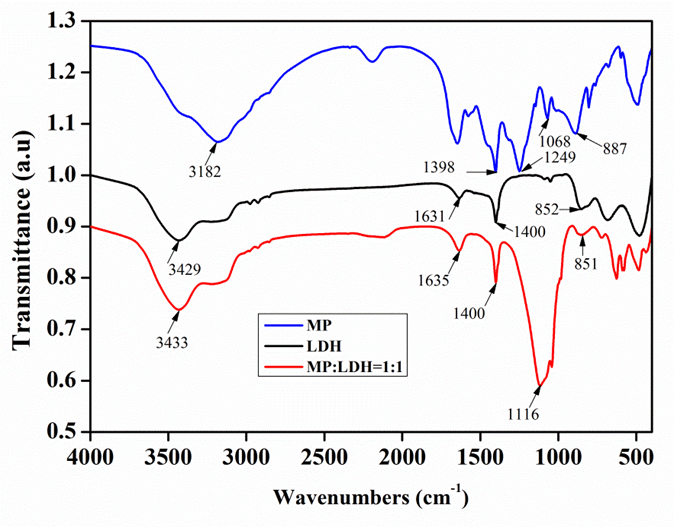
Fig. 1. FTIR spectra of pure MP, pure LDH, and compounds of MP and LDH (MP: LDH = 1:1)
The FTIR spectrum for the combined system prepared from MP and LDH was similar with that of LDH except for the differences of peaks at 1116 cm-1; the appearance of this new broad peak was attributed to the P-O-Mg or P-O-Al bonds. The FTIR results indicated some new characteristic absorptions of Mg-O and Al-O groups, and this suggested that Mg-O and Al-O bonds were formed because of the chemical reactions that lead to an obvious increase in the peaks of P-O-Mg/Al. The existence of P-O-Mg/Al bonds suggested the presence of flame-retardant efficiency in MP and LDH combinations.
Thermal Degradation of MP, LDH, and MP-LDH Compounds
Flame retardancy can be attributed to the carbonization occurring during the thermal degradation process, which will eventually exist in the form of carbon residue. The temperature of the thermal decomposition and the amount of carbon residues formed confirms the flame retardant effect and is determined by the application range and the conditions of the composite processing technology. The thermal degradation behaviors of MP, LDH, and the compounds with various proportions of MP and LDH were investigated by TG-DSC in nitrogen and air atmosphere, respectively. The results of the thermal analysis are shown in Figs. 2 and 3. For MP, the first step of dehydration process lasts up to 335 °C. The maximum fast decomposition temperature was at 413 °C, and a residue of about 36% of the original mass was formed at 800 °C. For LDH, the weight loss occurred up to 250 °C was attributed to the loss of adsorbed and interlayer water, and then, obvious thermal decomposition was observed after 400 °C, which involved dihydroxylation of the brucite-like layers followed by decomposition of the organic anions. However, compared with the thermal decomposition temperature obtained from the pure MP and LDH, the MP-LDH compounds with various proportions of MP and LDH showed two decomposition temperatures at low temperatures except for the proportion MP:LDH = 3:1. The lower decomposition temperature for the MP and LDH compounds suggested a faster decomposition of effective products that suppress the combustion of MDF.
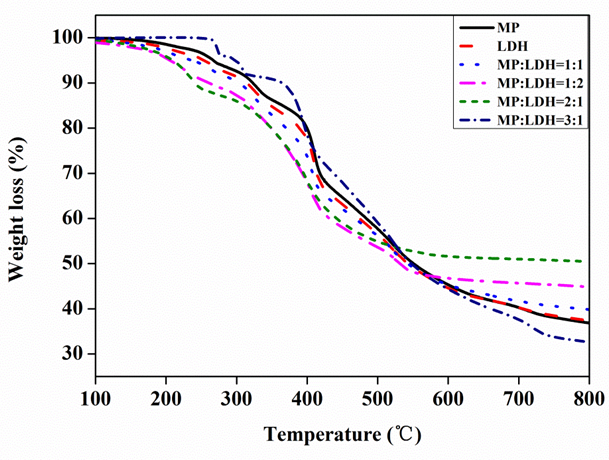
Fig. 2. TGA curves for pure MP, pure LDH, and compounds of MP and LDH (MP:LDH = 1:1; 1:2; 2:1; and 3:1)
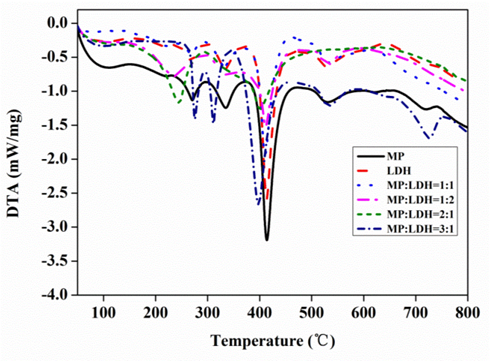
Fig. 3. DTA curves for pure MP, pure LDH, and compounds of MP and LDH (MP:LDH = 1:1; 1:2; 2:1; 3:1)
Limited Oxygen Index of MDF
The limited oxygen index (LOI) represents the minimum concentration of oxygen in a mixture of oxygen and nitrogen that will support the flaming combustion of a material (Xu et al. 2012). The LOI values of various samples with and without MP and LDH compounds are shown in Fig. 4.
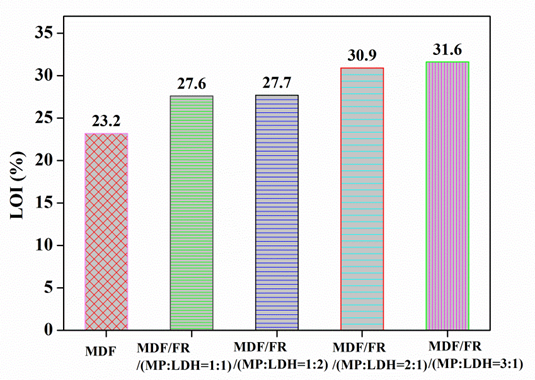
Fig. 4. The limited oxygen index (LOI) results for FR-MDF composites
The addition of MP and LDH compounds in the FR-MDFs apparently increased the LOI values compared with the control MDF sample. The LOI value of pure MDF is 23.2%, suggesting that MDF is easy to ignite as a wood material (Shunk 1977). After the addition of the FRs, the FR-MDF composites self-extinguished and formed a layer of char during the LOI tests. These phenomena strongly suggest that MP and LDH compounds have an effective FR effect on the MDFs. The LOI value increased to 27.6%, 27.7%, 30.9%, and 31.6% with the addition of 10 wt% of the FRs (MP:LDH = 1:1; 1:2; 2:1; and 3:1) to the MDFs. However, it had almost no effect on the LOI of the FR-MDF with a further small addition of LDH. Similar results reported that small amount of LDH addition does not obviously improve the LOI value (Costa et al. 2007).
Cone Calorimetric Experiments of MDF
The cone calorimetric test is the most effective method for laboratory evaluation of the fire behavior of composites. The results of this test are compiled in Table 2, and the HRR, THR, SPR, TSP, CO yield, and CO2 yield curves are shown in Fig. 5. It can be found from Table 2 that MDF has a shorter ignition time than FR-MDF. With the addition of the MP and LDH compounds, the ignition times of the four FR-MDF composites exhibited a slight increase. The ignition times of samples (a), (b), (c), and (d) are 29.5 s, 27.5 s, 29.0 s, and 29.5 s, respectively. The increase in the ignition time of the FR-MDFs was due to the early decomposition of LDH, which absorbs heat and partly decomposes into water vapor and CO2. However, the early degradation of MP resulted in intermolecular dehydration reaction that partly releases melamine pyrophosphate and phosphorus acid (Camino et al.1991; Ni et al. 2010). Moreover, the synergistic effect of the MP and LDH compounds leads to effective flame retardancy in the FR-MDFs.
Table 2. Cone Calorimetric Analysis Data for FR-MDFs
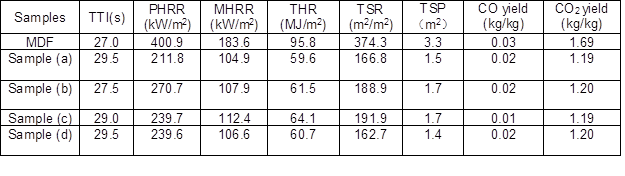
Figure 5(a) presents the thermodynamic curves of HRR for the MDF and FR-MDFs, which displayed two peaks. The first PHRR value (60 s, 360 kW/m2) of the MDF exemplified a wood material that was sensitive to flames when the surface was ignited, and the generated inflammable gas led to the release of combustion heat. The second PHRR was observed at 310 s for 400.9 kW/m2. This was primarily because of the higher heat released during the flameless combustion of wood pyrolysis carbon residue at high temperatures. However, the PHRR values were decreased with the addition of 10 wt% of the MP and LDH compounds. This addition affected the combustion of MDF, and the PHRR values were decreased by 47.2%, 32.5%, 40.2%, and 40.2%, respectively. The decrease in PHRR was higher for sample (a) than that for samples (b), (c), and (d). In addition, the MHRR curves of the FR-MDFs also exhibited a substantial decrease compared with those of MDF; the MHRR of MDF was 183.6 kW/m2, whereas the MHRR of the FR-MDFs with addition of the MP and LDH compounds in proportions of 1:1, 2:1, 1:2, and 3:1 were 104.9, 107.9, 112.4, and 106.6 kW/m2, respectively. This indicated that the flame retardancy effect was greater with the addition of the MP and LDH compounds with a proportion of 1:1 compared to the other proportions. Meanwhile, it can be seen from Table 2 and Fig. 5(b) that the average THR of sample (a), which was 37.8% lower than that of MDF, also demonstrated that MP and LDH addition with a proportion of 1:1 had a better flaming combustion. The results showed that PHRR, MHRR, and THR were decreased after adding MP and LDH compounds. Similar results have been obtained using MP and LDH handle to rosin-based rigid polyurethane foam (Gao et al. 2013).
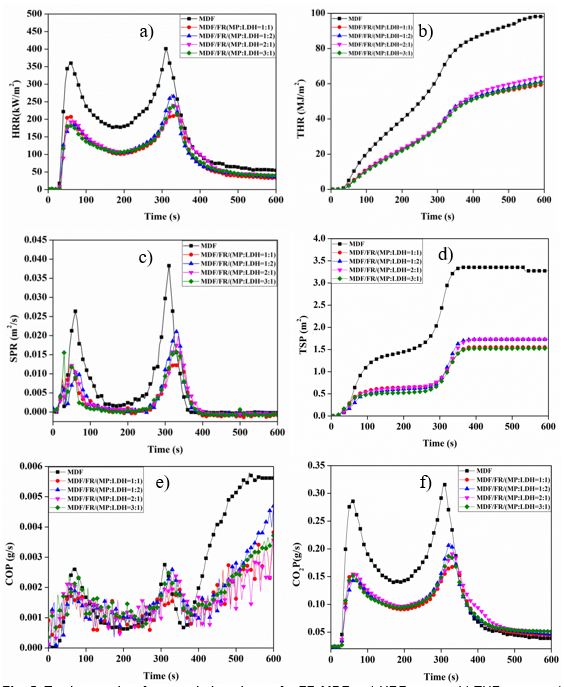
Fig. 5. Testing results of cone calorimetric test for FR-MDFs: a) HRR curves, b) THR curves, c) SPR curves, d) TSP curves, e) COP curves, and f) CO2P curves
Evaluation of fire performances of the FR-MDFs involved quantifying the smoke generated using the total smoke release (TSR), total smoke production (TSP), and the rate of smoke produced (SPR). From Table 2 and Figs. 5(c-f), the smoke released from FR-MDF composite was clearly lower than that of the MDF. The TSR values also decreased to 166.8, 188.9, 191.9, and 162.7, with the addition of 1:1, 2:1, 1:2, and 3:1 of the MP and LDH compounds, respectively. This result was very important for increasing the fleeing time and reducing the hazard in actual fires. Figure 5(e) shows the CO yield curve; a sharp increase from 360 s to 600 s was observed for MDF, and this phenomenon was not observed after the addition of the MP and LDH compounds. This was possibly because the FRs can effectively suppress the smoke production during MDF burning. The peculiar layer structure of LDH endow them with larger surface area and more surface adsorption activity center. During the thermal decomposition of LDH, the thermolysis of LDH produced solid alkali (aluminium oxide, magnesium oxide), which has highly dispersed and large specific surface area. The solid alkali has good adsorption acidic gases from the burning of FR-MDFs which would decrease the CO yield in flameless combustion. However, a slight change in the proportion of MP and LDH did not obviously increase or decrease the TSP, CO yield, and CO2 yield. This can be explained by the fact that the phosphorus and amino groups present in MP were converted into smoke more easily during burning, and LDH released CO2, thus partially increasing the CO2 yield. From the cone calorimetric tests, one synergistic effect of the addition of MP and LDH compounds was the decomposition of more charcoal and water during combustion because of MP, which has the function of low temperature catalytic conversion into charcoal for cellulose materials. The LDH decomposition also released H2O and CO2, absorbed a lot of heat, and was effectively isolated from oxygen, thus reducing the material surface temperature. Furthermore, the LDH formed a condensed phase in the MDF surface, generating MgO and Al2O3 because of the strong absorption of acidic gases from the combustion oxidation.
Morphological Observation of Char Layer
To illustrate the effect of char formation on the combustion behavior of the FR-MDFs, the morphology and structure of the chars after cone calorimetric testing were investigated using a digital camera and SEM. The micrographs of the inner and outer surfaces of the char structure are shown in Figs. 6 and 7, respectively. Figure 6 shows digital photographs of the residual chars of FR-MDF. For sample (a) (Fig. 6a), a layer of white powder covered with chars was observed after the cone calorimetric tests, and the char had a certain degree of hardness. For sample (b), the char was crushed owing to more LDH addition, which formed loose and separated particles. This was because of the stacked aluminum-magnesium oxide sheet composition of the decomposed substance from LDH (Huang et al. 2014). With an increase in MP addition, a more rigid and hard char was obtained because the MP exhibited an even stronger catalytic conversion into charcoal (Figs. 6c and 6d).
Figure 7 shows the SEM images of the chars for the FR-MDFs after combustion. Figure 7a showed that some magnesium-aluminum oxides and inorganic polymer (PON)x from the LDH and MP thermal decomposition were dispersed on the surface of the char. In contrast, more metallic oxides were generated with more LDH addition and it was observed clearly on surface of sample (b) (Fig. 7b). However, the increased amount of LDH (From MP:LDH = 1:1 to MP:LDH = 1:2) could not improve the hardness of the char layer because of more metallic oxides generation compared with Fig. 7a.
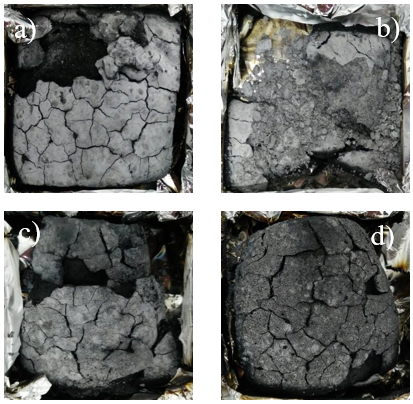
Fig. 6. Photographs of char formed after cone calorimetric tests: (a) MDF/FR/(MP:LDH=1:1);(b) MDF/FR/(MP:LDH=1:2); (c) MDF/FR/(MP:LDH=2:1) ; and (d) MDF/FR/(MP:LDH=3:1)

Fig. 7. SEM images of char residues after cone testing for FR-MDF composites: (a) MDF/FR/(MP:LDH=1:1); (b) MDF/FR/(MP:LDH=1:2); (c) MDF/FR/(MP:LDH=2:1); and (d) MDF/FR/(MP:LDH=3:1)
Samples shown in Figs. 7c and 7d had less magnesium-aluminum oxide than sample (a) and sample (b), possibly because most of the MP became decomposed into inorganic polymer (PON)x, carbon monoxide, ammonia gas, nitric oxide, and water during the combustion, and thus the wood material was carbonized to form harder and compact chars. The morphological characterization of the char layers further confirmed that the synergistic effect of MP and LDH compounds played a very important role, which led to the enhanced chars during the combustion of MDF. The same proportion of MP and LDH maintained in this study was useful for obtaining a more FR material.
CONCLUSIONS
- By incorporating various proportions of MP and LDH compounds as flame retardants (FRs), the compounds show lower thermal decomposition temperature when compared with pure MP and LDH.
- The addition of four proportions of MP and LDH flame retardant compounds into MDFs can clearly decrease the peak heat release rate (PHRR) and mean heat release rate (MHRR). The results indicated that the MP and LDH compounds exhibited a synergistic effect to improve the flame retardancy and smoke suppression in the MDFs.
- The combination of MP and LDH at a 1:1 ratio under 10 wt% FR addition displayed the better flame retardant base on the index of TTI, PHRR, MHRR, THR, CO2 and morphological char layer in the MDFs.
ACKNOWLEDGMENTS
The authors are grateful for financial support from the Fundamental Research Funds for the Central Non-profit Research Institution of Research Institute of Forestry New Technology, CAF (No. CAFINT2015C09).
REFERENCES CITED
Arao, Y., Nakamura, S., Tomita, Y., Takakuwa, K., Umemura, T., and Tanaka, T. (2014). “Improvement on fire retardancy of wood flour/polypropylene composites using various fire retardants,” Polym. Degrad. Stab. 100, 79-85. DOI: 10.1016/j.polymdegradstab.2013.12.022
Bai, G., Guo, C. G., and Li, L. P. (2014). “Synergistic effect of intumescent flame retardant and expandable graphite on mechanical and flame-retardant properties of wood flour-polypropylene composites,” Constr. Build. Mater. 50, 148-153. DOI: 10.1016/j.conbuildmat.2013.09.028
Camino, G., Costa, L., and Luda di Cortemiglia, M. P. (1991). “Overview of fire retardant mechanisms,” Polym. Degrad. Stab. 33(2), 131-154. DOI: 10.1016/0141-3910(91)90014-I.
Chen, L., and Wang, Y. Z. (2009). “A review on flame retardant technology in China. Part I: Development of flame retardants,” Polym. Adv. Technol. 21(1), 1-26. DOI: 10.1002/pat.155
Costa, F. R., Wagenknecht, U., and Heinrich, G. (2007). “LDPE/MgeAl layered double hydroxide nanocomposite: Thermal and flammability properties,” Polym. Degrad. Stab. 92(10), 1813-1823. DOI: 10.1016/j.polymdegradstab.2007.07.009
Gao, L. P., Zheng, G. Y., Zhou, Y. H., Hu, L. U., Feng, G. D., and Xie, Y. L. (2013). “Synergistic effect of expandable graphite, melamine polyphosphate and layered double hydroxide on improving the fire behavior of rosin-based rigid polyurethane foam,” Industrial Crops & Products 50(4), 638-647. DOI:10.1016/j.indcrop.2013.07.050
GB/T 2406.1. (2008). “Plastics – Determination of burning behaviour by oxygen index-Part 1: Guidance,” Standardization Administration of The People’s Republic of China
Huang, G. B., Fei, Z. D., Chen, X. Y., Qiu, F. L., Wang, X., and Gao, J. Y. (2012). “Functionalization of layered double hydroxides by intumescent flame retardant: Preparation, characterization, and application in ethylene vinyl acetate copolymer,” Appl. Surf. Sci. 258, 10115-10122. DOI: 10.1016/ j.apsusc.2012.06.088
Huang, G. B., Chen, S. Q., Song, P. G., Lu, P. P, Wu, C. L, and Liang, H. D. (2014). “Combination effects of graphene and layered double hydroxides on intumescent flame-retardant poly(methyl methacrylate) nanocomposites,” Appl. Clay Sci. 88-89, 78-85. DOI: 10.1016/j.clay.2013.11.002
ISO 5660-1. (2002). “Reaction-to-fire tests—Heat release, smoke production and mass loss rate—Part 1: Heat release rate (cone calorimeter method),” International Organization for Standardization, Geneva, Switzerland.
Khalil, H., Gläsel, H. J., and Buchmeiser, M. R. (2013). “High-mechanical-strength flame-retardant nanocomposites based on novel Al(III)- and Zr(IV)-melamine phosphates and sulfates,” Macromol. Mater. Eng. 298(6), 690-698. DOI: 10.1002/mame.201200168
Leroux, F., and Besse, J. P. (2001). “Polymer interleaved layered double hydroxide: A new emerging class of nanocomposites,” Chem. Mater. 13(10), 3507-3515. DOI: 10.1021/cm0110268
Liodakis, S., Tsapara, V., Agiovlasitis, I. P., and Vorisis, D. (2013). “Thermal analysis of Pinus sylvestris L. wood samples treated with anew gel–mineral mixture of short-and long-term fire retardants,” Thermochim. Acta 568, 156-160. DOI: 10.1016/j.tca.2013.06.011
Ni, X. M., Kuang, K. Q., Jin, X., Xiao, X. K., and Liao, G. X. (2010). “Large scale synthesis of porous microspheres of Mg-Al-layerd double hydroxide with improved fire suppression effectiveness,” Solid State Sci. 12(4), 546-551. DOI: 10.1016/j.solidstatesciences.2010.01.003
Nyambo, C., Kandare, E., Wang, D. Y., and Wilkie, C. A. (2008). “Flame-retarded polystyrene: Investigating chemical interactions between ammonium polyphosphate and MgAl layered double hydroxide,” Polym. Degrad. Stab. 93(9), 1656-1663. DOI: 10.1016/j.polymdegradstab.2008.05.029
Seefeldt, H., and Braun, U. (2012). “A new flame retardant for wood materials tested in wood-plastic composites,” Macromol. Mater. Eng. 297, 814-820. DOI: 10.1002/mame.201100382
Shunk, B. H. (1977). “The role of fire-retardant treated wood in the protection of life and property,” Wood Fiber Sci. 9(2), 89-95.
Tondi, G., Haurie, L., Wieland, S., Petutschnigg, A., Lacasta, A., and Monton, J. (2014) “Comparison of disodium octaborate tetrahydrate-based and tanninboron-based formulations as fire retardant for wood structures,” Fire Mater. 38(3), 381-390. DOI: 10.1002/fam.2186
Van den Eede, N., Dirtu, A. C., Neels, H., and Covaci, A. (2011). “Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust,” Environ. Int. 37(2), 454-461. DOI: 10.1016/j.envint.2010.11.010
Wang, Z. Y., Han, E. H., and Ke, W. (2005). “Influence of nano-LDH on char formation and fire-resistant properties of flame-retardant coating,” Prog. Org. Coat. 53(1), 29-37. DOI: 10.1016/j.porgcoat.2005.01.004
Wang, L. J., Su, S. P., Chen, D., and Wilkie, C. A. (2009). “Variation of anions in layered double hydroxides: Effects on dispersion and fire properties,” Polym. Degrad. Stab. 94(5), 770-781. DOI: 10.1016/j.polymdegradstab.2009.02.003
Wang, D. Y., Leuteritz, A., Kutlu, B., Landwehr, M. A. D., Jehnichen, D., Wagenknecht, U., and Heinrich, G. (2011). “Preparation and investigation of the combustion behavior of polypropylene/organomodified MgAl-LDH micro-nanocomposite,” J. Alloy Comp. 509(8), 3497-3501. DOI: 10.1016/j.jallcom. 2010.12.138
Xu, S. L., Zhang, L. X., Lin, Y. J., Li, R. S., and Zhang F. Z. (2012). “Layered double hydroxides used as flame retardant for engineering plastic acrylonitrile–butadiene–styrene (ABS),” J. Phys. Chem. Solids 73, 1514-1517. DOI: 10.1016/j.jpcs.2012.04.011
Yang, H. Y., Song, L., Tai, Q. L., Wang, X., Yu, B., Yuan, Y., Hua, Y., and Yuen, R. K. K. (2014). “Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites,” Polym. Degrad. Stab. 105, 248-256. DOI: 10.1016/j.polymdegradstab.2014.04.021.
Ye, L., Ding, P., Zhang, M., and Qu, B. J. (2008). “Synergistic effects of exfoliated LDH with some halogen-free flame retardants in LDPE/EVA/HFMH/LDH nanocomposites,” J. Appl. Polym. Sci. 107(6), 3694-3701. DOI: 10.1002/app.27526
Zhao, C. X., Liu, Y., Wang, D. Y., Wang, D. L., and Wang, Y. Z. (2008). “Synergistic effect of ammonium polyphosphate and layered double hydroxide on flame-retardant properties of poly (vinyl alcohol),” Polym. Degrad. Stab. 93(7), 1323-1331. DOI: 10.1016/j.polymdegradstab.2008.04.002
Article submitted: September 2, 2016; Peer review completed: October 22, 2016; Revised version received and accepted: November 17, 2016; Published: November 23, 2016.
DOI: 10.15376/biores.12.1.533-545
