Abstract
Xylan is the primary hemicellulose in most hardwood species, especially in birch. Research has highlighted the exploitation of xylans as a strength-enhancing additive to paper due to the current trend for the effective utilization of biomass. In this paper, a new pulping process was proposed, which involved the extraction of xylan prior to pulping, followed by the re-adsorption of the pulp after the final stage in the digester, followed by a suitable bleaching process. The aim of this work was to bleach hardwood kraft pulp (brown pulp) with adsorbed birch xylan via hydrogen peroxide and study the effect of the bleaching parameters on the paper properties. The results showed that the optical properties of paper decreased; however, the mechanical properties increased after the brown pulp adsorbed birch xylan. During the bleaching process, better mechanical properties were obtained with shorter bleaching times and lower bleaching temperatures, initial pHs, and MgSO4 dosages. However, the optical properties were improved as the bleaching time, temperature, initial pH, and MgSO4 dosage were increased. The adsorption of birch xylan could effectively modify the mechanical properties of paper made from brown pulp under various phases.
Download PDF
Full Article
H2O2 Bleaching of Brown Pulp with Adsorbed Xylan and Its Modifying Effects on the Mechanical Properties of Paper
Xue Zhang,a Lizi Li,b,c,* Junfei Tian,b Nanhua Li,c Leigang Zuo,c Luming Yan,c Jiang He,c and Xianyong Du c
Xylan is the primary hemicellulose in most hardwood species, especially in birch. Research has highlighted the exploitation of xylans as a strength-enhancing additive to paper due to the current trend for the effective utilization of biomass. In this paper, a new pulping process was proposed, which involved the extraction of xylan prior to pulping, followed by the re-adsorption of the pulp after the final stage in the digester, followed by a suitable bleaching process. The aim of this work was to bleach hardwood kraft pulp (brown pulp) with adsorbed birch xylan via hydrogen peroxide and study the effect of the bleaching parameters on the paper properties. The results showed that the optical properties of paper decreased; however, the mechanical properties increased after the brown pulp adsorbed birch xylan. During the bleaching process, better mechanical properties were obtained with shorter bleaching times and lower bleaching temperatures, initial pHs, and MgSO4 dosages. However, the optical properties were improved as the bleaching time, temperature, initial pH, and MgSO4 dosage were increased. The adsorption of birch xylan could effectively modify the mechanical properties of paper made from brown pulp under various phases.
Keywords: Hydrogen peroxide; Brown pulp; Birch xylan; Mechanical properties
Contact information: a: China National Pulp and Paper Research Institute Co. Ltd., Beijing 100102 China; b: State Key Laboratory of Pulp and Paper Engineering, School of Light Industry and Engineering, South China University of Technology, Guangzhou, Guangdong Province 510640 China; c: Zhejiang Kan Specialties Material Co., Ltd., Lishui, Zhejiang Province 323300 China;
* Corresponding author: lilizi1986@gmail.com
INTRODUCTION
As the primary raw material in the pulping and papermaking industry, the cell wall of wood is a complex hierarchical composite consisting primarily of rigid cellulose micro fibrils (40%) embedded in an amorphous matrix of hemicelluloses (30%) associated with lignins (25%) (Moreira et al. 2020; Zhu et al. 2020). Of these components, hemicelluloses are heteropolysaccharides represent the second most abundant biopolymer on Earth (Shen et al. 2021). The effect of the hemicellulose content of the pulp has been discussed by numerous authors, and it has been suggested that hemicelluloses increase the strength of the fiber-fiber joint by either increasing the swelling capacity of the fiber or acting as a “glue” between the fibers (Kaschuk and Frollini 2018). Xylan is the primary hemicellulose in birch, eucalyptus, and most other hardwood species. Figure 1a shows a representative formula for O-acetyl-(4-O-methylglucurono)-xylan, which is the primary hemicellulose in hardwoods, making up 27% of the dry mass of birch wood (Ban and Heiningen 2011). However, during kraft cooking, a large portion of the hemicelluloses are dissolved in the cooking liquor, which not only decreases the yield but also has a negative effect on the quality of the manufactured pulp (Magaton et al. 2011).
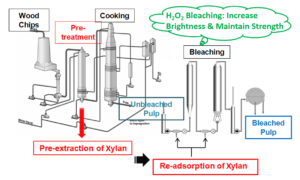
In the current trend for the greater utilization of biomass, the pre-isolation and utilization of xylan in cellulose fiber modification has gained a lot of attention (Wedin et al 2013; Corradini et al. 2018). Some studies have shown that xylan has considerable potential as an additive for the improvement of various pulp properties, e.g., tensile strength, beatability, and resistance to hornification (Testova et al. 2014; Budiarti et al. 2020). Accordingly, a new pulping process was proposed in this study, which involves the extraction of xylan prior to pulping, which is followed by the re-adsorption of xylan by the pulp before the bleaching process. The schematic diagram outlining the newly proposed pulping process is shown in Fig. 1b.
Fig. 1. (a) Representative formula of O-acetyl-(4-O-methylglucurono)-xylan; and (b) Schematic diagram of the newly proposed pulping process
Chlorine has been the most common delignifying chemical in chemical pulp bleaching. However, over the last two decades, chlorine-containing bleaching compounds have been withdrawn from the market and their usage has become limited. This is due to the formation of highly toxic chlorinated organic by-products during the bleaching process as well as effluent discharged. With increasing pressure on environment and safety issues, bleaching processes are becoming elemental chlorine free (ECF) or totally chlorine free (TCF) bleaching sequences (Comlekcioglu et al. 2014; Yao et al. 2015). In recent years, hydrogen peroxide (H2O2), which is an environmentally friendly chemical, has been the primary bleaching chemical for mechanical pulps, and it is also used for chemical pulps due to its final decomposition products being oxygen and water (Karlsson and Agnemo 2010). H2O2 primarily works in alkaline conditions when the perhydroxyl ion (OOH-) is formed. However, it can be decomposed by transition metals, e.g., iron, manganese, and copper, which is undesirable. Therefore, to reach higher brightness levels, a conventional hydrogen peroxide bleaching process often includes a chelating stage where detrimental metal ions are removed before bleaching (Liang et al. 2019; Li et al. 2019). During the peroxide bleaching process, the chemical and physical properties can be considerably modified; therefore, properly adjusting the bleaching parameters appears important to meet the specific requirements of different paper products (Behrooz et al. 2011; Akca 2019).
The objective of this work was to bleach hardwood kraft pulp with adsorbed birch xylan via H2O2 and study the effect of the bleaching parameters on the paper properties. After the adsorption of xylan, the pulp was bleached. The bleaching parameters studied included the bleaching temperature, bleaching time, initial pH, as well as the MgSO4 dosage. The optical properties, i.e., brightness, opacity, and post color number, and mechanical properties, i.e., tensile index, tearing index, and bulk, of paper made from the pulp bleached with different process variables were measured. Finally, the effect of the adsorption of birch xylan on the mechanical properties of paper made from hardwood kraft pulp with different treatment conditions was discussed.
EXPERIMENTAL
Materials
The brown (unbleached) hardwood kraft pulp used in this study was obtained from the Moorim Paper Company (Jinju-si, South Korea), which was composed of 31% mixed hardwood from Korea, 44% eucalyptus from Vietnam, and 25% acacia from Thailand. A bleaching sequence of D-EOP-D-PO-D was employed to produce the bleached pulp. The brown pulp was thoroughly washed (400-mesh), never-dried, and non-beaten. Birchwood xylan and poly diallyl dimethyl ammonium chloride (Poly-DADMAC), with a molecular weight of 400,000 to 500,000 g/mole was purchased from Sigma-Aldrich (St. Louis, MO). The DTPA, H2O2, MgSO4, and all other reagents for the bleaching process were bought from the Samchun Pure Chemical Company (Pyeongtaek-si, South Korea).
Adsorption of Birch Xylan by the Pulp Fibers
Poly-DADMAC was used to promote the interaction between xylan and the pulp fibers. To be specific, 0.30% Poly-DADMAC (based on the oven dried pulp) was added into the pulp slurry, which reversed the zeta potential of the cellulosic fibers to positive. The stock was then stirred for 20 min to ensure complete mixture and washed twice with 400-mesh to remove the unadsorbed Poly-DADMAC. The birch xylan was dissolved at a temperature of 90 ℃ for 15 min. After cooling down the solution to room temperature, 6.0% xylan (based on the oven dried pulp) was added into the pulp and mixed for 20 min to ensure sufficient adsorption. The schematic diagram of the adsorption of xylan by the pulp fibers is presented in Fig. 2.
Hydrogen Peroxide (H2O2) Bleaching Process
The chelation pretreatment was first performed in order to remove transition metal ions. Specifically, pulp with a 5% consistency was warmed at a temperature of 70 °C for 30 min, and then 0.30% DTPA (based on the oven dried pulp) was added and mixed well with the pre-warmed pulp. After that, the H2O2 bleaching process was conducted at a 10% pulp consistency with 2.0% H2O2 (based on the oven dried pulp) in plastic bags placed in a water bath. In addition, 3.0% Na2SiO3 (based on the oven dried pulp) was adopted as the H2O2 stabilizer. Before adding the bleaching chemicals, the pulp was pre-warmed to the desired temperature. The initial pH of pulp was measured before the bleaching process. During the bleaching experiment, the pulp was mixed every 15 min. The bleaching time, bleaching temperature, initial pH, and MgSO4 dosage varied in the ranges 30 min to 120 min, 40 ℃ to 80 ℃, 10 to 12, and 0% to 0.30%, according to different experiment designs. Sodium hydroxide was used to adjust the pH of the pulp to the desired value. After bleaching, the pulp was washed until a neutral pH was reached.
Fig. 2. Schematic diagram of the adsorption of birch xylan by the pulp fibers
Handsheet Forming and Measurement of Properties
According to TAPPI standard T 205 (2012), handsheets with a basis weight of 80 g/m2 were made from the pulp with different bleaching parameters. Then, the prepared handsheets were conditioned based on TAPPI standard T 402 (2013).
The brightness and opacity of the handsheets were measured in accordance with TAPPI standards T 452 (2018) and T 519 (2011). The post color number (P.C. number) was used to indicate the extent of the brightness reversion. The calculation of the P.C. number is shown in Eq. 1,
(1)
where R1 is the brightness before ageing and R2 is the brightness after ageing. The accelerated ageing test was based on ISO standard 5630-1 (1991), which utilized a dry heat treatment at a temperature of 105 ℃. The accelerated ageing test was carried out for 24 h for each sample.
The measurement of the mechanical properties of the handsheets, including the grammage, thickness, tensile index, and tear index, were in accordance with the TAPPI standard T 220 (2001). The bulk was expressed as cm3/g and calculated according to Eq. 2,
(2)
where δ is the mean thickness of the sheet (μm) and g is the grammage of the sheet (g/m2).
RESULTS AND DISCUSSION
Effect of Birch Xylan on Brown Pulp
The effect of the adsorption of birch xylan by the brown pulp (unbleached hardwood kraft pulp) is shown in Table 1. It could be observed clearly that the tensile index and tear index were increased, especially the tear index (which was increased by 28.7%). This is because the xylan adsorbed by the pulp fibers could effectively increase either the fiber-fiber joint strength, contact area, or number of joints between fibers, leading to enhanced strength properties (Köhnke and Gatenholm 2007; Borrega and Orelma 2019). In addition, the introduction of xylan into the pulp decreased the brightness by 5.5% but slightly increased the opacity as well as the bulk.
It is generally accepted that there are strong chemical and physical connections between hemicelluloses and lignins in wood, which causes a certain amount of lignins to remain in the extracted xylan fractions (Pedrazzi et al. 2010; Li et al. 2011; Dahlman et al. 2018). Therefore, the lignin residues in the xylan resulted in the reduction of the brightness and a minor improvement in the opacity. In addition, a distinct negative influence on the brightness reversion was noticed (the P.C. number was increased by 42.8%), which could be explained by the increased hemicellulose content in the pulp. During the ageing test, the formation of carbonyl groups and carboxyl groups via the oxidation of the added xylan associated with the newly produced chrophomores in the lignin residues of the xylan fractions contributed to the more significantly declined brightness stabilization (Gomes et al. 2014).
Table 1. Properties of Original Kraft Pulp and Xylan Treated Kraft Pulp
Effect of the Bleaching Time and Temperature on the Paper Properties
In order to study the effect of the bleaching time and temperature on the unbleached hardwood kraft pulp with adsorbed birch xylan, the hydrogen peroxide bleaching process was conducted with a 0.05% MgSO4 dosage at an initial pH of 11.
Figure 3 showed that the brightness was boosted significantly when the bleaching time was extended, and the bleaching temperature was increased. In the earlier stage of bleaching, the increase in brightness was much more pronounced than the later stages. More specifically, when the bleaching temperature was 80 °C, the brightness increased from 37.0% ISO to 56.2% ISO, with the improvement as high as 51.9% during the first 30 min, while it was only increased by 6.0% ISO in the following 90 min.
The effect of the bleaching time and temperature on the P. C. number is shown in Fig. 4. It could be clearly seen that a longer bleaching time and higher bleaching temperature yielded a lower P. C. number, which indicated less brightness reversion and more stable brightness. To be precise, at a bleaching temperature of 40 °C, the P. C. number constantly decreased with the bleaching time. However, when the bleaching temperature was 60 °C or 80 °C, the P. C. number considerably decreased in the first 30 min of the bleaching process, and then stabilized during the following 90 min.
Fig. 3. Effect of the bleaching time and temperature on the brightness of paper
Fig. 4. Effect of the bleaching time and temperature on the P. C. number
Figure 5 illustrated that the opacity constantly decreased as the bleaching time and temperature increased. This could be explained by the reduction of the scattering coefficient and adsorption coefficient caused by the hydrogen peroxide bleaching process, based on the Kubelka-Munk theory. First, during the delignification process, a large amount of lignins and other wood components involving pectin and extractives were dissolved, which could lead to the collapse of fiber lumens. In the meantime, pulp swelling in the alkaline bleaching liquor became more hydrophilic, softer, and more flexible. All of these structural changes contributed to the enhancement of the fiber-fiber contact area. In other words, the non-contact area between the fibers decreased, resulting in the reduction of the scattering coefficient of the formed paper (Miao et al. 2014). The decrease of the adsorption coefficient resulted from the elimination of light adsorbing colored groups through the bleaching process.
Fig. 5. Effect of the bleaching time and temperature on the opacity
The influence of the bleaching time and temperature on the mechanical properties of the paper is shown in Fig. 6.
Fig. 6. Effect of the bleaching time and temperature on the mechanical properties of paper:
As shown, increasing the retention time and bleaching temperature yielded a lower tensile index and tear index. This phenomenon could be attributed to two factors. First, the carbohydrates in the pulp, especially cellulose, were attacked by nucleophiles (HO- and HOO-) initially present in the bleaching system as well as by active oxygen species, e.g., hydroxyl radicals HO• and superoxide anions O2–• (Wuorimaa et al. 2009; Xu et al. 2015). The degradation of these carbohydrates could lead to undesirable strength loss in the fibers. Second, the adsorbed xylan by the fiber surface, as well as the original xylan located in the cell wall of the fibers, was dissolved into the alkaline bleaching liquor, which also had a negative effect on the strength of the bleached pulp (Li et al. 2015; Sun et al. 2017).
Effect of the Initial pH of the Bleaching Process on the Paper Properties
In order to study the effect of the initial pH on unbleached hardwood kraft pulp with adsorbed birch xylan, the hydrogen peroxide bleaching process was performed at a temperature of 80 °C, with a 0.05% MgSO4 dosage for 120 min.
The effect of the initial pH of the bleaching process on the brightness and brightness reversion of the paper is illustrated in Fig. 7. It was clear that the brightness was improved as the pH was increased up to 12. This might be due to the excess alkaline increasing the concentration of hydroperoxide ions, which in turn resulted in a greater delignification effect and the elimination of chromophores. The P. C. number decreased from 3.18 to 2.67 as the initial pH of the bleaching process increased from 10 to 11, but it slightly increased when the initial pH of the bleaching process increased further was increased up to 12. A possible reason for this was the offsetting chromophore formation that arose directly or indirectly from alkaline-induced reactions.
Fig. 7. Effect of the initial pH of the bleaching process on the brightness and P.C. number
Figure 8 indicates that when the initial pH of the bleaching process was increased, the bulk and opacity of the paper decreased; however, the tensile index and tear index of the paper increased. As discussed above, the higher the initial pH was, the stronger the alkaline bleaching condition, which led to a greater delignification effect, as well as the additional dissolving of wood components. The collapse of fiber lumens that resulted from these reactions was therefore intensified, which contributed to the decrease in the paper bulk. Moreover, the additional removal of the light adsorbing chromophores and the decrease of the bulk resulted in a lower opacity. However, the collapse of the fiber lumens increased the contact area between the fibers. This effect, in combination with the greater swelling of the pulp fibers caused by the alkaline bleaching environment, led to increases in the tensile and tear strength of the paper when the initial pH of the bleaching process was increased.
Fig. 8. Effect of the initial pH of the bleaching process on the mechanical properties of paper: (a) bulk and opacity; and (b) tensile index and tear index
Effect of the MgSO4 Dosage on the Paper Properties
In order to study the effect of the MgSO4 dosage on the unbleached hardwood kraft pulp with adsorbed birch xylan, the hydrogen peroxide bleaching process was performed at a temperature of 80 °C and an initial pH of 11 for 120 min.
The data shown in Fig. 9 reflects that the brightness of paper increased and then stabilized, while the P. C. number decreased and then stabilized with the increase of MgSO4 dosage. During the hydrogen peroxide process, the presence of transition metal ions, particularly manganese, copper, and iron, are known to catalyze peroxide decomposition. In contrast, magnesium is known to play an important role in preventing peroxide decomposition. It has been established that magnesium ions, e.g., Mg2+, has a comparable size to transition metal ions, e.g., Fe2+ and Mn2+; magnesium can be incorporated into a solid solution and deprived of the activity of the transition metal ions (Lapierre et al. 2003; Wickham et al. 2009; Shimoda et al. 2012; Das et al. 2016). Meanwhile, the deactivation of transition metal ions has a driving effect on the brightness preservation. Therefore, the brightness reversion was reduced, while the P.C. number decreased with the usage of MgSO4. As a result, an improvement in the brightness and brightness stableness of the paper was observed, which was directly led by a considerably more effective utilization of hydrogen peroxide and the deactivation of transition metal ions.
Fig. 9. Effect of the MgSO4 dosage on the brightness and P. C. number
According to Fig. 10, the bulk and opacity of the paper constantly decreased as the of MgSO4 dosage increased from 0% to 0.15%, and then it leveled off when the MgSO4 dosage was further increased.
Fig. 10. Effect of the MgSO4 dosage on the bulk and opacity
The effects shown in Fig. 10 could be explained by the fact that the delignification was strengthened due to the relatively higher usage rate of hydrogen peroxide, which could result in a decrease in the bulk and opacity. Based on previous research, the adsorption of carboxymethyl cellulose (CMC) by the pulp has shown that a higher electrical conductivity of the pulp slurry causes greater amounts of CMC to be adsorbed by the pulp (Liimatainen et al. 2009; Naderi et al. 2015). Similarly, the addition of MgSO4 could increase the electrical conductivity of the pulp, which was expected to increase the retention rate of the xylan by the fibers. The effects of this presumption would result in an increase in the bulk and opacity of the paper.
As shown in Fig. 11, the strength properties could be improved by increasing the MgSO4 dosage. This is due to the fact that the degradation of carbohydrates via hydrogen peroxide decomposition radicals is avoidable in the presence of magnesium salt. In addition, the introduction of MgSO4 was able to increase the retention rate of xylan by the pulp fibers, which also had a positive effect on the strength properties.
Fig. 11. Effect of the MgSO4 dosage on the tensile index and tear index
Modifying Effect of Birch Xylan on the Mechanical Properties of Paper
Figure 12 compares the mechanical properties of the original brown pulp (unbleached kraft pulp) and the brown pulp with adsorbed birch xylan under different bleaching conditions (“Q” refers to a chelating treatment while “P” refers to bleaching treatment). Without the bleaching treatment, the tensile index and tear index of the paper made from brown pulp were improved after the adsorption of birch xylan. Compared to the control conditions, the chelation stage with the addition of DTPA into the pulp system had a slightly negative effect on the mechanical properties of the paper. Since DTPA, an effective chelant, has higher binding affinities for the higher oxidation states of metal ions, e.g., Fe3+ and Mn3+, it could reduce the concentration of the transition metals within the pulp (Lapierre et al. 2003). However, the acidic condition during the chelation stage likely induced the degradation reaction of carbohydrates in the pulp, resulting in a decrease in the tensile index and tear index of the paper. As shown in Fig. 12, the mechanical properties of the paper made from xylan treated pulp were always better than the mechanical properties of the paper made from the original brown pulp under different bleaching temperatures. However, the tensile index and tear index of the papers made from the two types of pulp both decreased as the bleaching temperature increased. For example, the pulp adsorbed with xylan bleached at a temperature of 40 ℃ resulted in an increase in the tensile index of 8.0% and an increase in the tear index of 10.9% when compared to the original unbleached pulp.
Fig. 12. Modifying effect of birch xylan on the mechanical properties of the paper under different phases: (a) tensile index; and (b) tear index
The optical properties of paper before and after adsorption of xylan were also measured under different phases. As shown in Fig. 13, the adsorption of xylan decreased the brightness and brightness stableness of paper. Furthermore, the chelation stage with addition of DTPA into the pulp system improved the brightness and declined P. C. number. As mentioned before, DTPA could reduce the concentration of the transition metals within pulp. Therefore, the brightness and stableness of paper were improved slightly. Most importantly, the hydrogen peroxide bleaching could decrease the negative effect of adsorbed xylan on brightness and especially P. C. number. Comparing the bleached pulp with adsorbed xylan to the original unbleached pulp, it appears that the brightness was enhanced significantly, and the brightness reversion was declined. Take the pulp adsorbed with xylan bleached at temperature of 60 °C for example, the brightness was increased by 49.6% and the P. C. number was declined by 1.05% compared to the original unbleached pulp.
Fig. 13. The effect of birch xylan on the optical properties of the paper under different phases: (a) brightness; and (b) P.C. number
CONCLUSIONS
- The adsorption of birch xylan by hardwood unbleached kraft pulp fibers could improve the mechanical properties of paper. However, the adsorption treatment would reduce the brightness and increase the rate of brightness reversion of the paper.
- At different bleaching temperatures, the brightness of the paper was always increased with the extension of the bleaching time. However, the P.C. number, opacity, and mechanical properties (especially the tensile index and the tear index) of the paper decreased with the extension of the bleaching time.
- A relatively higher bleaching temperature could provide better performance in terms of the brightness of the paper, but would impact its opacity, tensile index, and tear index.
- The highest brightness could be obtained by bleaching at an initial pH of 12. The bulk and opacity of the paper decreased as the initial pH increased, while the tensile index and tear index of paper increased as the initial pH increased.
- The optical properties and mechanical properties of the paper were both improved by the addition of MgSO4.
- The modifying effect of birch xylan on the mechanical properties of the paper made from brown kraft pulp was obvious under different bleaching conditions.
- A H2O2 bleaching treatment was an appropriate method for bleaching hardwood kraft pulp with adsorbed birch xylan. Satisfactory optical properties and mechanical properties of the paper could be achieved through optimal H2O2 bleaching process conditions.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 32001276), the China Postdoctoral Science Foundation (Grant No. 2020M682709), the Postdoctoral Science Foundation of Zhejiang Province (Grant No. ZJ2020115), the Beijing Gold-Bridge Project, and the Doctor Special Project of China National Pulp and Paper Research Institute Co., Ltd.
REFERENCES CITED
Akca, C. (2019). “A new method: The usage of natural zeolite as a killer chemical for hydrogen peroxide during hydrogen peroxide bleaching,” Industria Textilă 70(6), 519-522. DOI: 10.35530/IT.070.06.1523
Ban, W., and Heiningen, A. V. (2011). “Adsorption of hemicellulose extracts from hardwood onto cellulosic fibers. I. Effects of adsorption and optimization factors,” Cellulose Chemistry and Technology 45(2), 57-65. DOI: 10.1515/HF.2010.121
Behrooz, R., Ghasemi, S., Atoii, G. A., and Fatehi, P. (2011). “Mg (OH)2-based hydrogen peroxide bleaching of CMP pulps at high consistency,” BioResources 7(1), 161-172. DOI: 10.15376/biores.7.1.161-172
Borrega, M., and Orelma, H. (2019). “Cellulose nanofibril (CNF) films and xylan from hot water extracted birch kraft pulps,” Applied Sciences 9(16), 3436-3442. DOI: 10.3390/app9163436
Budiarti, G. I., Fajariyanto, D., and Hendratno, O. (2020). “Quality improvement of recycled paper with extracted xylan from corncobs,” Key Engineering Materials 849(6), 67-71. DOI: 10.4028/www.scientific.net/KEM.849.67
Comlekcioglu, U., Tutus, A., Cicekler, M., Gunes, M., and Aygan, A. (2014). “Application of recombinant xylanase from Orpinomyces sp. in elemental chlorine-free bleaching of kraft pulps,” Romanian Biotechnological Letters 19(1), 8941-8950. DOI: 10.13140/2.1.3346.7680
Corradini, F. A. S., Baldez, T. O., Milessi, T. S. S., Tardioli, P. W., Ferreira, A. G., Giordano, R. d. C., and Giordano, R. d. L. C. (2018). “Eucalyptus xylan: An in-house-produced substrate for xylanase evaluation to substitute birchwood xylan,” Carbohydrate Polymers 197(4), 167-173. DOI: 10.1016/j.carbpol.2018.05.088
Dahlman, O., Sjoeberg, J., Jansson, U. B., and Larsson, P. O. (2018). “Effects of surface hardwood xylan on the quality of softwood pulps,” Nordic Pulp and Paper Research Journal 18(3), 310-315. DOI: 10.3183/npprj-2003-18-03-p310-315
Das, N., Bose, S. K., and Biswas, D. (2016). “Effect of magnesium-salts on hydrogen peroxide bleaching of non-wood pulps,” Bangladesh Journal of Scientific and Industrial Research 51(4), 291-296. DOI: 10.3329/bjsir.v51i4.30449
Gomes, V. J., Longue Jr., D., Colodette, J. L., and Ribeiro, R. A. (2014). “The effect of eucalypt pulp xylan content on its bleachability, refinability and drainability,” Cellulose 21(1), 607-614. DOI: 10.1007/s10570-013-0104-3
ISO 5630-1 (1991). “Paper and board — Accelerated ageing — Part 1: Dry Heat treatment at 105 degrees C,” International Organization for Standardization, Geneva, Switzerland.
Karlsson, A., and Agnemo, R. (2010). “High consistency hydrogen peroxide bleaching of mechanical pulps with varying amounts of fines,” Nordic Pulp and Paper Research Journal 25(3), 256-268. DOI: 10.3183/NPPRJ-2010-25-03-p256-268
Kaschuk, J. J., and Frollini, E. (2018). “Effects of average molar weight, crystallinity, and hemicelluloses content on the enzymatic hydrolysis of sisal pulp, filter paper, and microcrystalline cellulose,” Industrial Crops and Products 115(7), 280-289. DOI: 10.1016/j.indcrop.2018.02.011
Köhnke, T., and Gatenholm, P., (2007). “Modification of cellulose fibres by controlled xylan adsorption and its effect on softwood pulp properties,” in: Proceedings of the 14th International Symposium on Wood, Fibre and Pulping Chemistry, Durban, South Africa, pp. 25-28.
Lapierre, L., Berry, R., and Bouchard, J. (2003). “The effect of magnesium ions and chelants on peroxide bleaching,” Holzforschung 57(6), 627-633. DOI: 10.1515/HF.2003.094
Li, L., Lee, S., Lee, H. L., and Youn, H. J. (2011). “Hydrogen peroxide bleaching of hardwood kraft pulp with adsorbed birch xylan and its effect on paper properties,” BioResources 6(1), 721-736. DOI: 10.15376/biores.6.1.721-736
Li, M., Yin, J., Hu, L., Chen, S., Min, D., Wang, S., and Luo, L. (2019). “Effect of hydrogen peroxide bleaching on anionic groups and structures of sulfonated chemo-mechanical pulp fibers,” Colloids and Surfaces A: Physicochemical and Engineering Aspects 585(7), 1-9. DOI: 10.1016/j.colsurfa.2019.124068
Li, Z., Dou, H., Fu, Y., and Qin, M. (2015). “Improving the hydrogen peroxide bleaching efficiency of aspen chemithermomechanical pulp by using chitosan,” Carbohydrate Polymers 132(2), 430-436. DOI: 10.1016/j.carbpol.2015.06.062
Liang, F., Fang, G., Jiao, J., Deng, Y., Han, S., Li, H., Tian, Q., Pan, A., and Zhu, B. (2019). “Modified hydrogen peroxide bleaching of bamboo chemo-mechanical pulp using aqueous alcohol media,” BioResources 14(1), 870-881. DOI: 10.15376/biores.14.1.870-881
Liimatainen, H., Haavisto, S., Haapala, A., and Niinimäki, J. (2009). “Influence of adsorbed and dissolved carboxymethyl cellulose on fiber suspension dispersing, dewaterability, and fines retention,” BioResources 4(1), 321-340. DOI: 10.15376/biores.4.1.321-340
Magaton, A. S., Colodette, J, L., Piló-Veloso, D., and Gomide, J. L. (2011). “Behavior of eucalyptus wood xylans across kraft cooking,” Journal of Wood Chemistry and Technology 31(1), 58-72. DOI: 10.1080/02773813.2010.484123
Miao, Q., Zhong, G., Qin, M., Chen, L., and Huang, L. (2014). “Influence of alkaline treatment and alkaline peroxide bleaching of aspen chemithermomechanical pulp on dissolved and colloidal substances,” Industrial & Engineering Chemistry Research 53(6), 2544-2548. DOI: 10.1021/ie4040785
Moreira, R. L. P. O., Simão, J. A., Gouveia, R. F., and Strauss, M. (2020). “Exploring hierarchical structure and alignment of wood cellulose fibers for bioinspired anisotropic polymeric composites,” ACS Applied Bio Materials 3(4), 2193-2200. DOI: 10.1021/acsabm.0c00038
Naderi, A., Lindström, T., Sundström, J., Pettersson, T., Flodberg, G., and Erlandsson, J. (2015). “Microfluidized carboxymethyl cellulose modified pulp: A nanofibrillated cellulose system with some attractive properties,” Cellulose 22(2), 1159-1173. DOI: 10.1007/s10570-015-0577-3
Pedrazzi, C., Colodette, J. L., Gomide, J. L., and Muguet, M. C. D. S. (2010). “Kraft pulping of eucalyptus woods with varying contents of xylan,” Appita: Technology, Innovation, Manufacturing, Environment 63(2), 137-142.
Shen, F., Ling, H., Ge, W., Yang, Y., Wang, X., Ren, J., and Wang, X. (2021). “Self-assembly behavior and conformation of amphiphilic hemicellulose-graft-fatty acid micelles,” Carbohydrate Polymers 261(3), 1-9. DOI: 10.1016/j.carbpol.2021.117886
Shimoda, Y., Ito, H., and Tanaka, A. (2012). “Conversion of chlorophyll b to chlorophyll a precedes magnesium dechelation for protection against necrosis in Arabidopsis,” The Plant Journal 72(3), 501-511. DOI: 10.1111/j.1365-313X.2012. 05092.x
Sun, X., Hou, Q., Zhang, B., and Zhao, G. (2017). “Improving the efficiency of hydrogen peroxide bleaching of chemimechanical pulp by continuous replenishment of bleaching chemicals,” TAPPI Journal 16(6), 331-339. DOI: 10.32964/TJ16.6.331
TAPPI T 205 (2012). “Forming handsheets for physical tests of pulp,” TAPPI Press, Atlanta, GA.
TAPPI T 402 (2013). “Standard conditioning and testing atmospheres for paper, board, pulp handsheets, and related products,” TAPPI Press, Atlanta, GA.
TAPPI T 452 (2018). “Brightness of pulp, paper, and paperboard (directional reflectance at 457 nm),” TAPPI Press, Atlanta, GA.
TAPPI T 516 (2011). “Diffuse opacity of paper (d/0 paper backing),” TAPPI Press, Atlanta, GA.
Testova, L., Roselli, A., Costabel, L., Kovasin, K., Tenkanen, M., and Sixta, H. (2014). “Combined production of polymeric birch xylan and paper pulp by alkaline pre-extraction followed by alkaline cooking,” Industrial & Engineering Chemistry Research 53(19), 8302-8310. DOI: 10.1021/ie500694c
Wedin, H., Sevastyanova, O., Evtuguin, D. V., Ragnar, M., and Lindström, M. E. (2013). “Impact of extended-impregnation cooking on the xylan structure in Eucalyptus urograndis kraft pulps,” Nordic Pulp and Paper Research Journal 28(4), 498-505. DOI: 10.3183/NPPRJ-2013-28-04-p498-505
Wickham, J. R., Halye, J. L., Kashtanov, S., Khandogin, J., and Rice, C. V. (2009). “Revisiting magnesium chelation by teichoic acid with phosphorus solid-state NMR and theoretical calculations,” The Journal of Physical Chemistry B 113(7), 2177-2183. DOI: 10.1021/jp809313j
Wuorimaa, A., Jokela, R., and Aksela, R. (2009). “Recent developments in the stabilization of hydrogen peroxide bleaching of pulps: An overview,” Nordic Pulp and Paper Research Journal 21(04), 435-443. DOI: 10.3183/NPPRJ-2006-21-04-p435-443
Xu, Z. J., Liu, Y., and Wang, Z. (2015). “A survey of hydrogen peroxide bleaching and fas bleaching of liquid package recycled pulp,” Advanced Materials Research 1081(9), 48-52. DOI: 10.4028/www.scientific.net/AMR.1081.48
Yao, S., Cong, G., Zhu, H., Zhang, Y., Wang, S., and Qin, C. (2015). “Effects of additives on absorbable organic halide reduction in elemental chlorine-free bleaching of bagasse kraft pulp,” BioResources 11(1), 996-1006. DOI: 10.15376/biores.11.1.996-1006
Zhu, Z., Fu, S., Lavoine, N., and Lucia, L. A. (2020). “Structural reconstruction strategies for the design of cellulose nanomaterials and aligned wood cellulose-based functional materials – A review,” Carbohydrate Polymers 247(6), 116722-116737. DOI: 10.1016/j.carbpol.2020.116722
Article submitted: July 3, 2021; Peer review completed: August 14, 2021; Revised version received and accepted: August 21, 2021; Published: August 25, 2021.
DOI: 10.15376/biores.16.4.6814-6830
