Abstract
The black liquor generated from the alkaline pretreatment of lignocellulosic biomass is usually disposed of into wastewater, which could lead to environmental pollution. Alkaline black liquor (ALBL) contains a large amount of xylan with a small fraction of lignin, making it a promising raw material for the production of xylooligosaccharides (XOS). In this study, xylan was extracted from the ALBL generated upon treating the oil palm frond (OPF) with sodium hydroxide via two-stage precipitation for the separation of lignin and recovery of xylan. As a result, approximately 84.0% of xylan retrieved from the ALBL was recovered. Subsequently, enzymatic hydrolysis was optimized to recover the maximum amount of XOS from xylan. The results showed that enzymatic hydrolysis produced the highest XOS (62.5%) under optimal conditions of 50 °C, 4 U/mL xylanase, and 3% xylan loading for 48 h. The study provides insight for maximizing utilization of ALBL of OPF for future biorefinery economy.
Download PDF
Full Article
Green Production of Prebiotic Xylooligosaccharides via Enzymatic Hydrolysis from Xylan Black Liquor of Oil Palm Frond
Nur Syahirah Ahmad Sobri,a,b Shuhaida Harun,a,b,* Jamaliah Md Jahim,a,b Mohd Shahbudin Masdar,a,b Mohd Sobri Takriff,a,b Abdul Wahab Mohammad a,b and Mastura Abd Manaf a,b
The black liquor generated from the alkaline pretreatment of lignocellulosic biomass is usually disposed of into wastewater, which could lead to environmental pollution. Alkaline black liquor (ALBL) contains a large amount of xylan with a small fraction of lignin, making it a promising raw material for the production of xylooligosaccharides (XOS). In this study, xylan was extracted from the ALBL generated upon treating the oil palm frond (OPF) with sodium hydroxide via two-stage precipitation for the separation of lignin and recovery of xylan. As a result, approximately 84.0% of xylan retrieved from the ALBL was recovered. Subsequently, enzymatic hydrolysis was optimized to recover the maximum amount of XOS from xylan. The results showed that enzymatic hydrolysis produced the highest XOS (62.5%) under optimal conditions of 50 °C, 4 U/mL xylanase, and 3% xylan loading for 48 h. The study provides insight for maximizing utilization of ALBL of OPF for future biorefinery economy.
DOI: 10.15376/biores.18.1.1525-1544
Keywords: Xylooligosaccharides; Michaelis-Menten kinetics; Oil palm frond; Xylan; Enzymatic hydrolysis; Xylanase
Contact information: a: Department of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia; b: Research Centre for Sustainable Process Technology (CESPRO), Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia;
*Corresponding author: harun.shuhaida@ukm.edu.my
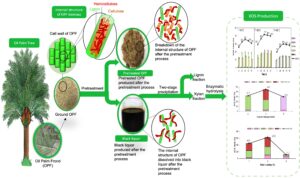
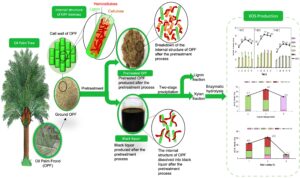
GRAPHICAL ABSTRACT
INTRODUCTION
The demand for production of prebiotics is booming globally, owing to its ability to selectively promote the growth of beneficial gut bacteria, and this has attracted manufacturers from the neutraceutical industries. Xylooligosaccharides (XOS) have emerged as favorable prebiotics due to their effects on human health and potential to combat several gastrointestinal disorders (Samanta et al. 2015).
XOS are oligosaccharides containing two to ten xylose monomer units linked by β 1-4 bonds (Vazquez et al. 2000; Moure et al. 2006; Makelainen et al. 2010; Reque et al. 2019). The XOS biological activity varies with the distribution of its molecular weight (Hughes et al. 2007). Prebiotic applications have been found for XOS with DP less than four monomer units; these molecules promote proliferation of bifidobacteria, which are beneficial microorganisms that play an important role in defending against pathogens in the digestive tract of humans and animals (Carvalho et al. 2013; Davani-Davari et al. 2019). The incorporation of XOS into food benefits the physiological properties of the body, including calcium absorption, lipid metabolism, prevention of dental caries, and reduction in the risk of colon cancer due to the formation of short-chain fatty acids (Grootaert et al. 2007; Wang et al. 2009; Le et al. 2020; Olaimat et al. 2020). However, the production of XOS is costlier than that of other prebiotics.
The exploitation of agricultural residues, namely lignocellulosic biomass (LCB), is increasing, as it is a strategy to reduce the cost of production due to its inexpensiveness and abundance. In contrast to other commercial prebiotics, such as inulin and fructooligo-saccharides, XOS is considered to be the only neutraceutical that can be produced from LCB. Abundant LCB is produced from the oil palm (Elaeis guineensis Jacq.) industry, including oil palm fronds (OPF) enriched with various carbohydrate sugars and other useful chemical compounds. These can be developed into various resources, for instance, bioenergy and other value-added products (Abdullah and Sulaiman 2013). Xylan is the most abundantly occurring heterogenous biomolecule present in hemicelluloses, and it is a promising biomaterial for pharmaceuticals.
Earlier studies have focused on the alkaline pretreated OPF using sodium hydroxide (NaOH), leaving the remaining black liquor as waste. The black liquor generated from the alkaline pretreatment process is an environmental pollutant. Such black liquor is composed mainly of xylan and a small fraction of lignin, which may lead to a huge waste of hemicelluloses. It is necessary to utilize the black liquor and bring the palm industry towards achieving zero waste strategies. The isolation of xylan was first performed by separation of lignin contained in the black liquor through pH adjustment, as lignin could impede the bioconversion process. Subsequently, xylan was recovered from the remaining black liquor using ethanol (Sobri et al. 2019). Xylan could serve as a raw material for XOS production.
The XOS can be produced by enzymatic hydrolysis, where the xylan in hemicellulose is broken down into XOS (Carrillo et al. 2018). Through the breaking of the glycosidic linkages of xylose, hemicellulose is segregated into oligosaccharides of a broad DP range in addition to monosaccharides in this method (de Freitas et al. 2019; Poletto et al. 2020). As reported by Samanta et al. (2016), xylan extracted from corn husk was converted at a 44 °C for 17.5 h of enzymatic hydrolysis to produce a maximum xylobiose concentration of 1.9 mg/mL. Enzymatic hydrolysis does not produce unwanted by-products or a large amount of monosaccharides. However, xylanases with different substrate specificities produce different end products of hydrolysis, and it is difficult to achieve the XOS production with the desired DP.
The functional food market is rapidly growing, and the rising number of other industrial applications (Moure et al. 2006) has forced researchers to explore a variety of sources and technologies for producing XOS with a defined DP distribution. In this study, the highest production of XOS from xylan extracted in ALBL was studied by investigating the factors affecting the XOS production, including hydrolysis time, temperature, enzyme dosage, and substrate loading.
EXPERIMENTAL
Preparation of Oil Palm Frond Sample
Oil palm frond as the raw material was collected from the oil palm plantation at the Universiti Kebangsaan Malaysia, Bangi, Selangor, Malaysia. The length of OPF petioles was cut to 1 to 3 m, and the juice was removed by pressing the OPF using a sugarcane presser machine model SCM (6.5 hp petrol-driven, Zheijiang Sheng, China). The samples were sun-dried for 72 h until the moisture content dropped below 10%, as measured by a moisture analyzer (IR35M, Denver Instrument, New York, USA). The dried OPF bagasse was ground to 2 mm particle-size using a grinder (Fritsch, Idar-Oberstein, Germany) and sieved through 0.5 mm mesh to remove the powdery particles. The OPF samples were sealed in a plastic bag and stored at 4°C. The composition of raw OPF bagasse was characterized as previously described (Hames et al. 2008; Sluiter et al. 2008; Sluiter et al. 2012; Sobri et al. 2019).
Extraction of Xylan from Oil Palm Frond
Prior to the extraction, the OPF samples were oven-dried at 45 °C overnight to guarantee that its moisture content was less than 10%. Xylan was extracted from dried OPF bagasse via alkaline extraction by preparing the solid-to-liquid ratio of 1:10 (g/L) at 6% sodium hydroxide (NaOH) (w/v) for 60 min at 100 °C. After the extraction, the black liquor was collected and subjected to two-stage precipitation using 6 M hydrochloric acid and adjusted to pH 5.5 to remove the lignin fraction (LF). The remaining acidified black liquor was precipitated with 95% ethanol to obtain high purity of xylan fraction (XF) to be used as substrate for XOS production. The XF was freeze-dried until the weight became constant. XF was obtained in the form of pellets and stored at room temperature prior to the hydrolysis. Previous work determined that approximately 84% of XF was recovered under optimal conditions of alkaline extraction (Sobri et al. 2019). The process for the bioconversion of xylan to XOS is illustrated in Fig. 1.
Fig. 1. Flow diagram for the bioconversion process of xylan extracted from ALBL of OPF to XOS
Scanning Electron Microscopy of Untreated and Treated Oil Palm Frond
The changes in structural and morphological structure of the untreated OPF (UOPF) and NaOH-treated OPF (TOPF) were observed by scanning electron microscopy (SEM) using an EVO MA10 instrument (Carl Zeiss, UK). The samples were freeze-dried and gold-coated using the sputter coating system, Synthetic Q150 RS (Quorum Technologies, UK). The samples were analysed under the range of magnification from 100x to 500x.
X-ray Diffraction of Extracted Xylan
The diffraction patterns of the lignin and xylan fractions (LF and XF) were examined with X-ray diffractometer (XRD) using a D8 Advance XRD (Bruker AXS, Germany) with a wavelength of 1.541 Å at 40 kV and 40 mA. The intensities were measured at 2θ ranging from 10° to 80°. The XRD patterns were recorded at room temperature. The crystallinity index of the samples was calculated as follows,
(1)
where I002 is the maximum intensity that represents both crystalline and amorphous material, and Iam is the intensity that represents amorphous material.
Nuclear Magnetic Resonance Analysis on Extracted Xylan
The solution-state 1H and 13C spectra were obtained by nuclear magnetic resonance (NMR) on a Bruker/AVANCE III HD 400 MHz spectrometer (MA, USA) at 400 MHz and 100 MHz, respectively, at 27 °C from 30 mg of sample dissolved in 0.6 mL D2O. A 90 °C pulse flipping angle, a 1.0 to 4.0 second acquisition time and a 1.0 to 2.0 second delay time between scans were used.
Enzyme Assay
Commercial enzyme of (Cellic® HTec2, Novozymes A/S, Bagsvaerd, Denmark) was assayed for the desired enzyme activities according to endo-xylanase activity (Bailey et al. 1992). Cellic Htec2 is an endoxylanase with high specificity toward soluble hemicellulose and it has a cellulase background. The xylan substrate was prepared by dissolving 1% of birchwood xylan in 0.05 M sodium citrate buffer, adjusted to pH 5.3. Then, 1.8 mL of xylan solution was mixed with 200 μL of diluted enzymes and incubated at 50 °C for 5 min. The 3,5-dinitrosalicyclic acid (DNS) was added into the solution and boiled for 15 min. The solution was analyzed under ultraviolet-visible (UV-VIS) spectrophotometer for the determination of its enzyme activity at 540 nm wavelength. One unit is defined as the quantity of enzymes which produced 1.0 μmol of xylose per min.
Enzymatic Hydrolysis of Extracted Xylan for Xylooligosaccharides Production
Enzymatic hydrolysis of extracted xylan using Cellic Htec2 xylanase was fixed at 15 mL and hydrolyzed in a 20 mL glass of scintillation vial. The pH was maintained at 4.8 using 0.05 M citrate buffer, and the hydrolysis was performed in an incubator shaker (Infors HT Ecotron, Switzerland) at 150 rpm. Different temperatures (40, 50, 60 °C), enzyme dosages (2, 4, 6 U/mL), and xylan loading (1, 3, 6%) were evaluated. The hydrolysates were collected at specific time intervals (4, 24, 48, 72, 96 h) and then heated to 100 °C for 15 min to inactivate the enzyme reaction. The samples were centrifuged at 9000 rpm for 10 min, and the supernatant was filtered through 0.22 μm Whatman membrane syringe filter prior to HPLC analysis for XOS determination. All of the experiments were duplicated and the results were calculated to average. The yield of XOS was calculated as follows,
(2)
where V is the volume of hydrolysis and mxylan is the mass of xylan loaded in the hydrolysis volume.
The Michaelis-Menten Kinetic Equation
The Michaelis-Menten model describes the kinetics of the vast majority of processes (Ariyawansha et al. 2018) catalysed by endo-1,4-ß-xylanases, as shown below,
(3)
The kinetics constant (Vmax and Km) were calculated from a Lineweaver-Burk double reciprocal (1/v versus 1/[S]) plot using xylan fraction from ALBL as the substrate (1, 3, 6%).
(4)
where vo, vmax, Km, and [S] are the initial velocity, maximal velocity, Michaelis constant and substrate concentration, respectively.
Analytical Methods
The concentration of XOS and monosaccharides after enzymatic hydrolysis were quantified using high performance liquid chromatography (HPLC) (UltiMate 3000 LC system, Dionex, Sunnyvale, California, USA), equipped with a refractive index (RI) detector (RefractoMax 520, ERC, Buchholz, Germany) that was set at 40 °C by using Rezex RSO-Oligosaccharides Ag+ column (200 x 10 mm; Phenomenex, California, USA) with a guard column (60 m x 10 mm; Phenomenex, California, USA). The column temperature was maintained at 60 C, and deionized water was isocratically eluted at a flow rate of 0.3 mL/min and pressure limit at 17 bars with the sample injection volume fixed at 20 μL. The sugars were quantified based on individually plotted calibration curves of standard xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6) for XOS and xylose and glucose for monosaccharides. The calibration curves as well as the concentrations of the samples were analysed using Chromeleon v. 7.2.2.6686 software (Dionex, Sunnyvale, California, USA). Furfural and hydroxymethylfurfural (HMF) in hydrolysates were quantified using an Agilent 1100 HPLC System (California, USA) equipped with ultraviolet-diode array detector (UV-DAD) set at 220 nm by using Gemini C-18 column (Phenomenex, California, USA). The temperature of the column was maintained at 40 °C and using 20 mM sulphuric acid/acetonitrile (1:10) as the mobile phase, which was isocratically eluted at a flow rate of 0.8 mL/min with the sample injection volume set at 20 μL. The mobile phase used was initially vacuum filtered and degassed.
RESULTS AND DISCUSSION
Characterization of Oil Palm Frond and Analysis of Extracted Xylan
The OPF was comprised of 34.7 ± 4.2% glucan, 19.4 ± 3.8% xylan, 20.7 ± 0.5% lignin, and 1.1 ± 0.1% ash (Sobri et al. 2019). The results obtained were similar to previous reports (Fauzi et al. 2016; Luthfi et al. 2017; Manaf et al. 2018), in which the glucan and xylan were the predominant structural carbohydrates present in raw OPF.
Under the optimal extraction conditions of 6% NaOH (w/v), 100°C, and 60 min, the alkaline black liquor (ALBL) generated was composed of 93.8 ± 0.6% xylan, 12.4 ± 0.7% glucan, and 29.5 ± 0.2% lignin based on their original content in raw OPF. The peeling and nucleophilic substitution are the phenomena that occurred during these alkaline processes, which resulted in the solubilization of xylan and lignin components into the black liquor. Using acidification, lignin in the black liquor was precipitated, and the composition of the lignin fraction (LF) and the xylan fraction (XF) extracted from ALBL was determined by hydrolysing it with sulphuric acid. The pure extracted xylan was obtained as a result of this process. The extracted LF was composed of 8.8 ± 0.4% xylan, 10.9 ± 0.1% glucan, 19.3 ± 0.1% lignin, and 1.1 ± 0.3% acetic acid, while the XF was composed of 84.0 ± 0.1% xylan, 0.3 ± 0.3% glucan, 7.7 ± 0.2% lignin, and 0.5 ± 0.2% acetic acid based on their original content in raw OPF (Sobri et al. 2019). The amount of lignin after the acidification process in LF was higher than in XF, resulting in a fraction that was primarily made of xylan in the residual fraction. Because the xylan employed in this study demonstrated low contamination of lignin, the process was carried out in bulk to be used as substrate for XOS production. Xylose was the most abundant monosaccharide detected in XF, accounting for 18.5 ± 0.1%, and glucose was found at a slight amount of 0.1 ± 0.1%. In sum, the extracted XF was composed of xylose as the primary unit, with glucose and acetic acid as substituents attached to the backbone of xylose molecule.
Scanning Electron Microscopy Analysis of Oil Palm Frond
The scanning electron microscope was used to investigate the interior structure of the OPF, which allowed further evaluation of the effectiveness of the pretreatment. The structural and morphological modifications of untreated OPF (UOPF) and NaOH-treated OPF (TOPF) are shown in Fig. 2. The surface of the cell walls of UOPF in Fig. 2a displayed rigid, uniform, and highly ordered fibrils. Upon the treatment with NaOH (Fig. 2b), the surface became fractured and showed non-uniform cracks on its surface, which were caused by the plant cell wall removal and disruption on the surface structure upon NaOH pretreatment. The disruption on the surface structure from the SEM images obtained was in agreement with Sukri et al. (2014). Apart from that, such disruption showed that the NaOH pretreatment was able to disrupt the structure of lignin, and this phenomenon indicated that pretreating the OPF with NaOH had damaged the rigid structure of OPF as it generated irregular cracks and pores. The formation of pores resulted from the degradation of lignin and hemicellulose, and these changes detached the amorphous components and altered the crystalline structure of biomass (Manaf et al. 2018).
Fig. 2. SEM images of (a) untreated OPF and (b) treated OPF at magnification of 500x
X-ray Diffraction Analysis of Extracted Xylan
The amount of amorphous and crystalline content in a solid sample can be calculated by integrating the crystalline peaks and amorphous background signal acquired from powder of X-ray diffraction (XRD) analysis (Kundu et al. 2020). Cellulose, in nature, appears to be highly crystalline, while hemicellulose exists in amorphous form in biomass (Mazlan et al. 2019). The XRD pattern of lignin fraction (LF) and xylan fraction (XF) are shown in Fig. 3.
Fig. 3. X-ray diffraction pattern of (a) lignin fraction; LF and (b) xylan fraction; XF
Based on the graphs, the crystallinity index (CrI) of LF after the acid precipitation of ALBL was greater than the crystallinity index of XF after alcohol precipitation of ACBL, where the CrI of LF and XF were 51.9% and 25.0%, respectively. The greater CrI value in LF may be attributable to its higher glucan content than XF, as demonstrated in the authors’ previous study (Sobri et al. 2019). This is also in line with the literature reports by Mazlan et al. (2019). The appearance of a sharp peak in LF at 2θ = 32° (Fig. 3a) indicated the crystallization of LF, and as observed, a broad diffraction peak at 2θ = 18.9° is typically attributed to xylan (Kundu et al. 2020). The XRD pattern of XF revealed no additional sharp peaks, suggesting that the xylan was structurally amorphous. Hence, it is possible that xylan is more accessible for hydrolysis to produce XOS than cellulose, which is in line with Oliveira et al. (2010), where the xylan derived from corn cob was reported as an amorphous polymer, as the XRD pattern of corn cob xylan displayed no sharp peaks.
Nuclear Magnetic Resonance Analysis of Extracted Xylan
Proton magnetic resonance (1H NMR) and carbon magnetic resonance (13C NMR) are used to characterize the molecular structure of polymers (Saleh and Aziz 2016). In the present study, the 1H and 13C NMR spectra of the xylan sub-fraction isolated were performed in D2O solvent. As shown in Fig. 4a, major signals attributed to β-D-xylopyranosyl units were detected at 4.5 ppm, 3.3 ppm, 3.6 ppm, 3.8 ppm, 3.4 ppm, and 4.1 ppm, and these were assigned to H-1 (anomeric), H-2, H-3, H-4, H-5ax, and H-5eq, respectively. In addition, the presence of 4-O-methylglucuronic acid (MeGlcA) was shown at 5.3 ppm, 3.4 ppm, 3.6 ppm, 3.3 ppm, 4.3 ppm and 3.49 ppm, which were assigned to H-1, H-2, H-3, H-4, H-5 and methoxy protons (-OCH3), respectively (Sousa et al. 2016).
Table 1. NMR Analysis of Xylan Extracted from Alkaline Black Liquor of Oil Palm Frond
The structural features of xylan sub-fractions were confirmed with 13C NMR analysis (Fig. 4b). The strong signals at 101.7, 76.4, 73.7, 72.7, and 63.0 ppm corresponded to the C-1, C-4, C-3, C-2, and C-5 positions, respectively, of β-D-xylopyranose, which represents the 1,4-linked xylopyranosyl unit of hemicelluloses (Sun and Tomkinson 2002). The signal at 59.9 ppm corresponded to the methoxy group that belonged to the glucuronic acid in the hemicellulose of xylan (Xu et al. 2004). The weak signals at 98.0, 86.4 80.2, 78.9, and 61.7 ppm were assigned to C-1, C-4, C-2, C-3, and C-5, respectively, corresponding to the glucopyranosyl residues linked to β-D-xylans. The chemical shift at 59.8 ppm was attributed to the methoxy group (-OCH3), which confirmed the methylation of glucuronopyranosyl residues at the O-4 position. The results are shown in Table 1. The NMR spectra showed that the hemicellulosic sub-fraction can be structurally defined as L-(4-O-methyl-D-glucurono) xylan. These typical signals for L-(4-O-methyl-D-glucurono) xylan demonstrated that the conditions used in the alkaline treatment had no effect on the overall structure of macromolecular hemicellulose, indicating that it is a promising source for XOS production.
Fig. 4. (a) 1H and (b) 13C NMR spectra of extracted xylan fraction (XF) from ALBL of OPF
Enzymatic Hydrolysis of Extracted Xylan to Produce Xylooligosaccharides
The extracted xylan fraction (XF) from the ALBL was subjected to hydrolysis due to its high xylan content that could facilitate the production of XOS. A low DP has been shown to have a stimulatory effect on the selective growth of beneficial microorganisms of human intestinal bifidobacteria and encourage the proliferation process and production of XOS among DP 2-6, which were known as low DP XOS (Xiao et al. 2013). Hence, this study focused at maximizing the XOS production with DP range from 2 to 6 by evaluating the effects of hydrolysis temperature, enzyme dosage, and substrate loading.
The enzymatic production of XOS was initially performed at different temperatures within the range of 40 to 60 °C for different time periods at fixed operating conditions of 1% xylan loading and 2 U/mL xylanase loading (Fig. 5a).
The results implied that a maximum of 41.7% XOS yield was attained at temperature of 50 °C during 48 h of hydrolysis where varied amounts of XOS ranging from DP X2-X5 were produced. A total decrement on the XOS yield was observed upon prolonging the reaction temperature to 60 °C. This occurrence was attributed to inactivation of the enzymes at higher temperature, whereby the XOS formed was hydrolyzed into monosaccharides. The results were in agreement with previous studies where the suitable temperature for enzymatic hydrolysis of xylan from wheat straw, cotton stalk, and tobacco stalk was at 50 °C (Akpinar and Bostanci 2009) and the reaction temperature of 50 °C was the most favorable for enzyme activity (Kamble and Jadhav 2012). Hence, hydrolysis temperature of 50 °C was considered as suitable for the efficient conversion of xylan to XOS, which consisted of X2 (0.5%), X3 (1.2%), X4 (18.1%), and X5 (21.9%).
Fig. 5. Enzymatic production of XOS (X2: xylobiose; X3: xylotriose; X4: xylotetraose; X5: xylopentaose; X6: xylohexaose) under varying operating conditions of (a) temperature at 40 °C, 50 °C, 60 °C; (b) enzyme dosage at 2 U/mL, 4 U/mL, 6 U/mL; and (c) xylan loading at 1%, 3%, 6%
Production of XOS was further studied with several enzyme dosages within the range of 2 to 6 U/mL at fixed operating conditions of 50 °C and 1% xylan loading for 48 h of hydrolysis (Fig. 5b). Further examination indicated that a high yield of XOS up to 44.9% was achieved at an enzyme dosage of 4U/mL during 48 h of hydrolysis, and increasing the dosage up to 6 U/mL decreased the release of XOS due to the binding of the enzyme proteins with the substrate that had reached a plateau. In addition, higher enzyme dosage gave an insignificant effect in the production of sugar due to unavailability of an easily accessible hydrolytic site in a xylan chain or reduction of endo-xylanase activity due to end-product inhibition (Akpinar et al. 2010). Under this condition, the highest XOS with a total yield of 44.9% within DP range of X2 (1.0%), X3 (1.6%), X4 (20.9%), X5 (0.2%), and X6 (21.2%) was produced at enzyme dosage of 4 U/mL.
Table 2. Summary of Enzymatic Production of XOS Under Varying Operating Conditions
Substrate loading plays a vital role in improving the bioconversion of carbohydrates though enzymatic hydrolysis. Figure 5c shows the production of XOS by various xylan loadings within the range of 1% to 6% for at fixed operating conditions of 50 °C, 4 U/mL, and 48 h of hydrolysis. The maximum XOS production of 62.5% was achieved at 3% xylan loading, consisting of X2 (9.5%), X3 (0.8%), X4 (24.1%), X5 (0.9%), and X6 (27.1%). The overall trend at 6% xylan loading showed the least production of XOS. The reason underlying this observation was related to the high viscosity and density of the reaction mixture, resulting in poor mixing efficiency of enzymes to substrate and low recovery of products (Lineweaver and Burk 1934; Price 1985; Li et al. 2019). It was also speculated that high substrate loading combined with insufficient enzyme loading resulted in the enzymes becoming restricted at saturating substrate concentrations. Hence, the activity no longer changes with the increasing of substrate. As this study showed that the extracted xylan fraction (XF) contained about 7.7 ± 0.2% lignin, a high conversion of xylan to XOS was obtained, which showed that the low amount of lignin in xylan did not have a significant effect on the conversion of xylan into XOS. The summary results of enzymatic production of XOS are tabulated in Table 2.
Xylan was initially degraded into polysaccharides with high DP values and then further hydrolyzed into XOS with low DP values, as well as monosaccharides, during the hydrolysis process of xylan. When it comes to monomeric sugars, increasing the xylan loading resulted in a significantly larger release of xylose (Fig. 6). In terms of acetic acid, only trace amounts ranging from 0.02% to 0.05% were found. The presence of acetic acid revealed that the acetyl groups on side chains of hemicelluloses were hydrolysed to acetic acid during hydrolysis. The highest XOS was produced under 3% xylan loading with a total monosaccharide yield of 6.5%. This indicated that the presence of monosaccharides did not impede the performance of enzymatic hydrolysis.
Fig. 6. Effect of xylan loading on xylose, glucose, and acetic acid during enzymatic hydrolysis
Table 3 summarizes the production of XOS via enzymatic hydrolysis using different types of biomass that had been alkaline pretreated. The results of study conducted by Singh et al. (2018) using pineapple peel resulted in a total of 25.7% of XOS yield under enzymatic conditions of 50 °C, 15 U of endo-xylanase for 24 h, whereas a study by de Figueiredo et al. (2017) using sugarcane bagasse yielded a total of 27.1% XOS using 120 U/g of endo-xylanase from A. fumigatus and T. reesei, under temperature of 50°C for 24 h. Li et al. (2019) explored the pretreatment for lignin removal using sugarcane bagasse by combining 1% of sodium hydroxide and 1% of hydrogen peroxide at 70 °C. The pretreated solids were enzymatically hydrolyzed by xylanase, yielding a high total of XOS (79.9%), at a temperature of 50 °C using 300 IU/g of xylanase. It is noteworthy that while other studies used the pretreated biomass to produce XOS, this study was able to recover a significant amount from black liquor and yield more than 60% of XOS, which is in line with the present goal of fully utilising the oil palm waste for its bioconversion into a value-added product.
Table 3. XOS Production via Enzymatic Hydrolysis Utilizing Different Types of Biomass
Comparison of Xylooligosaccharides Production from Extracted Xylan and Commercial Beechwood Xylan
Lignin amounting to 7.7 ± 0.2% was detected in the extracted xylan fraction (XF), yet the conversion of xylan to XOS was high. This demonstrated that the low amount of lignin in XF has no major impact on the conversion of xylan to XOS. The experiment was further studied by hydrolyzing the commercial beechwood xylan (BX) under the optimal conditions of enzymatic hydrolysis (Table 4). To put this in perspective, the total XOS production from BX (53.3%) via enzymatic treatment was lower than the extracted xylan used in the present study, as well as the production of each DP XOS. This suggests that the xylan from ALBL of OPF is a promising prebiotic for functional food application.
Table 4. Comparative View of Enzymatic Hydrolysis of Xylan Fraction Extracted from ALBL of OPF and Commercial Beechwood Xylan for XOS Production
Determination of The Kinetic Constants
Using the Lineweaver-Burk plot, the kinetic parameters were determined. The Michaelis-Menten equation was simplified to linear form, and the Lineweaver-Burk plot was created as a result. Additionally, the double-reciprocal is another term for this phenomenon (Lineweaver and Burk 1934).
Using the inverse of the y-axis interception point of the straight line, the maximum value of vmax was computed, and the value of Km was derived using the negative reciprocal of the x-axis interception point of the graph (Price 1985). From Lineweaver-Burk plotted in Fig. 7, the value obtained for vmax and Km were 0.025 g/L/h and 2.091 g/L, respectively. The maximum value of vmax will change depending on the amount of enzymes that are added into the reaction, but it will not be impacted by the concentration of the substrate. Km, on the other hand, was defined as the measurement of the enzyme’s affinity on its substrate. According to the Michaelis-Menten equation, the substrate concentration will have a significant impact on both Km and vmax. As previously stated, both kinetic parameters must be measured because they are especially crucial in enzyme kinetics (Fazary et al. 2010). From the results obtained, a low vmax value suggested that the reaction rate of the process was somewhat slow. Compared to processes with high reaction rates, processes with low reaction rates would necessitate longer reaction times. On the other hand, a low Km value is preferable because it indicates that the process would only need a small amount of substrate in order to accomplish specific rate of reaction (half of vmax). This implies that the process would require a small amount of substrate in order to achieve a high rate of XOS production.
Fig. 7. Lineweaver-Burk plot of Km estimation to produce XOS at 1%, 3% and 6% of xylan loading under enzymatic hydrolysis optimal condition at 50°C and 4 U/mL.
Mass Balance for Xylooligosaccharides Production from Xylan Extracted from Alkaline Black Liquor of Oil Palm Frond via Enzymatic Hydrolysis
The mass balance of the entire process for XOS production was computed and examined in a methodical manner, and the major steps are described in Fig. 8. 1000 g of oven-dried raw OPF bagasse comprising of 194 g xylan, 347 g glucan, and 207 g lignin was used as a basis or starting point for the mass balance of XOS production. The alkaline pretreatment of OPF bagasse under condition of 6% NaOH at 100 °C for 60 min, resulting in 68.3% recovery of solid fraction enriched in glucan (87.6%) and lignin (70.5%), based on their respective original content in raw OPF, while most of the xylan (93.8%) was hydrolysed into ALBL. The ALBL obtained corresponding to 31.7% of the original material was further subjected to two-stage precipitation for the removal of lignin and recovery of xylan. The acid precipitation of ALBL using 6M HCl generated two products in a solid and liquid form, namely LF and ACBL. A total of 67.2% lignin from its original content in ALBL was removed out in LF, while a total of 90.6% xylan remained in ACBL to be recovered via ethanol precipitation. The xylan content in ACBL was further recovered to be used as a substrate for the XOS production by using 95% ethanol and a total of 84.0% of xylan in the solid form. Thus, XF was successfully recovered from its original content in raw OPF. Enzymatic hydrolysis of XF took place under condition of 50 °C for 48 h using 3.0% xylan loading and 4 U/mL xylanase, releasing a total of 113 g of XOS, which consisted of xylobiose (17 g), xylotriose (2 g), xylotetraose (44 g), xylopentaose (2 g) and xylohexaose (50 g). In sum, the developed process was able to produce up to 62.5% XOS via enzymatic hydrolysis under mild process condition, and this study was able to achieve maximum utilization of oil palm waste.
Fig. 8. Mass balance for production of XOS from 1000 g of oil palm frond bagasse
Economic Potential of Xylan Extracted from Alkaline Black Liquor of Oil Palm Frond for Xylooligosaccharides Production
XOS is a preferred prebiotic, particularly in the pharmaceutical business, as it demonstrates prebiotic benefits at lower amounts than other prebiotics. It has become a motivating factor for the industry to concentrate on introducing alternative approaches for raising the efficiency of the process to fulfil the increased demand while simultaneously cutting costs. An analysis by Global Info Research (GIR) predicts that the global market for XOS will grow from 93 million USD in 2017 to 130 million USD in 2023, at a Compound Annual Growth Rate (CAGR) of approximately 5.3% (The Market Reports 2018). XOS, however, is considered to be pricey as compared to other prebiotics such as fructooligosaccharides (FOS), galactooligosaccharides (GOS), and inulin, where the market price is ranging between $5 to $10 per kilogramme (Amorim et al. 2019), while the market price for XOS is relatively high, ranging between $22 to $50 per kilogramme, with the price depending on the purity of the product (Otieno and Ahring 2012). The cost of XOS production is a big hurdle in incorporating them into daily meals; however this could be reduced by using economical substrates and efficient processes. This study was conducted by utilising renewable and abundant lignocellulosic residues as feedstock in the biorefinery process in order to carry out greener and efficient processes. The production of OPF in Malaysia is approximately 83 million metric tons (Zahari et al. 2012), in which the OPF availability is predicted to be 250 million metric tons per annum, globally. This was estimated from the ratio of Malaysia’s oil palm cultivation land (4.49 million hectares), which has been reported by the Malaysia Palm Oil Council (MPOC) (Malaysian Palm Oil Council, n.d.) to world oil palm cultivation land (13.46 million hectares), as reported by Sime Darby Plantation (Sime Darby Plantation Sustainability Report, 2014). OPF is the cheapest biomass ever reported, with a cost of only $19.4 per ton (Tan et al. 2014), owing to its abundant availability and carbohydrate enrichment. As a result, it is a better lignocellulosic feedstock in the biorefinery process for XOS production. Earlier findings conducted by other researchers mainly focused on the alkaline pretreated OPF, leaving the remaining black liquor being put to waste. The black liquor generated from the alkaline pretreatment process is often regarded as a pollutant to the environment. Hence, it is necessary to utilize the black liquor along with bringing the palm industry to achieve zero waste strategies. Thus, this study has examined the role of creating an economic value from agricultural residue, as a high production of XOS up to 62.5% was produced from ALBL of OPF.
CONCLUSIONS
- Xylan extracted from alkaline black liquor (ALBL) of oil palm fronds (OPF) offers an economical approach to biorefinery of OPF to xylooligosaccharides (XOS) by enzymatic hydrolysis.
- The total recovery of xylan and lignin, reaching up to 84% and 7.7%, respectively, was achieved under the optimal conditions of 6% NaOH (w/v), temperature of 100 °C, and extraction time at 60 min.
- The highest XOS production of up to 62.5%, containing XOS from DP range 2 to 6 was obtained via enzymatic hydrolysis.
- The study has in certain ways enhanced the current understanding of maximizing the utilization of ALBL of OPF and can be further integrated into biorefinery processes for prebiotic XOS product.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Higher Education, Malaysia through the grant provided under the grant Geran Universiti Penyelidikan (GUP-2019-016) and the Fundamental Research Grant Scheme (FRGS/1/2016/TK02/UKM/02/4).
REFERENCES CITED
Abdullah, N., and Sulaiman, F. (2013). “The oil palm wastes in Malaysia,” in: Biomass Now – Sustainable Growth and Use, pp. 75-100.
Akpinar, O., Erdogan, K., Bakir, U., and Yilmaz, L. (2010). “Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides,” LWT – Food Sci. Technol. 43, 119-125. DOI: 10.1016/j.lwt.2009.06.025
Akpınar, Ö., and Bostancı, Ș. (2009). “Xylooligosaccharide production from lignocellulosic wastes with Trichoderma longibrachiatum xylanase,” J. Food, Agric. & Environ. 7, 70-74.
Amorim, C., Silverio, S. C., Prather, K. L. J., and Rodrigues, L. R. (2019). “From
lignocellulosic residues to market: Production and commercial potential of
xylooligosaccharides,” Biotechnol. Adv. 37, article 107397 DOI:
10.1016/j.biotechadv.2019.05.003
Ariyawansha, R., Basnayake, B., Karunarathna, A., and Mowjood, M. (2018). “Extensions to Michaelis-Menten kinetic for single parameters,” Sci. Rep. 8, article 16586. DOI: 10.1038/s41598-018-34675-2
Bailey, M. J., Biely, P., and Poutanen, K. (1992). “Interlaboratory testing of methods for assay of xylanase activity,” J. Biotechnol. 23, 257-270. DOI: 10.1016/0168-1656(92)90074-J
Carrillo, I., Mendonça, R. T., Ago, M., and Rojas, O. J. (2018). “Comparative study of cellulosic components isolated from different Eucalyptus species,” Cellulose 25(2), 1011-1029. DOI: 10.1007/s10570-018-1653-2
Carvalho, A. F. A., Neto, P. de O., Silva, D. F., and Pastore, G. M. (2013). “Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis,” Food Res. Int. 51, 75-85. DOI: 10.1016/j.foodres.2012.11.021
Davani-Davari, D., Negahdaripour, M., Karimzadeh, I., Seifan, M., Mohkam, M.,
Masoumi, S. J., Berenjian, A. and Ghasemi, Y. (2019). “Prebiotics: Definition, types, sources, mechanisms, and clinical applications,” Foods 8(3), 92. DOI: 10.3390/foods8030092
de Freitas, C., Carmona, E., and Brienzo, M. (2019). “Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects,” Bioact. Carbohydr. 18, article 100184. DOI: 10.1016/j.bcdf.2019.100184
de Figueiredo, F. C., Carvalho, A. F. A., Brienzo, M., Campioni, T. S., and de Oliva-Neto, P. (2017). “Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production,” Bioresource Technol. 228, 164-170. DOI: 10.1016/j.biortech.2016.12.097
Dutta, S. K., and Chakraborty, S. (2015). “Kinetic analysis of two-phase enzymatic, hydrolysis of hemicellulose of xylan type,” Bioresource Technol. 198, 642-650. DOI: 10.1016/j.biortech.2015.09.066
Fauzi, N. A., Harun, S., and Jahim, J. (2016). “Physiochemical changes and mass balance of raw and alkaline pretreated oil palm frond: Pressed versus non-pressed sample,” Int. J. Appl. Eng. Res. 11, 9886-9893.
Fazary, A. E., Ismadji, S., and Ju, Y. H. (2010). “Studies on temperature dependent kinetics of Aspergillus awamori feruloyl esterase in water solutions,” Kinet. Catal. 51, 31-37. DOI: 10.1134/S0023158410010064
Grootaert, C., Delcour, J. A., Courtin, C. M., Broekaert, W. F., Verstraete, W., and Wiele, T. V. (2007). “Microbial metabolism and prebiotic potency of arabinoxylan oligosaccharides in the human intestine,” Trends Food Sci. Technol. 18, 64-71 DOI: 10.1016/j.tifs.2006.08.004
Hames, B., Ruiz, R., Scarlata, C., Sluiter, A., Sluiter, J., and Templeton, D. (2008). Preparation of Samples for Compositional Analysis: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42620), National Renewable Energy Laboratory, Golden, CO, USA.
Hughes, S. A., Shewry, P. R., Li, L., Gibson, G. R., Sanz, M. L., and Rastall, R. A. (2007). “In vitro fermentation by human fecal microflora of wheat arabinoxylans,” J. Agric. Food Chem. 55, 4589-4595. DOI: 10.1021/jf070293g
Kamble, R. D., and Jadhav, A. R. (2012). “Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation,” Int. J. Microbiol. 2012, 1-8. DOI: 10.1155/2012/683193
Le, B., Ngoc, A. P. T., and Yang, S. H. (2020). “Synbiotic fermented soymilk with
Weissella cibaria FB069 and xylooligosaccharides prevents proliferation in human colon cancer cells,” J. Appl. Microbiol. 128, 1486-1496. DOI: 10.1111/jam.14551
Li, H., Chen, X., Xiong, L., Luo, M., Chen, X., Wang, C., Huang, C., and Chen, X. (2019). “Stepwise enzymatic hydrolysis of alkaline oxidation treated sugarcane bagasse for the co-production of functional xylo-oligosaccharides and fermentable sugars,” Bioresource Technol. 275, 345-351. DOI: 10.1016/j.biortech.2018.12.063
Lineweaver, H., and Burk, D. (1934). “The determination of enzyme dissociation constants,” J. Am. Chem. Soc. 56, 658-666. DOI: 10.1021/ja01318a036
Luthfi, A. A. I., Manaf, S. F. A., Illias, R. M., Harun, S., Mohammad, A. W., and Jahim, J. M. (2017). “Biotechnological route for sustainable succinate production utilizing oil palm frond and kenaf as potential carbon sources,” Appl. Microbiol. Biotechnol. 101, 3055-3075. DOI: 10.1007/s00253-017-8210-z
Mäkeläinen, H., Forssten, S., Saarinen, M., Stowell, J., Rautonen, N., and Ouwehand, A. C. (2010). “Xylo-oligosaccharides enhance the growth of bifidobacteria and Bifidobacterium lactis in a simulated colon model,” Benef. Microbes 1, 81-91. DOI: 10.3920/BM2009.0025
Manaf, S. F. A., Jahim, J. M., Harun, S., and Luthfi, A. A. I. (2018). “Fractionation of oil palm fronds (OPF) hemicellulose using dilute nitric acid for fermentative production of xylitol,” Ind. Crop. Prod. 115, 6-15. DOI: 10.1016/j.indcrop.2018.01.067
Mazlan, N. A., Samad, K. A., Wan Yussof, H., Saufi, S. M., and Jahim, J. (2019). “Xylooligosaccharides from potential agricultural waste: Characterization and screening on the enzymatic hydrolysis factors,” Ind. Crops Prod. 129, 575-584. DOI: 10.1016/j.indcrop.2018.12.042
Moure, A., Gullón, P., Domínguez, H., and Parajó, J. C. (2006). “Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals,” Process Biochem. 41, 1913-1923. DOI: 10.1016/j.procbio.2006.05.011
Olaimat, A. N., Aolymat, I., Al-Holy, M., Ayyash, M., Ghoush, M. A., Al-Nabusi, A. A.,
Osaili, T., Apostolopoulus, V., Liu, S., and Shah, N. P. (2020). “The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19,” NPJ. Sci Food 4, 1-7. DOI: 10.1038/s41538-020-00078-9
Oliveira, E. E., Silva, A. E., Junior, T. N., Gomes, M. C. S., Aguiar, L. M., Marcelino, H. R., Araujo, I. B., Bayer, M. P., Ricardo, N. M. P. S., Oliveira, A. G., and Egito, E. S. T. (2010). “Xylan from corn cobs, a promising polymer for drug delivery: Production and characterization,” Bioresource Technol. 101, 5402-5406. DOI: 10.1016/j.biortech.2010.01.137
Otieno, D.O., and Ahring, B. K. (2012). “A thermochemical pretreatment process to produce xylooligosaccharides (XOS), arabinooligosaccharides (AOS) and mannooligosaccharides (MOS) from lignocellulosic biomasses,” Bioresource Technol. 112, 285-292. DOI: 10.1016/j.biortech.2012.01.162
Poletto, P., Pereira, G. N., Monteiro, C. R. M., Pereira, M. A. F., Bordignon, S. E., and de Oliveira, D. (2020). “Xylooligosaccharides: Transforming the lignocellulosic biomasses into valuable 5- carbon sugar prebiotics,” Process Biochem. 91, 352-363. DOI: 10.1016/j.procbio.2020.01.005
Price, N. C. (1985). “The determination of Km values from Lineweaver-Burk Plots,” Biochem. Educ. 95, 28184.
Reque, P. M., Pinilla, C. M. B., Gautério, G. V., Kalil, S. J., and Brandelli, A. (2019).
“Xylooligosaccharides production from wheat middlings bioprocessed with Bacillus subtilis,” Food Res Int 126, article 108673. DOI: 10.1016/j.foodres.2019.108673
Saleh, S. H., and Aziz, A. A. (2016). “Polymer characterization of cellulose and hemicellulose,” Polym. Sci. Res. Adv. Pract. Appl. Educ. Asp. 404-411.
Samanta, A. K., Jayapal, N., Jayaram, C., Roy, S., Kolte, A. P., Senani, S., and Sridhar, M. (2015). “Xylooligosaccharides as prebiotics from agricultural by-products : Production and applications,” Bioact. Carbohydrates Diet. Fibre 5, 62-71. DOI: 10.1016/j.bcdf.2014.12.003
Samanta, A. K., Kolte, A. P., Elangovan, A. V., Dhali, A., Senani, S., Sridhar, M., Suresh, K. P., Jayapal, N., Jayaram, C., and Roy, S. (2016). “Value addition of corn husks though enzymatic production of xylooligosaccharides,” Brazilian Arch. Biol. Technol. 59, 1-8. DOI: 10.1590/1678-4324-2016160078
Singh, R. D., Banerjee, J., Sasmal, S., Muir, J., and Arora, A. (2018). “High xylan recovery using two stage alkali pre-treatment process from high lignin biomass and its valorisation to xylooligosaccharides of low degree of polymerisation,” Bioresource Technol. 256, 110-117. DOI: 10.1016/j.biortech.2018.02.009
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008). Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42622), National Renewable Energy Laboratory, Golden, CO, USA.
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008). Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42619), National Renewable Energy Laboratory, Golden, CO, USA.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2012). Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42618), National Renewable Energy Laboratory, Golden, CO, USA.
Sobri, N. S. A., Harun, S., Ishak, N. S., Jahim, J. M., and Mohammad, A. W. (2019). “Enhancement of high xylan recovery from black liquor of alkaline pretreated oil palm frond and its physicochemical properties,” BioResources 14, 5400-5421. DOI: 10.15376/biores.14.3.5400-5421
Sousa, S., Pedrosa, J., Ramos, A., Ferreira, P. J., and Gamelas, J. A. F. (2016). “Surface properties of xylan and xylan derivatives measured by inverse gas chromatography,” Colloids Surfaces A Physicochem. Eng. Asp. 506, 600-606. DOI: 10.1016/j.colsurfa.2016.07.006
Sukri, S. S. M., Rahman, R. A., Md Illias, R., and Yaakob, H. (2014). “Optimization of alkaline pretreatment conditions of oil palm fronds in improving the lignocelluloses contents for reducing sugar production,” Rom. Biotechnol. Lett. 19, 9006-9018.
Sun, R. C., and Tomkinson, J. (2002). “Characterization of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw,” Carbohydr. Polym. 50, 263-271. DOI: 10.1016/S0144-8617(02)00037-1
Tan, J. P., Md. Jahim, J., Wu, T. Y., Harun, S., Kim, B. H., and Mohammad, A. W. (2014). “Insight into biomass as a renewable carbon source for the production of succinic acid and the factors affecting the metabolic flux toward higher succinate yield,” Ind. Eng. Chem. Res. 53, 16123-16134. DOI: 10.1021/ie502178j
Vazquez, M. J., Alonso, J. L., Dominguez, H., and Parajo, J. C. (2000). “Xylooligosaccharides: Manufacture and applications,” Trends Food Sci. Technol. 387-393. DOI: 10.1016/S0924-2244(01)00031-0
Wang, J., Yuan, X., Sun, B., Cao, Y., Tian, Y., and Wang, C. (2009). “On-line separation and structural characterisation of feruloylated oligosaccharides from wheat bran using HPLC-ESI-MSn,” Food Chem. 115, 1529-1541. DOI: 10.1016/j.foodchem.2009.01.058
Xiao, X., Bian, J., Peng, X. P., Xu, H., Xiao, B., and Sun, R. C. (2013). “Autohydrolysis of bamboo (Dendrocalamus giganteus Munro) culm for the production of xylo-oligosaccharides,” Bioresource Technol. 138, 63-70. DOI: 10.1016/j.biortech.2013.03.160
Xu, F., Sun, R. C., Sun, X. F., Geng, Z. C., Xiao, B., and Sun, J. X. (2004). “Analysis and characterization of acetylated sugarcane bagasse hemicelluloses,” Int. J. Polym. Anal. Charact. 9, 229-244. DOI: 10.1080/10236660490920228
Zahari, M. A. K. M., Zakaria, M. R., Ariffin, H., Mokhtar, M. N., Salihon, J., Shirai, Y., and Hassan, M. A. (2012). “Renewable sugars from oil palm frond juice as an alternative novel fermentation feedstock for value-added products,” Bioresource Technol. 110, 566-571. DOI: 10.1016/j.biortech.2012.01.119
Article submitted: November 4, 2022; Peer review completed: December 15, 2022; Revised version received: December 21, 2022; Accepted: January 2, 2023; Published: January 10, 2023.
DOI: 10.15376/biores.18.1.1525-1544
