Abstract
Microcrystalline cellulose (MCC) is a green material that has widespread applications in pharmaceuticals, food, cosmetics, and other industries because of its biocompatibility, biodegradability, hydrophilicity, and acid-insolubility. In this study, MCC was prepared from cotton waste via alkaline treatment and sulfuric acid hydrolysis. Further, the synthesized cotton-based MCC was characterized using Fourier transform infrared (FTIR), X-ray photoelectron, and energy dispersive X-ray spectroscopies. Based on these results, the major components were identified as carbon and oxygen. This finding was evidenced by the FTIR analysis, which displayed peak wavenumbers at 3446.9, 2891.1, 1649.5, 1380.1, 1061.2, and 1050 to 1150 cm-1. The surface morphology was also examined by field emission scanning electron microscopy and field emission transmission electron microscopy, which showed that the prepared MCC has a smooth surface and a consistent, rod-like shape. In addition, the MCC exhibited the typical diffraction peaks of a crystalline structure of cellulose II at 12.2°, 20°, and 22.03°, which correspond to the diffraction planes of 1-10, 110, and 020, respectively, and had a crystallinity index of 78.7%. Moreover, the prepared MCC had a diameter of 37.8 µm and exhibited good stability with a peak at -76.5 mV. Further, the cotton-based MCC exhibited high thermal stability, as revealed by the TGA.
Download PDF
Full Article
Generation of Microcrystalline Cellulose from Cotton Waste and its Properties
Wan Yuen Tan,a Subash C. B. Gopinath,a,b,c,d,* Periasamy Anbu,e,* Palaniyandi Velusamy,f Ahmad Anas Nagoor Gunny,a,g Yeng Chen,h and Sreeramanan Subramaniam d,i,j
Microcrystalline cellulose (MCC) is a green material that has widespread applications in pharmaceuticals, food, cosmetics, and other industries because of its biocompatibility, biodegradability, hydrophilicity, and acid-insolubility. In this study, MCC was prepared from cotton waste via alkaline treatment and sulfuric acid hydrolysis. Further, the synthesized cotton-based MCC was characterized using Fourier transform infrared (FTIR), X-ray photoelectron, and energy dispersive X-ray spectroscopies. Based on these results, the major components were identified as carbon and oxygen. This finding was evidenced by the FTIR analysis, which displayed peak wavenumbers at 3446.9, 2891.1, 1649.5, 1380.1, 1061.2, and 1050 to 1150 cm-1. The surface morphology was also examined by field emission scanning electron microscopy and field emission transmission electron microscopy, which showed that the prepared MCC has a smooth surface and a consistent, rod-like shape. In addition, the MCC exhibited the typical diffraction peaks of a crystalline structure of cellulose II at 12.2°, 20°, and 22.03°, which correspond to the diffraction planes of 1-10, 110, and 020, respectively, and had a crystallinity index of 78.7%. Moreover, the prepared MCC had a diameter of 37.8 µm and exhibited good stability with a peak at -76.5 mV. Further, the cotton-based MCC exhibited high thermal stability, as revealed by the TGA.
DOI: 10.15376/biores.18.3.4884-4896
Keywords: Microcrystalline cellulose; Natural fiber; Biopolymer; Acid hydrolysis; Cotton waste
Contact information: a: Faculty of Chemical Engineering & Technology, Universiti Malaysia Perlis (UniMAP), 02600 Arau, Perlis, Malaysia; b: Institute of Nano Electronic Engineering, Universiti Malaysia Perlis (UniMAP), 01000 Kangar, Perlis, Malaysia; c: Micro System Technology, Centre of Excellence (CoE), Universiti Malaysia Perlis (UniMAP), Pauh Campus, 02600 Arau, Perlis, Malaysia; d: Centre for Chemical Biology (CCB), Universiti Sains Malaysia, Bayan Lepas, 11900 Penang, Malaysia; e: Department of Biological Engineering, Inha University, Incheon – 402-751, South Korea; f: Research and Development Wing, Central Research Laboratory, Sree Balaji Medical College and Hospital, Bharath Institute of Higher Education and Research (BIHER), Chennai- 600 044, Tamil Nadu, India; g: Centre of Excellence for Biomass Utilization, Universiti Malaysia Perlis (UniMAP), Kompleks Pusat Pengajian Jejawi 3, 02600 Arau, Perlis, Malaysia; h: Department of Oral & Craniofacial Sciences, Faculty of Dentistry, University of Malaya, 50603 Kuala Lumpur, Malaysia; i: School of Biological Sciences, Universiti Sains Malaysia, Georgetown, 11800 Penang, Malaysia; j: Department of Biology, Faculty of Science and Technology Universitas Airlangga, Surabaya 60115, Indonesia; *Corresponding authors: subash@unimap.edu.my; anbu25@yahoo.com
GRAPHICAL ABSTRACT

INTRODUCTION
Cellulose is a widely available biopolymer that has received increased attention because of its biosynthesis, chemistry, and ultrastructure (Fattahi et al. 2014; Suk et al. 2019; Gobalu et al. 2021; Lim et al. 2021; Vasudevan et al. 2021). As a natural polymer, cellulose is renewable, eco-friendly, inexpensive, non–toxic, biodegradable, and biocompatible (Olivera et al. 2016; Gunny et al. 2021). It is an organic carbohydrate component, composed of repeating β-D-glucopyranose units that have three hydroxyl groups per anhydroglucose unit, yielding a high degree of functionality for cellulose molecules (Kusumattaqiin and Chonkaew 2015; Gopinath et al. 2016; Yuen et al. 2019). As shown in Fig. 1a, the basic structure of cellulose exhibits a crystalline region depending on the hydrogen bonding and can also exist in an amorphous state. Moreover, cellulose is usually insoluble in most solvents and has hydrophilic properties due to the presence of hydrogen bonding. Recently, it has become a popular research topic as a green biomaterial because of its excellent mechanical and chemical characteristics (Song et al. 2016). Further, it can be easily obtained from plants (e.g., cotton, flax, abaca, coir, jute, hemp, kenaf, and ramie), wood, bacteria, algae, and marine fauna (e.g., tunicates) (Li et al. 2015). Currently, plants are the main source of cellulose, which is present in plant cell walls.
Microcrystalline cellulose (MCC) is applied widely in cosmetics, plastic, food, pharmaceuticals, and other industries because of its biocompatibility, hydrophilicity, biodegradability, and acid-insolubility (Widiarto et al. 2017). “Microcrystalline” refers to cellulose chains in natural polymers; a region with a high order degree is named microcrystalline, whereas a region with a low order degree is named amorphous (Haque et al. 2015). Various studies have extracted MCC on a large scale from cotton and wood cellulose using dilute mineral acids (Adel et al. 2010).
The MCC can be prepared by different methods depending on the type of cellulosic material, including acid hydrolysis, enzyme-mediated methods, explosive autohydrolysis, and alkaline oxidation (Kushnir et al. 2015; Kusumattaqiin and Chonkaew 2015). The MCC is normally prepared via the acid hydrolysis method, which uses a strong acid, such as HCl and sulfuric acid (H2SO4), as an acid catalyst to eliminate the amorphous part of the cellulose to yield particles consisting of microcrystals (Kusumattaqiin and Chonkaew 2015; Suryadi et al. 2017). Acid hydrolysis has many advantages, including low cost, short duration, continuous process, limited quantity of consumed acid, and fine particles of fine products (El-Sakhawy and Hassan 2007; Chauhan et al. 2009). The elimination of non-cellulosic components via alkaline treatment provides several advantages for the quality of the MCC produced. First, it increases the MCC’s whiteness and brightness, making it more suited for usage in a variety of industries such as pharmaceuticals and cosmetics. Second, it enhances the MCC’s surface area and porosity, allowing it to be a more effective absorbent and dispersion in applications such as medication delivery and food processing.
In the present study, MCC was prepared from cotton waste using two steps: alkaline treatment and acid hydrolysis. The cotton was treated with an alkaline solution of sodium hydroxide to remove impurities, such as hemicellulose and lignin, before acid hydrolysis. Then, the treated cotton was hydrolysed with acid to decrease the size of the crystalline cellulose. When the cellulose polymer is treated with the acid solution, the β-1,4-glycosidic bond is broken; subsequently, the chain is hydrolyzed, as shown in Fig. 1b. This process reduces the degree of cellulose polymerization. The novelty of producing new materials from cotton waste lies in the fact that cotton is a readily available and abundant raw material that is often discarded as waste. As a result, the current study sought to develop a sustainable and environmentally beneficial approach of turning cotton waste materials into MCC.
EXPERIMENTAL
Cotton waste was used as the source material for MCC production. The cotton waste was originally supplied to the laboratory by a local supplier in Malaysia. H2SO4 (95%) and sodium hydroxide (NaOH) pellets were purchased from Sigma-Aldrich (St. Louis, MO, USA). Carbon-coated copper grids were purchased for the transmission electron microscopy (TEM) analysis from Electron Microscopy Sciences (Hatfield, UK). Morphological analysis was performed to characterize the MCC using field emission scanning electron microscopy (FESEM) and field emission transmission electron microscopy (FETEM), and structural analyses were performed using Fourier transform infrared (FTIR), energy dispersive X-ray (EDX), X-ray photoelectron spectroscopies (XPS), X-ray diffraction (XRD), thermogravimetric analyses (TGA), zeta potential, and a particle size analyzer.
Production of MCC Powder: Alkaline and Acid Treatments
The cotton waste was cut into small pieces (approximately 1 cm), which were turned into MCC powder after treatment with NaOH (treatment with alkaline) and H2SO4 (acid hydrolysis). Specifically, the small pieces of cotton were added with NaOH (5%) and stirred constantly for 4 h at room temperature to obtain a homogenous mixture. Then, the alkali-treated MCC powder was filtered and then washed by distilled water many times to reach the neutral pH. This ensured lignin and hemicellulose impurities in the cotton were eliminated. Next, the filtrate was dried at 80 °C in an oven for 24 h (Theivasanthi et al. 2018).
The alkali-treated dried cotton samples were further treated by H2SO4. Specifically, the alkali-treated sample was mixed with a 35.5 M concentrated H2SO4 solution and stirred constantly at 40 °C for 60 min to obtain a well-mixed solution. After mixing, cold water was added to stop the hydrolysis reaction and then the solids were suspended and washed by distilled water until the neutral pH. The suspended (bottom) layer was centrifuged for 15 min with the speed of 10,000 × g for 15 min and then the suspended MCC was dried at 80 °C in an oven for 24 h to obtain the powder form of MCC (Theivasanthi et al. 2018).
Surface Characterization of MCC: Morphology Analysis
The morphology of the MCC samples was characterized using FESEM (Hitachi, S-4300SE, Tokyo, Japan), which scanned the sample at high-energy beams (15 kV). The morphologies of the samples were examined using FETEM (JEM 2100F, Jeol Ltd., Kyoto, Japan), in which the samples were prepared by dropping the MCC solution on a carbon-coated copper grid and dried.
Surface Characterization of MCC: Surface Chemistry Analysis
The synthesized MCC was further characterized using structural and functional analyses. An FTIR analysis (Vertex 80V, Bruker, Bremen, Germany) was employed to find the functional groups, record the emission spectrum of the MCC sample, and a kBr pellet was added to the sample to study the FTIR spectra in the range of 500 to 4000 cm-1. Absorption was identified with various stretching modes of functional groups between 1500 and 4000 cm-1. Meanwhile, peaks below 1500 cm-1 often denote bending, ring vibrations, and deformation are commonly used as the fingerprint region of the spectrum. Moreover, FESEM equipped with EDX analysis (EDAX, Mahwah, NJ, USA) was performed to confirm the elements in the synthesized MCC at an accelerating voltage of 15 kV.
X-ray photoelectron spectroscopy (Thermo Scientific, K-Alpha, Hemel Hempstead, UK) with monochromatic Al Ka micro-focused was utilized to identify the elemental composition on the surface of the MCC. XPS was also used to analyze the chemical composition of the samples from the outer 10 nm of the surface. The synthesized MCC was subjected to XRD (Rigaku, DMAX-2500, Tokyo, Japan) with a Cu-Kα wavelength of 1.5406 to 1.5444 A, a voltage of 40 kV, and a current of 100 mA. The sample was scanned at a rate of 2 °C/min in a Cu radiation source at 2θ = 5° to 50°. The crystallinity index (CrI) was calculated with SmartLab Studio II software (Rigaku Corporation, Tokyo, Japan).
The thermal stability of the MCC was verified by a progressive enhancement in temperature in the TGA (TG 209 F3 Tarsus, Netzsch, Berlin, Germany). A total of 6.30 mg of each synthesized MCC was placed in an alumina pan and heated from 30 °C to 600 °C at a rate of 10 °C min-1. The size distributions and particle dispersion stability were analyzed by using particle size analyzer (ELS2-2000, Otsuka Electronics, Tokyo, Japan) and dynamic light scattering (DLS) with a zeta potential.
RESULTS AND DISCUSSION
Production of MCC from Waste Cotton
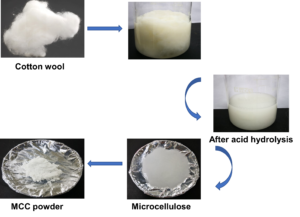
The naturally obtained cellulose material is composed of glucose units connected by β-1,4 glycosidic bonds (Fig. 1a and b). To prepare MCC from the cotton waste, the samples were first treated with NaOH for alkaline treatment and then H2SO4 for acid hydrolysis (Fig. 1c-i-iii). Next, the treated samples were dried at 80 °C (24 h) to obtain the MCC powder (Fig. 1c through iv and v). The synthesized MCC was further characterized by morphological analyses to examine the size, surface, and surface structure to confirm the chemical composition, functional group, thermal stability, particle size distribution, and stability of the MCC.
Fig. 1. a) The structure of cellulose; b) Acid hydrolysis of cellulose into MCC; c) Synthesis of MCC from cotton waste. i) Cotton wool; ii) After alkali treatment; iii) After acid hydrolysis; iv) Microcellulose; v) MCC powder
Characterization of MCC: Morphology Analysis
A morphological analysis of the MCC sample was performed utilizing FESEM and TEM. The FESEM results revealed a uniform particle size distribution and rod-like structure particles (Fig. 2a and b). Further, the shape of the particles determined the outer surface area. Natural MCC was used to determine the surface morphology of the prepared MCC to obtain the uniform size of particle, and a slightly rough formation was observed, which was attributed to the chemical treatment during the delignification process. The surface morphology of the prepared MCC was further examined using FESEM with accelerated electrons under 15 kV of energy. Note that MCC is produced by breaking the fibers via acid hydrolysis (Kale et al. 2018). The results showed a particle size range of 22.1 µm to 37.2 µm with an average size of 29.4 µm. FETEM was also used to study morphology at different magnification scales. The results revealed irregularly shaped particles with individual molecules in different ranges (Fig. 2c and d). Moreover, at a higher magnification, the cotton-based MCC shape was different from the FESEM image.
Fig. 2. Morphological analysis of the MCC. a) FESEM was examined at 100 µm magnification; b) FESEM was examined at 20 µm magnification; c) FETEM – scale bar at 1 µm; d) FETEM – scale bar at 500 nm
Surface Chemical Bonds Analysis: FTIR
The FTIR spectra of the MCC sample were examined in the range of 500 to 4000 cm-1 (Fig. 3a). According to the infrared spectra of the MCC, O−H stretching was observed at 3446.9 cm-1, and C=O carbonyl ring stretching occurred at 1649.5 cm-1. Thus, the alkali treatment decreased the hydrogen bonding of cellulose by removing hydroxyl groups (–OH) when reacting with NaOH, whereas the C−H and C−O bonds of cellulose are present in the polysaccharide aromatic rings (Theivasanthi et al. 2018). C−H stretching in the cellulose structure was noticed at 2891.1 cm-1, whereas C−O stretching was noted in the range of 1050 to 1150 cm-1. Moreover, a C−O−C pyranose ring in cellulose occurred at 1061.2 cm-1. Further, the peak at 1381.1 cm-1 confirms the presence of cellulose in the MCC sample. Moreover, the peak at 900 cm-1 occurred because of the glycosidic linkage between the sugar units (Trilokesh and Uppuluri 2019).
The EDX equipped with FESEM was employed to find the chemical composition of the MCC. The EDX spectra peaks of MCC correspond to the energy levels of C and O, as shown in Fig. 3b, wherein C and O are major peaks at 0.3 and 0.6 KeV, respectively, indicating the adequate synthesis of MCC from the cotton waste. The percentages of O and C were 57.5% and 42.5%, respectively, in the MCC.
Fig. 3. a) FTIR spectrum of MCC, explaining the chemical bond examined in the MCC, b) EDX analysis of MCC shows the presence of elements
Surface Elemental Composition and Chemical State: XPS
The XPS analysis was used to find the chemical composition of the MCC surface (Fig. 4a through c). The results clearly show that O and C were the dominant peaks at binding energies of 531.74 eV and 284.68 eV, respectively, which are attributed to the presence of C and O in the MCC. The deconvolution of C1 spectrum’s shows several distinct peaks that correspond to various carbon habitats for binding states. These peaks can be deconvoluted to reveal the distinct functional groups, such as the C=O, C-C/C=C, and -COO groups (Fig. 4b).
Fig. 4. XPS pattern of MCC from cotton waste: a) Survey scan, b) Carbon C1s, c) Oxygen O1s
In addition, the O1s spectrum can be deconvoluted to reveal the contributions of different oxygen-containing functional groups, such as C=O and -COO groups (Fig. 4c). The relative intensities of these peaks can reveal the details about the surface composition and the presence of different functional groups (Khili et al. 2019).
Crystallographic Structure Analysis: XRD
The XRD method was used to elucidate the crystallinity of the MCC. Generally, the polymer material of MCC was semi-crystalline because it still contained amorphous material in addition to the dominant crystalline parts (Alavudeen et al. 2017). Crystalline polymers produce sharp peaks, whereas amorphous polymers tend to produce blunt or widened peaks (Fig. 5a). The MCC created from cotton waste exhibited the typical diffraction peaks of a crystalline cellulose II structure at 12.2°, 20°, and 22.03°, which represent the diffraction planes of 1-10, 110, and 020, respectively (French 2014). After alkaline treatment and acid hydrolysis on cotton, the cellulose I peak 22.03° (020) was split into two peaks 20° (110) and 22.03° (020), indicating the formation of cellulose II (French 2014; Trilikesh and Uppuluri 2019). The CrI of MCC was reported as the percentage of crystallinity calculated according to the diffraction patterns. Based on the diffraction patterns, the CrI of the prepared MCC was decreased to 78.7% after alkaline treatment and acid hydrolysis, as compared with that of cotton (81.2%) (Table 1). This decrease in CrI is caused by the conversion of the structure from cellulose I to cellulose II, which is associated with the destruction of the cellulose I structure in cotton by molecular chain cleavage and the subsequent reformation of the crystalline structure to cellulose II (Yue et al. 2013).
Thermal Stability: TGA
The thermal stability of MCC was confirmed using TGA. The TGA curves of the synthesized MCCs are shown in Fig. 5b. The initial weight loss of the materials began within the range of 50 to 100 °C because of the evaporation of moisture on the sample surface (Haque et al. 2015; Nasution et al. 2017). Meanwhile, the thermal decomposition of MCC began at 250 °C to 370 °C, accounting for a total weight loss of 70%. These results indicate that the MCC created from cotton has high thermal stability (Table 1).
Fig. 5. a) XRD pattern of MCC from cotton waste. b) Thermal analysis of MCC; TGA curve indicates the reduction of weight as the temperature increased from 270 to 600 °C.
Table 1. Comparison of the MCC from Cotton Waste with Other Studies
Stability and Size Distribution: Zeta Potential and Particle Size Analysis
A zeta potential analysis was used to elucidate the colloidal stability of the particles in an aqueous suspension of the MCC sample by measuring the surface charge of the particles. The zeta potential for the MCC was -76.5 mV, which indicates the stability of the particles (Fig. 6a). This value is consistent with that reported by Mahajan and Ramana (2014). Thus, MCC is suitable for the encapsulation of enzymes and other molecules. In addition, a particle size analyzer was used to examine the MCC particle size. When MCC is dispersed in a solution, it exhibits Brownian motion, in which smaller particles exhibit faster motions. The scattered light from the particles displays fluctuations corresponding to individual particles when the laser light illuminates the particles under the effect of Brownian motion. This fluctuation is detected using the pinhole-type photon detection method and can determine the particle size and particle size distribution. The particle size of MCC had a diameter of 37.8 µm, as shown in Fig. 6b, with a polydispersity index (PDI) of 0.304. The low PDI value of the prepared MCC indicates a narrow particle size distribution, suggesting an even particle size. Therefore, this study confirmed that cotton waste could be used as a potential raw material for isolating MCC for use as a green material in many industrial applications.
Fig. 6. a) Zeta potential of MCC from cotton waste; b) Dynamic light scattering of the MCC
CONCLUSIONS
The main objective of this research was to prepare MCC from cotton waste. The samples were first treated with NaOH for alkaline treatment and then H2SO4 for acid hydrolysis. The alkaline treatment step is critical in the production of MCC from waste cotton; it helps to improve the purity, whiteness, and functional properties of the MCC, making it a versatile material for various applications. The synthesized MCC was characterized using morphological and structural analyses, and the major components of the sample were confirmed using FTIR, XPS, and EDX. Moreover, the FTIR peaks of MCC were observed at 3446.9, 2891.1, 1649.5, 1380.1, 1061.2, and 1050 to 1150 cm-1, indicating it was a cellulose material. The surface morphology of the prepared MCC was examined using AFM, FESEM, and TEM, which revealed that the prepared MCC had a smooth surface and a consistent, rod-like shape. In addition, the synthesized MCC exhibited the typical diffraction peaks of the crystalline structure of cellulose II at 12.2°, 20°, and 22.03°, which represent the diffraction planes of 1-10, 110, and 020, respectively, and the CrI was 78.7%. Based on the results of zeta potential and particle size analyses, the cotton-based MCC with a diameter of 37.8 µm displayed excellent colloidal stability, with a peak at -76.51 mV. Moreover, the synthesized MCC had high thermal stability. Based on the results of this research, cotton waste could be a suitable source for preparing MCC as a green material for potential applications in various industries.
ACKNOWLEDGMENTS
The author Periasamy Anbu thanks Inha University for research support.
REFERENCES CITED
Adel, A. M., Abd El-Wahab, H. Z., Ibrahim, A. A., and Al-Shemy, M. T. (2010). “Characterization of microcrystalline cellulose prepared from lignocellulosic materials,” Bioresource Technol. 101(12), 4446-2255. DOI: 10.1016/j.biortech.2010.01.047
Chauhan, Y. P., Sapkal, R. S., Sapkal, V. S., and Zamre, G. S. (2009). “Microcrystalline cellulose from cotton rags (waste from garment and hosiery industries),” Int. J. Chem. Sci. 7(2), 681-688.
Chuayjuljit, S., Su-Uthai, S., Tunwattanaseree, C., and Charuchinda, S. (2009). “Preparation of microcrystalline cellulose from waste-cotton fabric for biodegradability enhancement of natural rubber sheets,” J. Reinforced Plast. Composite 28, 1245-1254. DOI: 10.1177/0731684408089129
Deng, S., Binauld, S., Mangiante, G., Frances, J. M., Charlot, A., Bernard, J., Zhou, X., and Fleury, E. (2016). “Microcrystalline cellulose as reinforcing agent in silicone elastomers,” Carbohyd. Polym. 151, 899-906.
El-Sakhawy, M., and Hassan, M. L. (2007). “Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues,” Carbohyd. Polym. 67(1), 1-10. DOI: 10.1016/j.carbpol.2006.04.009
Fattahi Meyabadi, T., Dadashian, F., Mir Mohamad Sadeghi, G., and Zanjani Asl, H. E. (2014). “Spherical cellulose nanoparticles preparation from waste cotton using a green method,” Powder Technol. 261, 232-240. DOI: 10.1016/j.powtec.2014.04.039
French, A. D. (2014). “Idealized powder diffraction patterns for cellulose polymers,” Cellulose 21, 885-896. DOI: 10.1007/s10570-013-0030-4
Gobalu, K., Vasudevan, M., Gopinath, S. C. B., Perumal, V., and Ovinis, M. (2021). “Molybdenum disulphide/cellulose acetate nanofiber composite on screen printed electrodes for detecting cardiac troponin by electrical impedance spectroscopy,” Cellulose 28, 5761-5774. DOI: 10.1007/s10570-021-03911-w
Gopinath, S. C. B., Goh, C. S., Citartan, M., Lakshmirpriya, T., Arshad, M. K. K., Faudzi, F. N. M., Rahim, R. A., Hashim, U., Chinni, S. V., and Tang, T. H. (2016). “Micro-encapsulation of antibiotic in cellulose nanoparticle inhibits bacteria,” Micro. Nanosys. 8(1), 41-46. DOI: 10.2174/1876402908666160720104019
Gunny, A. A. N., Arbain, D., Javed, M., Baghaei-Yazdi, N., Gopinath, S. C. B., and Jamal, P. (2021). “Deep eutectic solvents-halophilic cellulase system: An efficient route for in situ saccharification of lignocellulose,” Proc. Biochem. 81, 99-103. DOI: 10.1016/j.procbio.2019.03.003
Haque, S., Chowdhury, A., Rana, A., Masum, S., Ferdous, T., Rashid, M. A., Sarker, M., and Karim, M. M. (2015). “Synthesis of microcrystalline cellulose from pretreated cotton obtained from Bombax ceiba L. and its characterization,” Bangladesh J. Sci. Ind. Res. 50(3), 199-204. DOI: 10.3329/bjsir.v50i3.25586
Hou, W., Ling, C., Shi, S., and Yan, Z. (2019). “Preparation and characterization of microcrystalline cellulose from waste cotton fabrics by using phosphotungstic acid,” Int. J. Biol. Macromol. 123, 363-368.
Kale, R. D., Bansal, P. S., and Gorade, V. G. (2018). “Extraction of microcrystalline cellulose from cotton sliver and its comparison with commercial microcrystalline cellulose,” J. Polym. Environ. 26, 355-364. DOI: 10.1007/S10924-017-0936-2
Khili, C., Borges, J., Almeida, P. L., Boukherroub, R., and Omrani, A. D. (2019). “Extraction of cellulose nanocrysttal with structure I and II and their applications for reduction of graphene oxide and elaboration,” Waste Biomss Volor. 10, 1913-1927.
Kushnir, E. Y., Autlov, S. A., and Bazarnova, N. G. (2015). “Preparation of microcrystalline cellulose directly from wood under microwave radiation,” Russian J. Bioorg. Chem. 41(7), 713-718. DOI: 10.1134/S1068162015070079
Kusumattaqiin, F., and Chonkaew, W. (2015). “Preparation and characterization of microcrystalline cellulose (MCC) by acid hydrolysis using microwave assisted method from cotton wool,” Macromol. Symposia 354(1), 35-41.
Li, M. C., Wu, Q., Song, K., Lee, S., Qing, Y., and Wu, Y. (2015). “Cellulose nanoparticles: Structure-morphology-rheology relationships,” ACS Sustain. Chem. Eng. 3(5), 821-832. DOI: 10.1021/acssuschemeng.5b00144
Li, M., He, B., and Zhao, L. (2019). Isolation and characterization of microcrystalline cellulose from cotton stalk waste,” BioResources 14(2), 3231-3246.
Liang, Y., Liu, X., Wang, L., and Sun, J. (2017). “The fabrication of microcrystalline cellulose-nanoZnO hybrid composites and their application in rubber compounds,” Carbohyd. Polym. 169, 324-331.
Lim, W. L., Gunny, A. A. N., Kasim, F. H., Gopinath, S. C. B., Kamaludin, N. H. I., and Arbain, D. (2021). “Cellulose nanocrystals from bleached rice straw pulp: Acidic deep eutectic solvent versus sulphuric acid hydrolyses,” Cellulose 28, 6183-6199. DOI: 10.1007/s10570-021-03914-7
Mahajan, A., and Ramana, E. (2014). “Patents on magnetoelectric multiferroics and their processing by electrophoretic deposition,” Recent Pat. Mater. Sci. 7(2), 109-130. DOI: 10.2174/1874464807666140701190424
Merci, A., Urbano, A., Grossmann, M. V. E., Tischer, C. A., and Mali, S. (2015). “Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion,” Food Res. Int. 73, 38-43.
Nasution, H., Yurnaliza, Veronicha., Irmadani, and Sitompul, S. (2017). “Preparation and characterization of cellulose microcrystalline (MCC) from fiber of empty fruit bunch palm oil,” in: IOP Conference Series: Materials Science and Engineering, Badung, Indonesia, article ID 012007. DOI: 10.1088/1757-899X/180/1/012007
Olivera, S., Muralidhara, H. B., Venkatesh, K., Guna, V. K., Gopalakrishna, K., and Yogesh Kumar, K. (2016). “Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review,” Carbohyd. Polym. 153, 600-618. DOI: 10.1016/j.carbpol.2016.08.017
Rasheed, M., Jawaid, M., Karim, Z., and Abdullah, L. C. (2020). “Morphological, physiochemical and thermal properties of microcrystalline cellulose (MCC) extracted from Bamboo fiber,” Molecules 25, article 2824.
Shi, S., Zhang, M.-L., Ling, C., Hou, W.-S., and Yan, Z.-F. (2018). “Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method,” Waste Manag. 82, 139-146.
Song, Y. K., Leng Chew, I. M., Yaw Choong, T. S., and Tan, K. W. (2016). “Nanocrystalline cellulose, an environmental friendly nanoparticle for pharmaceutical application – A quick study,” MATEC Web of Conf. 60, Article Number 1006.
Suk, K. H., Gopinath, S. C. B., Anbu, P., and Lakshmipriya, T. (2019). “Cow urine encapsulated cellulose nanoparticles for effective inhibition of pathogens,” Powder Technol. 328, 140-147. DOI: 10.1016/j.powtec.2018.01.010
Suryadi, H., Sutriyo Sari, H. R., and Rosikhoh, D. (2017). “Preparation of microcrystalline cellulose from water hyacinth powder by enzymatic hydrolysis using cellulase of local isolate,” J. Young Pharm. 9, S19-S23. DOI: 10.5530/jyp.2017.1s.6
Tarchoun, A. F., Trache, D., and Klapotke. T. M. (2019). “Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization,” Int. J. Biol. Macromol. 138, 837-845. DOI: 10.1016/j.ijbiomac.2019.07.176
Theivasanthi, T., Christma, F. L. A., Toyin, A. J., Gopinath, S. C. B., and Ravichandran, R. (2018). “Synthesis and characterization of cotton fiber-based nanocellulose,” Int. J. Biol. Macromol. 109, 832-836. DOI: 10.1016/j.ijbiomac.2017.11.054
Trilokesh, C., and Uppuluri, K. B. (2019). “Isolation and characterization of cellulose nanocrystals from Jackfruit peel,” Sci. Rep. 9, article 16709. DOI: 10.1038/s41598-019-53412-x
Vasudevan, M., Tai, M. J. Y., Perumal, V., Gopinath, S. C. B., Murthe, S. S., Ovinis, M., Mohammed, N. M., and Joshi, N. (2021). “Cellulose acetate-MoS2 nanopetal hybrid: A highly sensitive and selective electrochemical aptasensor of Troponin I for the early diagnosis of acute myocardial infarction,” J. Taiwan Institute Chem. Eng. 118, 245-253. DOI: 10.1016/j.jtice.2021.01.016
Widiarto, S., Yuwono, S. D., Rochliadi, A., and Arcana, M. (2017). “Preparation and characterization of cellulose and nanocellulose from agro-industrial waste – cassava peel,” in: IOP Conference Series: Materials Science and Engineering, 176(1), Solo, Indonesia, article 012052. DOI: 10.1088/1757-899X/176/1/012052
Yue, Y., Han, G., and Wu, Q. (2013). “Transitional properties of cotton fibers from cellulose I to cellulose II structure,” BioResources 8(4), 6460-6471.
Yuen, T. W., Gopinath, S. C. B., Anbu, P., Kasim, F. H., Yaakub, A. R. W., Lakshmipriya, T., and Lee, C. G. (2019). “Encapsulation of fungal extracellular enzyme cocktail in cellulose nanoparticles: Enhancement in enzyme stability,” Ind. J. Biochem. Biophys. 56(6), 475-483. DOI: 10.56042/ijbb.v56i6.29251
Article submitted: February 28, 2023; Peer review completed: April 1, 2023; Revised version received and accepted: May 12, 2023; Published: May 25, 2023.
DOI: 10.15376/biores.18.3.4884-4896
