Abstract
The unique physico-chemical properties of silver nanoparticles (Ag NPs) have opened up the opportunity for their use in many promising physico-chemical and biomedical applications. In this study, an economically feasible approach to green synthesis of silver nanoparticles (Ag NPs) is described using hemicellulose from bamboo. Simultaneously, glucose was used as the reducing agent, and distilled water was used as the reaction medium. UV-vis spectroscopy and transmission electron microscopy were used to investigate the surface plasmon behavior, morphology, and particle size of the Ag NPs. Hemicellulose alone could not reduce Ag+ to Ag0. The formation rate of the nanoparticles was accelerated when high amounts of glucose were added. Small particle sizes were obtained when high concentrations of hemicellulose were used. The stability of the Ag NPs synthesized using different concentrations of hemicellulose from 0.5 mg/mL to 8.0 mg/mL was also evaluated. Higher initial hemicellulose concentrations produced more stable Ag NPs. The average particle sizes of the NPs after storing for 160 days at 4 °C were still smaller than 10 nm when the initial hemicellulose concentrations were 6.0 and 8.0 mg/mL.
Download PDF
Full Article
Green Synthesis and Stability Evaluation of Ag Nanoparticles Using Bamboo Hemicellulose
Hong Peng,a,b,* Yang Liu,a Wenyi Peng, b Jinsheng Zhang,a and Roger Ruan a,c
The unique physico-chemical properties of silver nanoparticles (Ag NPs) have opened up the opportunity for their use in many promising physico-chemical and biomedical applications. In this study, an economically feasible approach to green synthesis of silver nanoparticles (Ag NPs) is described using hemicellulose from bamboo. Simultaneously, glucose was used as the reducing agent, and distilled water was used as the reaction medium. UV-vis spectroscopy and transmission electron microscopy were used to investigate the surface plasmon behavior, morphology, and particle size of the Ag NPs. Hemicellulose alone could not reduce Ag+ to Ag0. The formation rate of the nanoparticles was accelerated when high amounts of glucose were added. Small particle sizes were obtained when high concentrations of hemicellulose were used. The stability of the Ag NPs synthesized using different concentrations of hemicellulose from 0.5 mg/mL to 8.0 mg/mL was also evaluated. Higher initial hemicellulose concentrations produced more stable Ag NPs. The average particle sizes of the NPs after storing for 160 days at 4 °C were still smaller than 10 nm when the initial hemicellulose concentrations were 6.0 and 8.0 mg/mL.
Keywords: Hemicellulose; Ag nanoparticles; Glucose; Particle size; Stability
Contact information: a: Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang University, Nanchang, Jiangxi 330047, PR China; b: School of Materials Science and Engineering, Nanchang University, Nanchang, Jiangxi 330031, P R. China; c: Center for Biorefining and Dept. of Bioproducts and Biosystems Engineering, University of Minnesota, St. Paul, MN 55108, USA;
* Corresponding authors: penghong@ncu.edu.cn
INTRODUCTION
Silver nanoparticles (Ag NPs), which exhibit special physico-chemical properties of both nanosize- and colloidal-metal Ag particles, have been utilized in many physico-chemical and biomedical applications, such as catalyst (Gangula et al. 2011), antibacterial agent (Bindhu and Umadevi 2013; Jannoo et al. 2015), sensors (Wang et al. 2012), and lithium-ion batteries (Das and Bhattacharyya 2011). Along with such promising applications of Ag NPs, the synthesis of nanosized Ag particles is a growing research field in material and chemical sciences (He et al. 2013). Until now, various radiation, chemical, and biological methods have been developed to synthesize AgNPs (Kasthuri et al. 2009; Liu et al. 2009; Zhang et al. 2011; Jannoo et al. 2015). Upon the production of Ag NPs, the utilization of a stabilizer is essential to enhance the stability of the formed Ag NPs. Various stabilizers have been proposed, including chemical surfactants (AL-Thabaiti et al. 2008; Abd-Elaal et al. 2015), polyvinylpyrrolidone (PVP) (Wang et al. 2014; Kyrychenko et al. 2015), and organic polymers (Suber et al. 2005). However, most of these conventional agents exhibit difficulty in separating particles from stabilizers. These agents are also toxic, non-biodegradable, non-biocompatible, and they easily pollute the environment. Therefore, progress in selecting environmental-friendly and easily removed stabilizing agents are still needed.
Polysaccharides are natural polymers produced from renewable natural resources. In the last decade, much attention has been directed to the green synthesis of noble metal NPs using polysaccharides, such as starch (Cheviron et al. 2014), chitosan (Jannoo et al. 2015), and lentinan (Li et al. 2011). During the process of synthesizing Ag NPs by adding polysaccharides, it has been shown that polysaccharides act mainly as stabilizing agents to stabilize the formed Ag NPs (Peng et al. 2013). Several kinds of polysaccharides even behave as stabilizing and reducing agent at the same time (Bakar and Bakar 2008; Amin et al. 2013). It has been suggested that polysaccharides are adsorbed on silver colloid crystalline surfaces preferably via the nonbonding electrons of the hydroxyl group to prevent agglomeration of the particles (Li et al. 2011). Compared with the conventional stabilizing agents, the stabilizer of polysaccharides are non-toxic, biodegradable, biocompatible, and easily separated from the stabilizers. Synthesizing Ag NPs by using polysaccharides may be an environmental-friendly alternative to existing potentially toxic chemical and physical methods of preparing Ag NPs.
Bamboo is widely distributed in different parts of the world. Hemicellulose is an important component of bamboo that accounts for around 25% to 30% by mass (Scurlock et al. 2000). The bamboo hemicellulose mainly consists of arabinoxylans, which are rich in hydroxyl groups and exist as helical chains or random-coil chains in an aqueous solution (Peng et al. 2012). Thus, metal particles can be encapsulated by the special structure and the numerous hydroxyl groups. In addition, it is easy to remove hemicellulose from Ag NPs by using enzymatic hydrolysis when hemicellulose is used as stabilizer during preparing Ag NPs. Therefore, bamboo hemicellulose is potentially a good stabilizer suitable for green preparation of Ag NPs.
In this study, Ag+ ions were reduced to metallic Ag0 by using glucose, and the formed Ag NPs were stabilized by bamboo hemicellulose in a distilled water medium. The formed Ag NPs samples were characterized via UV-vis spectrometry and transition electron microscopy (TEM) equipped with an energy-dispersive X-ray spectrometry (EDX). Finally, the stability of Ag NPs in aqueous medium was also evaluated.
EXPERIMENTAL
Materials
Hemicellulose was obtained from bamboo (Phyllostachys pubescens Mazel) via the same separation method described in previous studies (Peng et al. 2013). The bamboo hemicellulose was used as a stabilizer and glucose was used as a reducing agent. The silver nitrate (AgNO3) and glucose used were of analytical reagent grade. Redistilled deionized water was used in all of the experiments performed. All glassware items were thoroughly cleaned and dried in an oven.
Methods
Green synthesis of AgNPs
Carefully weighed amounts of glucose and bamboo hemicellulose were placed in a 100 mL flask containing 20 mL of aqueous AgNO3 precursor solution. The resultant solution was heated in a water bath with continuous stirring. The solution was immediately cooled down to room temperature when the reaction was completed. The reaction dynamics during the synthesis of the AgNPs were systematically investigated by varying the experimental factors as follows: (i) initial glucose concentrations from 0.0 mg/mL to 3.0 mg/mL (0.004 mmol/mL of AgNO3 and 2.0 mg/mL of hemicellulose and reaction temperature of 60 °C for 4 h); (ii) hemicellulose concentrations from 0.5 mg/mL to 8.0 mg/mL (glucose and AgNO3 concentrations of 2.0 mg/mL and 0.004 mmol/mL, respectively, and reaction time of 1 h at 60 °C).
Stability evaluation of AgNPs in aqueous medium
The Ag NPs were synthesized using 2.0 mg/mL of glucose, 0.004 mmol/mL of AgNO3, and different concentrations of hemicellulose from 0.5 mg/mL to 8.0 mg/mL. The reaction temperature and heating time employed were 60 °C and 1 h, respectively. The aqueous dispersions of the Ag NPs stabilized by different amounts of hemicellulose were stored undiluted for 40 and 160 days at 4 °C in a refrigerator. The samples were then evaluated via UV-vis spectroscopy and transmission electron microscopy (TEM).
Characterization of AgNPs
The surface plasmon resonance (SPR) spectra of the Ag NPs were obtained using a LabTech 300563 UV-visible spectrophotometer. The Ag NP suspensions (suspensions synthesized using initial hemicellulose concentrations of 4.0, 6.0, and 8.0 mg/mL) after storage for 160 days at 4 °C in a refrigerator were diluted with 1.5 volumes of water before they were subjected to UV-vis analysis (solution:water = 1:1.5, mL/mL). All other Ag NP suspensions were analyzed directly using the UV-vis spectrophotometer without any dilution.
The morphology and size of the silver nanoparticles were determined by TEM using a JEM 2010 instrument at an accelerating voltage of 200 kV. The sample for TEM analysis was prepared by placing a drop of silver nanoparticle solution onto a carbon–coated copper grid, followed by water evaporation in air at room temperature. The presence of elemental silver in the nanoparticles was analyzed using a TEM equipped with an energy-dispersive X-ray (EDX) spectrum.
RESULTS AND DISCUSSION
Effect of Initial Glucose Concentration on Formation of Ag NPs
Figure 1a shows the Ag NP dispersions synthesized by varying the initial glucose concentration from 0.0 mg/mL to 3.0 mg/mL, while keeping initial hemicellulose concentration (2.0 mg/mL), AgNO3 concentration (0.004 mmol/mL), reaction time (4 h), and temperature (60 °C) constant. The preliminary detection of the Ag NPs was performed by visually observing the color changes of the reaction solutions. This procedure was conducted according to the excitation of surface plasmon resonance (SPR) in the metal nanoparticles (Natarajan et al. 2010). Therefore, the reduction of Ag+ to Ag0 was evident from the color change. When no glucose was added, the solution appeared colorless after 4 h of reaction, suggesting that there were no Ag NPs formed (Fig. 1a). However, the color of the solutions changed from light yellow, yellow, andred to deep red with increased concentrations of glucose from 0.5 mg/mL to 3.0 mg/mL, indicating the formation of Ag NPs. The results imply that the bamboo hemicellulose alone could not reduce the Ag+ to Ag0 without the addition of glucose under the conditions mentioned above. The results also demonstrate that glucose played an important role for Ag NPs formation in the presence of hemicellulose since no color change was observed when only hemicellulose was added. The results are in accordance with the previous observation (Peng et al. 2013). Glucose probably acted as a reducing agent, resulting in the reduction of Ag+ to Ag0. Figure 1b shows the UV-vis absorption spectra of the Ag NP solutions prepared using different initial glucose concentrations. The absorption peaks were centered at 418 nm. It is well known that typical Ag NPs exhibit characteristic SPR between 390 and 450 nm. Simultaneously, SPR absorbance of Ag NPs is sensitive to the nature, size, and shape of particles present in solution. The observed maximum absorption at 418 nm further confirmed the presence of Ag NPs in the prepared solutions. The absorption band intensity was enhanced with the increase of initial glucose concentrations from 0.5 mg/mL to 3.0 mg/mL, indicating that the concentration of Ag NPs increased. The results suggest that a high initial glucose concentration exposed more aldehyde groups to electrostatically interact with Ag+, which resulted in a high reduction rate of Ag+ to Ag0 by glucose.
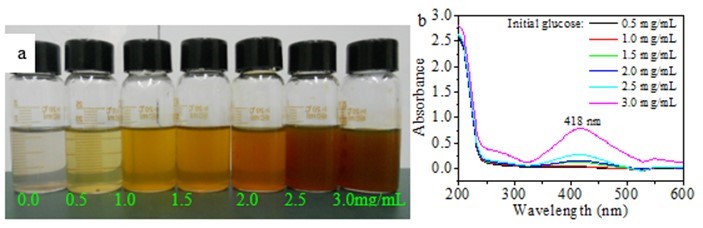
Fig. 1. (a) Image of Ag nanoparticles obtained using different initial amounts of glucose and (b) corresponding UV-vis spectra of (a) (synthesis conditions: 2.0 mg/mL of hemicellulose and 0.004 mmol/mL of AgNO3 heated for 4 h at 60 °C)
Effect of Initial Hemicellulose Added on Formation of Ag NPs
Figure 2a shows the Ag NP dispersions synthesized using different initial hemicellulose concentrations, 2.0 mg/mL of glucose, 0.004 mmol/mL of AgNO3, and reaction time and temperature of 1 h and 60 °C, respectively.The color of the sample changed from yellow to deep red with increased concentrations of hemicellulose used. Differences in color of the samples were attributed to the different sizes, shapes, and numbers of particles. As shown in Fig. 2b, the sample solutions obtained using different initial hemicellulose concentrations showed similar SPR with maximum absorption peak in the range of 416 nm to 424 nm. Blue shift occurred when the initial hemicellulose concentration was increased from 0.5 mg/mL to 8.0 mg/mL.
According to Kelly et al. (2003), the SPR properties of Ag metal particles are extremely sensitive to size, shape, environment, and interactions of particles. Thus, it is proposed that the particle size decreased when high hemicellulose concentration was employed. The intensity of the peaks increased with the increase of initial hemicellulose dosages from 0.5 mg/mL to 8.0 mg/mL. The results imply that higher hemicellulose concentration accelerated the formation of Ag NPs, resulting in the corresponding increase of particle number.
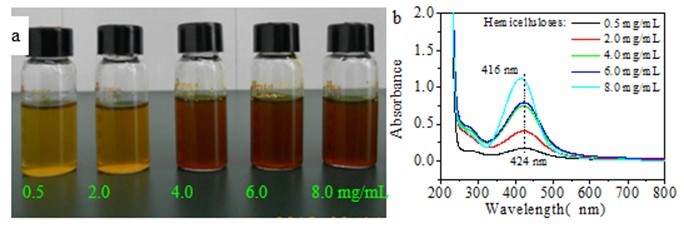
Fig. 2. (a) Image of Ag nanoparticles obtained using different initial amounts of hemicellulose and (b) corresponding UV-vis spectra of (a) (synthesis conditions: 2.0 mg/mL of glucose and 0.004 mmol/mL of AgNO3 heated for 1 h at 60 °C)
The sizes and shapes of the Ag NPs formed were visualized via TEM. Figures 3a through e show the typical TEM images of the Ag NPs synthesized using different concentrations of hemicellulose from 0.5 mg/mL to 8.0 mg/mL. The morphology of the AgNPs showed that most of the particles were spherical in shape. Most of the particles were nearly spherical when the initial hemicellulose concentration was 0.5 mg/mL. The edges of the particles were lighter than the centers, suggesting that the hemicellulose capped the Ag NPs and adhered to the surfaces of the Ag NPs (Jagtap and Bapat 2013). Small and monodispersed spherical particles formed when the hemicellulose concentration used was 2.0 mg/mL (Fig. 3b). The Ag NPs were smaller and further well dispersed when the initial hemicellulose concentrations used were higher than 2.0 mg/mL (Figs. 3c through e). The results suggest that higher amounts of hemicelluloses produced smaller Ag particles. Furthermore, the bamboo hemicellulose stabilized the Ag particles, and growth of the particles was inhibited to some extent.
A high-resolution-TEM (HR-TEM) image of a single Ag nanoparticle shows that the synthesized Ag NPs were spherical and consisted of well-ordered single crystals with distinct lattice fringes (Fig. 3f). The presence of clear and uniform lattice fringes confirmed that the spherical particles were highly crystalline. The HR-TEM image also displayed a d spacing of 2.39 Å, which corresponds to the lattice spacing of the {110} plane of Ag (Taleb et al. 1997; Li et al. 2011), which is characteristic of one of the three most intense d spacings, namely, 2.36, 2.04, and 1.44 Å reported for zero-valent Ag (Abdel-Halim and Al-Deyab 2011; Chulovskaya et al. 2009). Figure 3g shows the selected area electron diffraction (SAED) pattern of the Ag NPs. The crystalline structure of the particles was confirmed by the SAED pattern, which exhibited bright circular spots that correspond to the (111), (200), (220), and (311) reflection planes. The diffraction rings also suggest that the particles were crystalline.
Figure 3h presents the typical EDX spectrum of the Ag NPs. The presence of the elemental Ag signal was confirmed in the EDX spectrum, suggesting that Ag NPs were formed. The metallic Ag nanocrystals exhibited a typical optical absorption peak at approximately 3.010 keV because of SPR. The spectral signals observed for C and O indicate that the organic moieties (i.e., from hemicelluloses or glucose) were observed on the surface or in the vicinity of the metallic Ag NPs. The spectral signals for sodium, silicon, and chlorine, probably came from the hemicellulose which was extracted from bamboo powder using NaOH (Peng et al. 2013). The presence of copper (Cu) signal was due to the Cu grid used.
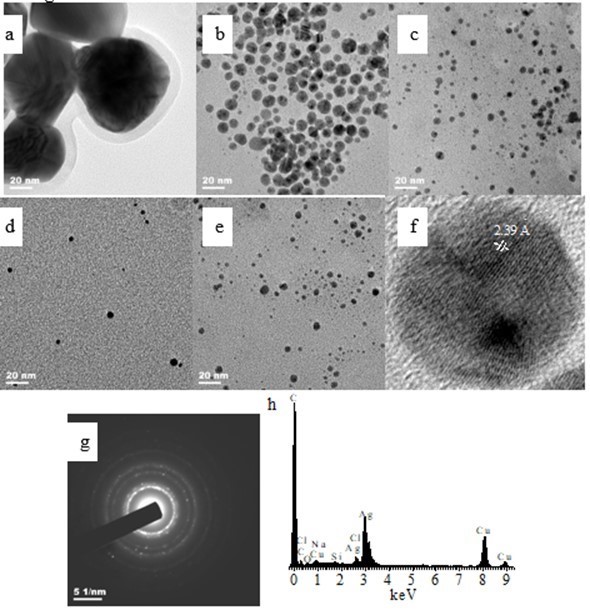
Fig. 3. TEM images of Ag NPs synthesized using: (a) 0.5 mg/mL of hemicellulose; (b) 2.0 mg/mL of hemicellulose; (c) 4.0 mg/mL of hemicellulose; (d) 6.0 mg/mL of hemicellulose; and (e) 8.0 mg/mL of hemicellulose (synthesis conditions: 2.0 mg/mL of glucose and 0.004 mmol/mL of AgNO3 heated for 1 h at 60 °C) and (f) typical HR-TEM image of (b); (g) typical SAED pattern of AgNPs; and (h) typical EDX spectrum of AgNPs of (b)
Figure 4 shows the histograms of particle size distribution recorded from silver nanoparticle solutions prepared using different concentrations of hemicellulose. The stabilized Ag particles produced using bamboo hemicellulose and glucose were indeed nanoparticulate with diameters ranging from 1.6 nm to 100 nm. A wide range of particle sizes from 3.33 nm to 100.00 nm was observed when the initial hemicellulose concentration was only 0.5 mg/mL (Fig. 4a). The particle sizes ranged from 3.33 nm to 15.83 nm, 1.67 nm to 12.50 nm, and 1.67 nm to 9.17 nm when the initial hemicellulose amounts used were 2.0, 4.0, 6.0, and 8.0 mg/mL, respectively (Figs. 4b–e). Only 3.82% of the particles with sizes smaller than 10 nm were present when the hemicellulose concentration was only 0.5 mg/mL (Fig. 4f). The percentage of the particles with sizes smaller than 10 nm significantly increased to 66.48% when the initial hemicellulose concentration was 2.0 mg/mL,and this value reached 100% after 6.0 and 8.0 mg/mL of hemicelluloses were added (Fig. 4f). The average particle sizes, which were measured from the TEM images, were 54.63, 8.43, 4.67, 4.38, and 3.45 nm when the initial hemicellulose concentrations were 0.5, 2.0, 4.0, 6.0, and 8.0 mg/mL, respectively. The average particle size decreased from 54.63 nm to 3.45 nm when the initial hemicellulose concentration was increased from 0.5 mg/mL to 8.0 mg/mL (Fig. 4f). Small-sized Ag nanoparticles were obtained when high amounts of initial bamboo hemicellulose were used. This phenomenon may be attributed to the high stability of Ag NPs observed with increased hemicellulose amounts, which resulted in a smaller particle size. These results further imply that the bamboo hemicellulose behaved as a stabilizer during the synthesis of Ag NPs.
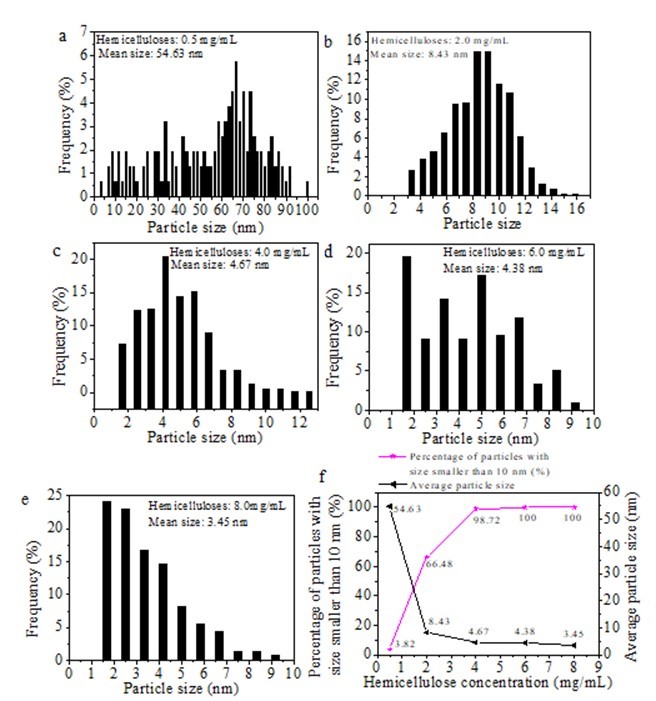
Fig. 4. (a-e) Histograms of particle size distribution of Ag NPs prepared using different initial amounts of hemicellulose and (f) percentage of particles with sizes smaller than 10 nm and average particle size (synthesis conditions: 2.0 mg/mL glucose and 0.004 mmol/mL AgNO3 heated for 1 h at 60 °C)
Evaluation of Stability of Ag NPs
The aqueous suspensions of AgNPs synthesized using different concentrations of hemicellulose were stored at 4 °C for 40 and 160 days in a refrigerator to evaluate the stability of the synthesized AgNPs. Figure 5a shows the UV-vis spectra of the Ag NPs obtained after 40 days of storage at 4 °C. All the spectra exhibited characteristic surface plasmon band for Ag NPs. The UV-vis spectra of the Ag NPs exhibited similar maximum absorption peak at about 405 nm with a shoulder at approximately 540 nm when the initial hemicellulose concentrations used were 0.5 and 2.0 mg/mL. According to the Mie theory, spherical nanoparticles exhibit only one SPR absorption peak (Mie 1908). The presence of additional peaks at about 540 nm corresponds to the dipole and quadrupole modes of Ag because of the non-spherical nanoparticles (Nadagouda et al. 2014). Contrary to the isotropic (spherical) particles that were present in the freshly prepared aqueous suspensions (Fig. 2b), an anisotropic (non-spherical) feature was observed after 40 days of storage at 4 °C. The other three samples synthesized using initial hemicellulose concentrations higher than 2.0 mg/mL and after being stored for 40 days at 4 °C exhibited almost a similar maximum absorption peak at about 430 nm, and no shoulders were observed. The result implies that the Ag NPs synthesized using high initial bamboo hemicellulose amounts were greatly stabilized by the hemicellulose. Figure 5b presentsthe UV spectra of the synthesized AgNPs after storing them in a refrigerator for 160 days at 4 °C. Compared with the spectra of corresponding Ag NPs given in Fig. 5a, the shapes of the spectra of the different products synthesized did not significantly change except that for the particle synthesized using 0.5 mg/mL hemicellulose. The difference in the UV-vis spectra obtained may offer information on the role of bamboo hemicellulose on the green synthesis of Ag NPs. The stability of the Ag NPs was greatly enhanced by using high concentrations of bamboo hemicelluloses, and some Ag NPs agglomerated when low amounts of hemicellulose were used.
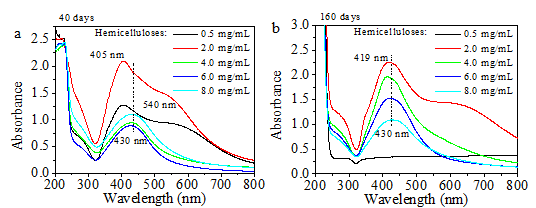
Fig. 5. UV-vis spectra of Ag NPs obtained after storage in a refrigerator at 4 °C (synthesis conditions: 2.0 mg/mL glucose, 0.004 mmol/mL AgNO3, and 0.5 mg/mL to 8.0 mg/mL of hemicellulose heated for 1 h at 60 °C): (a) after 40 days of storage; (b) after 160 days of storage
Figure 6 shows the representative TEM images of the Ag NPs obtained after 40 days of storing them in a refrigerator at 4 °C. The experimental conditions in preparing the Ag NPs were as follows: 2.0 mg/mL of hemicellulose, 0.004 mmol/mL of AgNO3, 2.0 mg/mL of glucose, temperature of 60 °C, and time of 1 h. As shown in Fig. 6a, the spherical particles exhibited irregular nanoparticle sizes. Furthermore, new particles with hexagonal, pentagonal, and elongated shapes were also observed compared with the freshly synthesized particles shown in Fig. 3b (circled in red color in Fig. 6a). The TEM results are in accordance with the UV-vis analysis results shown in Fig. 5a. This result further confirms that new particles with non-spherical shape were formed. The mechanism of the formation of hexagonal, pentagonal, and elongated shapes should be investigated further. The average diameter of the NPs was 19.97 nm (Fig. 6b), and the average particle size of the Ag NPs greatly increased from 8.43 nm (not stored for 40days at 4 °C, as shown in Fig. 4b) to 19.97 nm after they were stored for 40 days at 4 °C. This finding suggests that the Ag NPs were not stable in the solution containing 2.0 mg/mL of bamboo hemicellulose. Compared with the histogram of the particle size distribution of Ag NPs that were not stored in a refrigerator for 40 days at 4 °C (Fig. 4b), the histogram of the particle size distribution of the Ag NPs after stored for 40 days was greatly broadened (Fig. 6b), ranging from 2.7 nm to 75.0 nm.
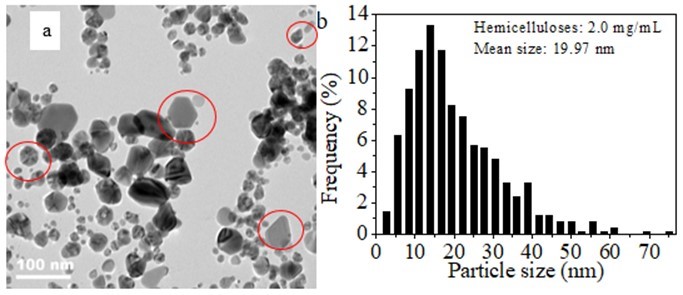
Fig. 6. (a) RepresentativeTEM image of Ag NPs after 40 days of storage in a refrigerator at 4 °C and (b) corresponding histogram of particle size distribution (synthesis conditions: 2.0 mg/mL of hemicellulose, 0.004 mmol/mL of AgNO3, and 2.0 mg/mL of glucose heated for 1 h at 60 °C)
Figure 7 shows the TEM image and particle size distributions of the various AgNPs obtained after storing them in a refrigerator at 4 °C for 160 days. Compared with the narrow particle size distribution (ranging from 3.33 nm to 15.83 nm) of the freshly synthesized AgNPs using 2.0 mg/mL hemicellulose (Figs. 3b and 4b), the particle size distribution became broader after the same particle solution was stored for 160 days at 4 °C, and its distribution ranged from 4.5 nm to 72.7 nm (Figs. 7a and b). When 4.0 mg/mL of initial hemicellulose was used to synthesize the AgNPs, the particles were nearly spherical although the obtained AgNP suspension had been stored in a refrigerator at 4 °C for 160 days. The particle size observed was between 30 and 70 nm with an average particle size of 11.78 nm (Figs. 7c and d). Figures 7e and f show the TEM image and the corresponding particle size distribution of the Ag NPs obtained using 6.0 mg/mL hemicellulose after storing them for 160 days at 4 °C. The particle size observed was around 3 nm to 29 nm with an average value of 9.14 nm. The particles synthesized using 8.0 mg/mL hemicellulose exhibited the highest stability. Compared with Figs. 7a through f, the most uniform Ag nanoparticle shape and size were observed in Fig. 7g. The average particle size ranged from 3.0 nm to 21.2 nm with an average size of 7.86 nm (Fig. 7h). Obvious aggregation occurred to form large particles when the hemicellulose concentration was only 0.5 mg/mL, as shown in Fig. 7i. Although a few single spherical particles were observed, the particles were greatly aggregated together that the average particle size was not determined. At the same time, after 160 days of storage at 4 °C, the particles with sizes smaller than 10 nm accounted for 24.89%, 50.51%, 33.51%, and 67.75%, respectively, when the various Ag NPs were synthesized using 2.0, 4.0, 6.0, and 8.0 mg/mL bamboo hemicelluloses, respectively (Fig. 7j). The percentage of all the particles smaller than 10 nmdecreased compared withthat of the freshly prepared Ag particles (Fig. 4f). Furthermore, large amounts of Ag particles with sizes smaller than 10 nm remained when initial hemicellulose of 8.0 mg/mL was used. Simultaneously, the average particle sizes of Ag NPs decreased with the increase of initial amounts of hemicellulose (Fig. 7j). Low concentrations of hemicellulose caused large aggregations of AgNPs that resulted in the formation of large Ag particles after storage in a refrigerator at 4 °C for 160 days. High amounts of hemicellulose caused a slow aggregation of AgNPs. These results further demonstrate that the bamboo hemicellulose was responsible for the stability of the synthesized Ag NPs.
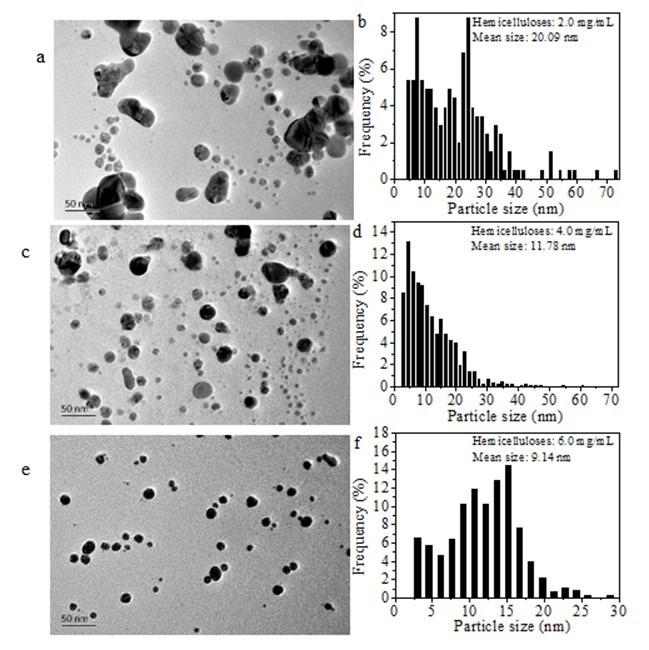
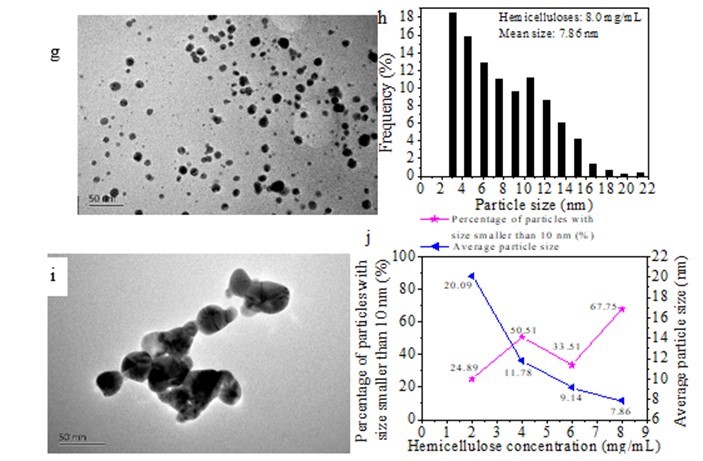
Fig. 7. TEM images andhistograms of particle size distribution of Ag NPs after 160 days of storage in a refrigerator at 4 °C (0.004 mmol/mL of AgNO3, 2.0 mg/mL of glucose, temperature of 60 °C, time of 1 h, and initial hemicellulose amounts ranging from 0.5 to 8.0 mg/mL): (a) and (b) 2.0 mg/mL of hemicellulose; (c) and (d) 4.0 mg/mL of hemicellulose; (e) and (f) 6.0 mg/mL of hemicellulose; (g) and (h) 8.0 mg/mL of hemicellulose; (i) 0.5 mg/mL of hemicellulose; (j) percentage of particles with sizes smaller than 10 nm and average particle size
The solutions of the dispersed Ag nanoparticles synthesized using bamboo hemicellulose were not highly stable, and they exhibited signs of aggregation after storage in a refrigerator at 4 °C for 160 days. The study conducted by Amin et al. (2013) showed that Ag nanoparticles were produced (5 nm to 20 nm) using arabinoxylans from ispaghula (Plantago ovata) seed husk and these nanoparticles were stable for more than 2 years; in the cited study arabinoxylans behaved as a reducing and stabilizing agent. In the present study, the bamboo hemicellulose (Phyllostachys pubescens Mazel), which was extracted using NaOH, mainly consists of arabinoxylans (Peng et al. 2012). Although both stabilizers were arabinoxylans, the arabinoxylans obtained from ispaghula (Plantago ovata) seed husk by Amin et al. (2013) were mainly water-soluble, whereas the arabinoxylans used in the present study were alkali-soluble and insoluble in neutral water. Thus, a possible reason for the lower stability exhibited by the Ag NPs synthesized in the present study was the lower solubility of the bamboo hemicellulose in neutral water. Therefore, the solubility of the alkali-extracted bamboo hemicellulose in neutral water should be modified.
Illustration of Proposed Mechanism of Ag NPs Formation
The possible formation mechanism of Ag NPs is given in Fig. 8. According to the authors’ earlier study, the bamboo hemicellulose mainly consists of arabinoxylans with 4-O-methyl-α-D-glucuronic acid as side chains (Peng et al. 2012). Thus, the bamboo hemicellulse is rich in hydroxyl groups, and also contains a certain nember of carboxylate groups. On the other hand, the oxygen atom of both hydroxyl group and carboxylate group has a lone pair of electrons; thus both the hydroxyl groups and carboxylate groups from hemicelluose exhibit electronegativity, making them possible to complex with Ag+ ions to form complexation compounds in water medium (Raveendran et al. 2003). Subsequently, the Ag+ ions were reduced to Ag0 by the reducing hemiacetal groups of glucose. Meanwhile, the glucose was oxided to form gluconic acid. Because its high unstability, the elemental silver aggregated together immediately through nucleation, resulting in the formation of Ag NPs. Since the oxygen atoms of hydroxyl groups and carboxylate groups from hemicellulose molecules and gluconic acid presented strong electronegativity, there was a non-covalent interaction between the hemicellulose molecules and Ag NPs, and also there was a non-covalent interaction between the formed gluconic acid and Ag NPs. Furthermore, these interactions were much stronger than that among Ag NPs themselves (Li et al. 2011). As a result, the excessive growth of Ag NPs was prevented by the hemicelluloses and gluconic acid, leading to the production of silver particles in nanometer size. When higher concentrations of hemicelluloses were used, the initially formed Ag NPs were capped by more electronegative hydroxyl groups and carboxylate groups of hemicelluloses. It was further noticed that these Ag NPs capped by much more hydroxyl groups and carboxylate groups had less chance to contact with each other to form a bigger one. Thus, smaller nanoparticle size and narrow size distribution occured when higher concentration of hemicellulose was used (Figs. 3 and 4), and also the Ag NPs prepared with higher concentration of hemicellulose were more stable (Fig. 7).
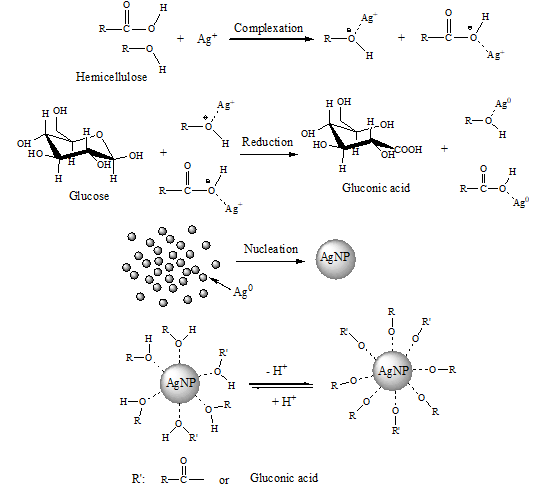
Fig. 8. Proposed mechanism of Ag NPs formation using hemicelluloses as stabilizer and glucose as reducer
CONCLUSIONS
- Spherical Ag nanoparticles were successfullysynthesized using bamboo hemicellulose as a stabilizer and glucose as a reducing agent in aqueous medium. Hemicellulose alone could not reduce Ag+ to Ag0. Addition of high amounts of glucose stimulated the formation of the Ag NPs.
- High amounts of hemicellulose resulted in narrow particle size distribution and small average particle size. Both initial glucose and hemicellulose concentrations affected the formation rate of Ag NPs.
- Adding higher amounts of hemicellulose produced particles that exhibited stronger stability in an aqueous medium. The method proposed is an environmentally safe method for the synthesis of Ag nanoparticles.
ACKNOWLEDGMENTS
The authors are grateful for financial support of the Natural Science Foundation of China (21306076), of the postdoctoral research project of China (2013M540537), of the postdoctoral research project of Jiangxi Province of China (2013KY41), and of the Natural Science Foundation of Jiangxi Province (20122BAB204001, 20151BBG70049).
REFERENCES CITED
Abd-Elaal, A. A., Tawfik, S. M., and Shaban, S. M. (2015). “Simple one step synthesis of nonionic dithiol surfactants and their self-assembling with silver nanoparticles: Characterization, surface properties, biological activity,” Appl. Surf. Sci. 342, 144-153. DOI: 10.1016/j.apsusc.2015.03.038
Abdel-Halim, E. E., and Al-Deyab, S. S. (2011). “Utilization of hydroxypropyl cellulose for green and efficient synthesis of silver nanoparticles,” Carbohydr. Polym. 86(4), 1615-1622. DOI: 10.1016/j.carbpol.2011.06.072
AL-Thabaiti, S. A., Al-Nowaiser, F. M., Obaid, A. Y., Al-Youbi, A. O., and Khan, Z. (2008). “Formation and characterization of surfactant stabilized silver nanoparticles: A kinetic study,” Colloid. Surf. B: Biointerfaces 67(2), 230-237. DOI: 10.1016/j.colsurfb.2008.08.022
Amin, M., Iram, F., Iqbal, M. S., Saeed, M. Z., Raza, M., and Alam, S.(2013). “Arabinoxylan-mediated synthesis of gold and silver nanoparticles having exceptional high stability,”Carbohydr. Polym. 92(2), 1896-1900. DOI: 10.1016/j.carbpol.2012.11.056
Bakar, A., and Bakar, M. B. (2008). “Preparation of chitosan-gold nanoparticles: Part 2, the role of chitosan,” Indo. J. Chem. 8, 320-326.
Bindhu, M. R., and Umadevi, M. (2013).“Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity,” Spectrochim.Acta A 101, 184-190. DOI : 10.1016/j.saa.2012.09.031
Cheviron, P., Gouanvé, F., and Espuche, E. (2014). “Green synthesis of colloid silver nanoparticles and resulting biodegradable starch/silver nanocomposites,” Carbohydr. Polym. 108, 291-298. DOI: 10.1016/j.carbpol.2014.02.059
Chulovskaya, S. A., Garas’ko, E. V., and Parfenyuk, V. I. (2009). “Electrochemical preparation and properties of ultradisperse silver powder,” Russ. J. Appl. Chem. 82(8), 1396-1400. DOI: 10.1134/S107042720908014X
Das, S. K., and Bhattacharyya, A. J. (2011). “Electrochemical performance of mixed crystallographic phase nanotebes and nanosheets of titania and titania-carbon/silver composites for lithium-ion batteries,” Mater. Chem. Phys. 130(1-2), 569-576. DOI: 10.1016/j.matchemphys.2011.07.026
Gangula, A., Podila, R., Ramakrishna, M., Karanam, L., Janardhana, C., and Rao, A. M. (2011). “Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides,” Langmuir27(24), 15268-15274. DOI: 10.1021/la2034559
He, D., Ikeda-Ohno, A., Boland, D. D., and Waite, T. D. (2013). “Synthesis and characterization of antibacterial silver nanoparticle-impregnated rice husks and rice husk ash,” Environ. Sci. Technol. 47(10), 5276-5284. DOI: 10.1021/es303890y
Jagtap, U. B., and Bapat, V. A. (2013). “Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. Seed extract and its antibacterial activity,” Ind. Crop. Prod. 46, 132-137. DOI: 10.1016/j.indcrop.2013.01.019
Jannoo, K., Teerapatsakul, C., Punyanut, A., and Pasanphan, W. (2015). “Electron bean assisted synthesis of silver nanoparticle in chitosan stabilizer: Preparation, stability and inhibition of building fungi studies,” Radiat. Phys. Chem. 112, 177-188. DOI: 10.1016/j.radphyschem.2015.03.035
Kasthuri, J., Veerapandian, S., and Rajendiran, N. (2009). “Biological synthesis of silver and gold nanoparticles using apiin as reducing agent,” Colloid. Surf. B: Biointerfaces 68(1), 55-60. DOI: 10.1016/j.colsurfb.2008.09.021
Kelly, K. L., Coronado, E., Zhao, L. L., and Schatz, G. C. (2003). “The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment,” J. Phys. Chem. B, 107(3), 668-677.
Kyrychenko, A., Korsun, O. M., Gubin, I. I., Kovalenko, S. M., and Kalugin, O. N. (2015). “Atomistic simulations of coating of silver nanoparticles with poly(vinylpyrrolidone) oligomers: Effect of oligomer chain length,” J. Phys. Chem. C 119, 7888-7899. DOI: 10.1021/jp510369a
Li, S., Zhang, Y. Y., Xu, X. J., and Zhang, L. N. (2011). “Triple helical polysaccharide-induced good dispersion of silver nanoparticles in water,” Biomacromolecules12(8), 2864-2871. DOI: 10.1021/bm2001439
Liu, Y. S., Chen, S. M., Zhong, L., and Wu, G. Z. (2009). “Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation,” Radiat. Phys. Chem. 78(4), 251-255. DOI: 10.1016/j.radphyschem.2009.01.003
Mie, G. (1908). “Beitrage zur optic trüber Medien, speziel kolloidaler Metallosungen,” Ann. Phys. 25, 377-445. DOI: 10.1002/andp.19083300302
Nadagouda, M. N., Iyanna, N., Lalley, J., Han, C., Dionysiou, D. D., andVarma, R. S. (2014). “Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate, and turmeric extracts,” Sustain.Chem. Eng. 2(7), 1717-1723. DOI: 10.1021/sc500237k
Natarajan, K., Selvaraj, S., and Murty, V. R. (2010). “Microbial production of silver nanoparticle,” Dig. J. Nanomater. Bios. 5, 135-140.
Peng, H., Yang, A. S., andXiong, J. H. (2013). “Green, microwave-assisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium,” Carbohydr. Polym. 91(1), 348-355. DOI: 10.1016/j.carbpol.2012.08.073
Peng, H., Wang, N., Hu, Z. R., Yu, Z. P, Liu, Y. H., Zhang, J. S., and Ruan, R. (2012). “Physicochemical characterization of hemicelluloses from bamboo (Phyllostachys pubescens Mazel) stem,” Ind. Crop. Prod. 37(1), 41-50. DOI: 10.1016/j.indcrop.2011.11.031
Raveendran, P., Fu, J., and Wallen, S. L. (2003). “Completely “green” synthesis and stabilization of metal nanoparticles,” J. Am. Chem. Soc. 125(46), 13940-13941.
Scurlock, J. M. O., Dayton, D. C., and Hames, B. (2000). “Bamboo: An overlooked biomass resource?,” Biomass Bioenerg. 19(4), 229-244. DOI: 10.1016/S0961-9534(00)00038-6
Suber, L., Sondi, I., Matijević, E., and Goia, D. V. (2005). “Preparation and the mechanisms of formation of silver particles of different morphologies in homogeneous solutions,” J. Colloid. Interf. Sci. 288(2), 489-495. DOI: 10.1016/j.jcis.2005.03.017
Taleb, A., Petit, C., and Pileni, M. P. (1997). “Synthesis of highly monodisperse silver nanoparticles from AOT reverse micelles: A way to 2D and 3D self-organization,” Chem. Mater. 9(4), 950-959. DOI: 10.1021/cm960513y
Wang, D. J., Ge, L. Q., He, J. Z., Zhang, W., Jaisi, D. P., and Zhou, D. M. (2014). “Hyperexponential and nonmonotonic retention of polyvinylpyrrolidone-coated silver nanoparticles in an ultisol,” J. Contam. Hydrol. 164, 35-48. DOI: 10.1016/j.jconhyd.2014.05.007
Wang, G. L., Zhu, X. Y., Jiao, H. J., Dong, Y. M., Wu, X. M., and Li, Z. J. (2012). “Oxidative etching-aggregation of silver nanoparticles by melamine and electron acceptors: An innovative route toward ultrasensitive and versatile functional colorimetric sensors,” Anal. Chim. Acta747, 92-98. DOI: 10.1016/j.aca.2012.08.019
Zhang, Q., Li, N., Goebl, J., Lu, Z. D., and Yin, Y. D. (2011). “A systematic study of the synthesis of silver nanoparticles: Is citrate a “magic” reagent?,” J. Am. Chem. Soc.133(46), 18931-18939. DOI: 10.1021/ja2080345
Article submitted: August21, 2015; Peer review completed: October 5, 2015; Revised version received: October 26, 2015; Accepted: November 2, 2015; Published: November 17, 2015.
DOI: 10.15376/biores.11.1.385-399
