Abstract
Hot water extraction of hemicelluloses from aspen wood chips of different sizes and wood meal was performed, and the extracted hemicelluloses were characterized. With decreasing wood chip size, the total sugars and total poly- and oligosaccharides extracted with hot water increased. The dissolution of hemicelluloses was mainly limited by diffusion in the fiber wall and mass transfer from fiber to fiber during hot water extraction of the wood chips. The extraction yield of high molecular weight hemicelluloses was less than that of low molecular weight hemicelluloses, and the reduction of wood chip size benefited the extraction of high-molecular weight hemicelluloses. Compared to the wood chips, the high molecular weight hemicelluloses extracted from wood meal had a higher extraction yield and an increased average molecular weight.
Download PDF
Full Article
Hot Water Extraction of Hemicelluloses from Aspen Wood Chips of Different Sizes
Zongquan Li,a Menghua Qin,a,b,* Chunlin Xu,c and Xiaoqian Chen a
Hot water extraction of hemicelluloses from aspen wood chips of different sizes and wood meal was performed, and the extracted hemicelluloses were characterized. With decreasing wood chip size, the total sugars and total poly- and oligosaccharides extracted with hot water increased. The dissolution of hemicelluloses was mainly limited by diffusion in the fiber wall and mass transfer from fiber to fiber during hot water extraction of the wood chips. The extraction yield of high molecular weight hemicelluloses was less than that of low molecular weight hemicelluloses, and the reduction of wood chip size benefited the extraction of high-molecular weight hemicelluloses. Compared to the wood chips, the high molecular weight hemicelluloses extracted from wood meal had a higher extraction yield and an increased average molecular weight.
Key words: Hot water extraction; Aspen; Wood chip size; Hemicelluloses; Molecular weight
Contact information: a: Key Laboratory of Paper Science & Technology of Ministry of Education, Qilu University of Technology, University Park of Science and Technology, Jinan 250353, China; b: Laboratory of Organic Chemistry, Taishan University, Taian 271021, China; c: Laboratory of Wood and Paper Chemistry, Åbo Akademi University, 20500 Åbo/Turku, Finland;
* Corresponding author: qmh@spu.edu.cn
INTRODUCTION
Hemicelluloses are important components present in wood materials. In native softwood, the main hemicellulose is galactoglucomannan (Willför et al. 2005a), and in native hardwood, the main hemicellulose is glucuronoxylan (Willför et al. 2005b). In recent years, extraction of hemicelluloses from wood has gained growing attention, and many applications have been developed for hemicelluloses. For example, hemicelluloses can be used as the raw material for biopolymers such as hydrogels (Karaaslan et al. 2011), controlled release drug coatings (Karaaslan et al. 2010), and oxygen barrier materials (Hartman and Albertsson 2006); they can also be used as a source of sugars to fuel fermentation after hydrolysis (Vena et al. 2010; Liu et al. 2012). In conventional alkaline or kraft pulping processes, a portion of the hemicelluloses is dissolved in the cooking liquor, together with the lignin, and is burned in the chemical recovery process, while the remaining hemicelluloses remain in the pulp.
The concept of a biorefinery has been put forward as an effective separation of lignocellulosic materials for high value-added production (van Heiningen 2006). For hemicellulose separation from wood or non-wood materials, hot water extraction, which is also called autohydrolysis or hydrothermal treatment, has been considered an effective process (van Heiningen et al. 2008; Song et al. 2008). No chemicals other than water or steam are needed in this step, so it is considered an environmentally friendly technology (Garrote et al. 1999). During the production of dissolved pulp, hot water extraction is an effective method for hemicellulose removal from wood chips prior to kraft pulping (Li et al. 2010). Hot water extraction has also been used to remove the hemicelluloses prior to kraft pulping or soda-AQ pulping for hemicellulose separation and normal pulp production (Vena et al. 2010; Vila et al. 2011). Most hemicelluloses and some lignin are dissolved in the autohydrolysis liquor. Extracted wood chips are easier to delignify than untreated wood chips, and the resulting pulp has a better bleachability (Chirat et al. 2012).
Hot water extraction of hemicelluloses from wood has been extensively investi-gated, including the effects of hot water processing conditions on the extraction of hemicellulose and the properties of extracted hemicelluloses (Song et al. 2008, 2011a,b; Cristóbal et al. 2012). Hot water extraction temperatures vary from 120 °C to 240 °C, and the extraction time varies from several minutes to several hours. Based on the end use of the isolated hemicelluloses, the molecular mass characteristics and yield of extracted hemicelluloses depend on the extraction temperature and pH (Leppänen et al. 2011; Magnus and Guido 2003; Song et al. 2011b). Higher temperatures and longer extraction times lead to higher extraction yields. The results obtained by Song et al. (2008) showed that a high yield of galactoglucomannans (GGM) can be obtained by pressurized hot water extraction of ground spruce wood at temperatures of 170 to 180 °C with an extraction time of 60 min. While high temperatures (> 200 °C) can lead to near total dissolution of woody biomass, the amount of sugars present in the extraction liquor decreases rapidly with temperature (Liu et al. 2012). The extraction yield is also dependent on the residual pH of the extracts, and the maximum yield can be obtained at a pH of about 3.5, below which the extraction yield rapidly decreases (Yoon et al. 2008). The pH of extraction liquor can be controlled by the addition of sodium bicarbonate in the hot water extraction to enhance extraction of high-molar mass hemicelluloses from wood by partially inhibiting the autohydrolysis of acetyl groups and glycosidic bonds (Song et al. 2011b). During the hot water extraction, the extracted lignin amount increases with increasing liquid-to-wood (L/W) ratio, but the L/W ratio has little effect on the extraction of carbohydrates (Borrega and Sixta 2011).
During hot water extraction of hemicelluloses from wood, wood chips or ground wood have been used as raw materials. The extraction of hemicelluloses is limited mainly by the diffusion in the fiber wall (Song et al. 2012). Compared to ground wood, hot water extraction from spruce wood chips gives a lower yield of GGM (Song et al. 2008). The particle size also significantly affects the extraction of ground wood. The total extraction yield, as well as the yields of hemicelluloses and monosaccharides, increases clearly with decreasing particle size; however, ground spruce sapwood, heartwood, and thermomechanical pulp (TMP) exhibit only small differences in hemicellulose extraction (Song et al. 2012).
In recent years, fast-growing aspen has been widely planted in North China and has been used as a raw material for chemical and mechanical pulp production (Xu et al. 2010). The main hemicellulose in aspen is xylan, and the hemicelluloses have been extracted and used in novel industrial applications. The extracted hemicelluloses should be in high-molar mass form if they are to be used for novel industrial products such as biopolymers, biofilms, and hydrogels (Hartman and Albertsson, 2006; Ebringerová et al. 2008; Gabrielii et al. 2000). In this work, the main objective was to evaluate both the effect of aspen wood chip size on the hot water extraction of hemicelluloses (mainly xylan) and the properties of the extracted hemicelluloses, especially high molecular weight hemicelluloses.
EXPERIMENTAL
Materials
Aspen (a mixture of Populus×euramericana ‘Guariento’ and Populus× euramericana ‘Neva’) chips were obtained from MCC Paper Yinhe Co. Ltd, Shandong Province, China. The average size of wood chips was about 32.5 mm × 40.0 mm × 3.9 mm. The wood chips were stored in a freezer at -20 °C before they were used. They contained 21.30% pentosan, 19.87% Klason lignin, and 2.65% acid-soluble lignin, as determined by TAPPI T 223, TAPPI T 222, and TAPPI UM 250, respectively.
Methods
Preparation of wood chips with different sizes
The wood chips were extruded and split with a laboratory single-screw extruder (JS10) after being soaked in water at room temperature for 24 h. Three fractions with different sizes were selected by hand. In addition, the wood chips were ground, and the fraction between 40- and 60-mesh (0.38 to 0.25 mm) was prepared. Therefore, five fractions of wood chips, including untreated wood chips with different sizes and ground wood, were obtained and were numbered from large to small size (Fig. 1).
Fig. 1. Wood chips/wood meal and their average sizes
Hot water extraction
Hot water extraction of the wood chips or wood meal of different sizes was performed in a 500-mL stainless steel reactor. Thirty grams (oven dry basis) of wood chips were placed in the reactor, and preheated deionized water was added to reach a liquid-solid ratio of 6:1.
The loaded reactor was placed in an oil bath that was preheated to 85 °C, and the reactor was heated to 170 °C and kept for 60 min. At the end of extraction, the reactor was cooled and the extracted mixture was filtered with three layers of 20- to 25-μm pore size filter paper and then washed with 60 mL of 85 °C water. The filtrate was collected as extracted liquor.
Analyses of extracted liquor
Total dissolved solids (TDS) were determined gravimetrically from 30 mL of extracted liquor by freeze-drying to constant weight. The ash content of the extracted liquor was determined according to TAPPI T 211. The lignin content of the extracted liquor was measured based on the UV/Vis spectrometric method at a wavelength of 205 nm (TAPPI UM 250). The samples were diluted using a sulfuric acid solution of the same pH as the samples to be tested (Shi et al. 2011). Two milliliters of extracted liquor were freeze-dried, and the total sugar composition of each sample was analyzed by acid methanolysis in 2 M HCl/methanol for 3 h at 100 °C, followed by silylation and GC analysis of the silylated sugar monomers according to Sundberg et al. (1996).
To determine the monosaccharide content in the extracted liquor, the samples were freeze-dried and then directly silylated. The monosaccharides were determined by GC-FID on a 25 m × 0.2 mm i.d. column coated with crosslinked methyl polysiloxane (HP-1) according to Holmbom and Örså (1993). The total polysaccharides and oligosac-charides in the extracted liquor were obtained by subtracting the total monosaccharides from the total sugar content.
Furfural content in the extracted liquor was analyzed by HPLC (Prominence LC-20A, Shimadzu Corporation, Japan) equipped with a SPD-20A UV-Vis detector, and the detection wavelength was 210 nm. A SHIM-PACK VP-ODS (250 mm * 4.6 mm) column was used as the separation column. The eluent was 0.1% H3PO4 at a flow rate of 0.7 mL/min. The samples were filtered through a 0.22-μm nylon syringe filter before injection. The injection volume was 25 µL.
All of the results were calculated on an oven-dried wood basis, and both hemicelluloses and monosaccharide content were calculated as anhydrosugars.
Separation and purification of hemicelluloses in the extracted liquor
Extracted liquor was treated with XAD-7 resin to remove the lignin (Xu et al. 2007), and ethanol was then added to the treated liquor at an ethanol: water ratio of 4:1 to precipitate the hemicelluloses. After standing for 24 h, the precipitated white powder was separated and then further purified by washing with ethanol, acetone, and MTBE and was finally dried in a vacuum oven at 40 ºC for 24 h (Buranov and Mazza 2010; Song et al. 2011a). High molecular weight hemicelluloses were obtained by this method because the monosaccharides and low molecular weight hemicelluloses in the extracted liquor remained in the liquor phase rather than being precipitated (Buranov and Mazza 2010).
Determination of molecular weight of purified hemicelluloses
The average molecular weight of purified carbohydrates, Mw, was determined by Agilent 1100 Series size-exclusion chromatography (SEC) (Agilent Technologies, Santa Clara, USA) in tandem with a multi-angle laser-light-scattering (MALLS) instrument (miniDAWN Tristar, Wyatt Technology, Santa Barbara, USA) with a refractive index (RI) detector (Shimadzu Corporation, Japan). A two-column system, 2×UltrahydrogelTM linear 7.8 mm × 300 mm column (Waters, Milford, USA) with an effective Mw range of 103 to 106 Da in series equipped with an Ultrahydrogel 6 mm × 40 mm guard column (Waters, Milford, USA) was used. The eluent was 0.1 M NaNO3 at a flow rate of 0.5 mL min-1. The samples were filtered through a 0.22-μm nylon syringe filter before injection. The injection volume was 100 μL. A dn/dc value of 0.146 mL g-1 was applied (Michielsen 1999). The data analysis was performed using Astra software (Wyatt Technology, Santa Barbara, USA).
RESULTS AND DISCUSSION
Effect of Wood Chip Size on the pH of Extracted Liquor and the Extraction Yield of Ash, Lignin, and TDS
As shown in Table 1, the pH of hot water extracted liquor from different sizes of wood chips and wood meal was 3.6 to 3.7 due to the deacetylation of hemicelluloses and the formation of acetic acid during extraction (Grénman et al. 2011). The TDS of extracted liquor slightly increased with a reduction in chip size. Though the smaller size of wood chips is beneficial to the dissolution of cell wall substances, the TDS amount does not have a linear correlation with wood chip size. As wood chip size decreased from No. 1 to No. 4, the TDS amount in the extracted liquor increased from 124.2 mg/g to 139.7 mg/g, respectively. The wood meal with the smallest size did not increase the TDS amount further. The lignin concentration increased with a reduction in wood chips size, and the lignin concentration of wood meal (No. 5) was 20% higher than that of the normal wood chips (No. 1).
Table 1. The pH of Extracted liquor and Extraction Yield of Ash, Lignin, and TDS
Effect of Wood Chip Size on the Sugar Content in the Extracted Liquor
Total sugars and monosaccharides in the extracted liquor were analyzed by GC with and without acid methanolysis, and the furfural content in the extracted liquor was analyzed by HPLC. The total poly- and oligosaccharides in the extracted liquor were obtained by subtracting the total monosaccharides from the total sugar content. The results are shown in Tables 2 and 3 (all results are given on an oven-dried wood basis).
The total sugar content accounted for more than 55% of the corresponding TDS. Xylose was the dominant sugar, accounting for about 67% of total sugars in the extracted liquor. The yield of total sugar extracted from different size wood chips was higher than that extracted from aspen wood chips under alkaline conditions as reported in the literature (Jun et al. 2012; Al-Dajani and Tschirner 2007; Liu et al. 2011). The total sugar content increased with decreasing chip size due to easier diffusion of hemicelluloses and faster mass transfer in the wood matrix during extraction (Song et al. 2012). However, it should be noted that the total sugar content from wood meal (No. 5) was slightly lower than that from wood chips of Nos. 3 and 4. The reduction of total sugars was mainly caused by the reduction of xylose. During the hot water extraction of hardwood, a small part of monomeric xylose was further dehydrated to form furfural (Garrote et al. 2004; Li et al. 2010). As shown in Table 2, the furfural content in extracted liquor increased with the reduction of wood chips size. Compared to the wood chips of different size, wood meal produced much more furfural during the extraction. For wood meal, the higher concentration of monosaccharides (mainly xylose, shown in Table 3) caused more decomposition of monosaccharides to furfural, which resulted in the slight decrease in total sugars in the extracted liquor.
Break-down of hemicelluloses and the dissolution of the corresponding oligomers occurred in turn during the hot water extraction, followed by the degradation of oligomeric sugars to monomeric sugars (Li et al. 2010). It is shown in Table 3 that the monosaccharide concentration was slightly affected by the wood chip size. However, the monosaccharide concentration was much higher in wood meal than in wood chips of different sizes. With decreasing wood chip size, the poly- and oligosaccharides concentration increased. The highest concentration was obtained from the smallest wood chips (No. 4), accounting for about 92% of the total sugars in the extracted liquor. Unexpectedly, the poly- and oligosaccharides obtained from the wood meal decreased sharply, accounting for only 68% of the total sugars. Therefore, the reduction of wood chip size was helpful in the extraction of poly- and oligosaccharides; however, compared to the wood chips of different sizes, fewer poly- and oligosaccharides were extracted from wood meal.
Table 2. Sugar Composition Analyzed by GC after Methanolysis and Furfural Content in Extracted Liquor
Rha: Rhamanose, Ara: Arabinose , Xyl: Xylose, GluA: Glucuronic acid,GalA: Galacturonic acid, 4-O-MeGlcA: 4-O-methyl-glucuronic acid, Man: Mannose, Gal: Galactose, Glu: Glucose
Table 3. Monosaccharides and Total Poly- and Oligosaccharides in the Extracted Liquor (mg/g)
The ratio of total xylose (mono-, oligo-, and polysaccharides) to the total sugars in the extracted liquor was more than 65% (by weight), but the ratio of xylose (monosaccharide) to the total monosaccharides in the extracted liquor from wood chips was much less than 65%, indicating that the xylan in the extracted liquor autohydrolysed to monosaccharides less efficiently compared to other carbohydrates during hot water extraction conditions.
High Molecular Weight Hemicelluloses Isolated and Purified from Extracted Liquor
The average molecular weight (Mw) and molecular weight distribution of purified hemicelluloses were determined by size-exclusion chromatography (SEC), and the results are shown in Figs. 2 and 3. With decreasing wood chip size, both the amount and average molecular weight of high molecular weight hemicelluloses increased. The wood meal gave the highest yield and average molecular weight of the purified hemicelluloses.
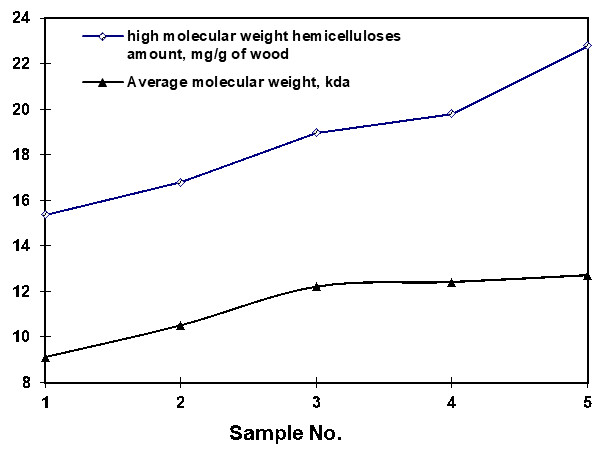
Fig. 2. Amount and average molecular weight of high molecular weight hemicelluloses extracted from wood chips of different sizes and wood meal
Fig. 3. Molecular weight distribution of purified hemicelluloses extracted from wood chips of different sizes and wood meal
The amount of high molecular weight hemicelluloses increased from 15.4 mg/g with the largest wood chip to 19.8 mg/g with the smallest wood chip and to 22.8 mg/g with wood meal. High molecular weight hemicelluloses accounted for about 30% of the total sugars in the extracted liquor from the wood meal, but only about 22% of that in the largest wood chips. The average molecular weight of the extracted hemicelluloses was 9.1 kDa for the largest wood chip and 12.2 kDa and 12.4 kDa for wood chips of smaller size (Nos. 3 and 4, respectively), and it was 12.7 kDa for wood meal. Compared to the wood chips of different sizes, both the average molecular weight and the yield of high molecular weight hemicelluloses extracted from wood meal at 170 °C for 60 min were the highest. The results in Table 3 show that the yield of total poly- and oligo-saccharides extracted from wood meal was much lower than that from wood chips of different size. The difference between the total poly- and oligo-saccharides and high molecular weight hemicelluloses was the low molecular weight hemicelluloses (oligo-saccharides included) in the extracted liquor, so it could be deduced that the low molecular weight hemicelluloses extracted from wood meal was much less than that extracted from wood chips of different size. During the extraction of wood materials, the dissolution of the broken hemicelluloses was limited by diffusion in the fiber wall and mass transfer in the wood matrix (Song et al. 2012). Compared to low molecular weight hemicelluloses and monosaccharides, less high molecular weight hemicelluloses was extracted due to their larger size, which was caused by a low diffusion rate from fiber to fiber. Compared to larger chips, smaller chips, in which the diffusion distance of mass transfer was smaller, aided the extraction of high-molecular weight hemicelluloses. Therefore, if the purpose is to extract the hemicelluloses in high molecular weight form prior to pulping, the wood chips should be cut into sizes that are as small as possible while still retaining pulping feasibility.
CONCLUSIONS
- The size of wood chips affects the hot water extraction of hemicelluloses and the properties of the extracted hemicelluloses. Both the total extracted sugars and total poly- and oligo-saccharides increased with decreasing wood chip size when the wood chips were extracted at 170 °C for 60 min.
- The extraction yield of high molecular weight hemicelluloses is less than that of low molecular weight hemicelluloses and monosaccharides. A reduction of wood chip size is beneficial for the extraction of hemicelluloses, especially the high-molecular weight hemicelluloses, due to the shorter diffusion distance of mass transfer during hot water extraction. Both the yield and the average molecular weight of high-molecular weight hemicelluloses extracted from wood meal are higher than that from wood chips.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support from the Natural Science Foundation of China (grant No. 31370581, and Shandong Provincial Outstanding Youth Scholar Foundation for Scientific Research (grant No. BS2012CL029).
REFERENCES CITED
Al-Dajani, W. W., and Tschirner, U. (2007). “Alkaline extraction of hemicelluloses from aspen chips and its impact on subsequent kraft pulping,” TAPPI 2, 958-966.
Borrega, M., and Sixta, H. (2011). “Production of cellulosic pulp by subcritical water extraction followed by mild alkaline pulping,” Proceedings of 16th International Symposium on Wood, Fiber and Pulping Chemistry. Tianjing, China. 1, 651-654.
Buranov, A. U., and Mazza, G. (2010). “Extraction and characterization of hemicelluloses from flax shives by different methods,” Carbohydrate Polymers 79(1), 17-25.
Chirat, C., Lachenal, D., and Sanglard, M. (2012). “Extraction of xylans from hardwood chips prior to kraft cooking,” Process Biochemistry 47(3), 381-385.
Cristóbal, C., Encarnación, R., Florbela, C., Patrícia, M., Ignacio, B., Eulogio, C., and Francisco, G. (2012). “Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis,” Industrial Crops and Products 40(1), 225- 231.
Ebringerová, A., Hromádková, Z., Hríbalová, V., Xu, C., Holmbom, B., Sundberg A., and Willför, S. (2008). “Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities,” International Journal of Biological Macromolecules 42(1), 1-5.
Gabrielii, I., Gatenholm, P., Glasser, W. G., Jain, R. K., and Kenne, L. (2000). “Separation characterization and hydrogel- formation of hemicellulose from aspen wood,” Carbohydrate Polymers 43(4), 367-374.
Garrote, G., Dominguez, H., and Parajó, J. C. (1999). “Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood,” Journal of Chemical Technology and Biotechnology 74(11), 1101-1109.
Garrote, G., Dominguez, H., and Parajó, J. C. (2004). “Production of substituted oligosaccharides by hydrolytic processing of barley husks,” Industrial and Engineering Chemistry Research 43(7), 1608-1614.
Grénman, H., Eränen, K., Krogell, J., Willför, S., Salmi, T., and Murzin, D. Y. (2011). “Kinetics of aqueous extraction of hemicelluloses from spruce in an intensified reactor system,” Industrial & Engineering Chemistry Research 50(7), 3818-3828.
Hartman, J., and Albertsson, A. C., Lindblad M. S., Sjöberg J. (2006). “Oxygen barrier materials from renewable sources: Material properties of softwood hemicellulose-based films,” Journal of Applied Polymer Science 100(4), 2985-2991.
Holmbom, B., and Örså, F. (1993). “Methods for analysis of dissolved and colloidal wood components in papermaking process waters and effluents,” Proceedings of the 7th Intern. Symp. Wood Pulping Chem. Beijing, China 2, 810-817.
Jun, A., Tschirner, U.W., and Tauer, Z. (2012). “Hemicellulose extraction from aspen chips prior to kraft pulping utilizing kraft white liquor,” Biomass and Bioenergy 37, 229-236.
Karaaslan, M. A., Tshabalala, M. A., and Buschle-Diller, G. (2010). “Wood hemicellulose/ chitosan-based semi: Interpenetrating network hydrogels: Mechanical, swelling and controlled drug release properties,” BioResources 5(2), 1036-1054.
Karaaslan, M. A., Tshabalala, M. A., and Buschle-Diller, G. (2011). “Hydrogels prepared from wood hemicellulose and cellulose nanocrystals,” TAPPI International Conference on Nanotechnology for Renewable Materials 2011, 459-475.
Leppänen, K., Spetz, P., Pranovich, A., Hartonen, K., Kitunen, V., and Iivesniemi, H. (2011). “Pressurized hot water extraction of Norway spruce hemicelluloses using a flow-through system,” Wood Science and Technology 45(2), 223-236.
Li, H., Saeed, A., Jahan, M. S., Ni, Y., and Van Heiningen, A. (2010). “Hemicellulose removal from hardwood chips in the pre-hydrolysis step of the kraft-based dissolving pulp production process,” Journal of Wood Chemistry and Technology 30(1), 48-60.
Liu, S., Lu, H., Hu, R., Shupe, A., Lin, L., and Liang, B. (2012). “A sustainable woody biomass biorefinery,” Biotechnology Advances 30(4), 785-810.
Liu, W., Yuan, Z., Mao, C., Hou, Q., and Li, K. (2011). “Removal of hemicelluloses by NaOH pre-extraction from aspen chips prior to mechanical pulping,” BioResources 6, 3469-3480.
Michielsen, S. (1999). “Specific refractive index increments of polymers in dilute solutions,” in: Polymer Handbook (4th Edition), J. Brandrup, E. H. Immergut, and E. A. Grulke (eds.), Wiley, New York.
Shi, H., Fatehi, P., Xiao, H., and Ni, Y. (2011). “A combined peo flocculation and acidification process for lignin removal from pre-hydrolysis liquor of mixed harwood,” Proceedings of 16th International Symposium on Wood, Fiber and Pulping Chemistry. Tianjing, China 2, 1049-1054.
Song, T., Pranovich, A., Sumerskiy, I., and Holmbom, B. (2008). “Extraction of galactoglucomannan from spruce wood with pressurised hot water,” Holzforschung 62(6), 659-666.
Song, T., Pranovich, A., and Holmbom, B. (2011a). “Effects of pH control with phthalate buffers on hot-water extraction of hemicelluloses from spruce wood,” Bioresource Technology102(22), 10518-10523.
Song, T., Pranovich, A., and Holmbom, B. (2011b). “Characterisation of Norway spruce hemicelluloses extracted by pressurised hot-water extraction (ASE) in the presence of sodium bicarbonate,” Holzforschung 65(1), 35-42.
Song, T., Pranovich, A., and Holmbom, B. (2012). “Hot water extraction of ground spruce wood of different particle size,” BioResources 7(3), 4214-4225.
Sundberg, A., Sundberg, K., Lillandt, C., and Holmbom, B. (1996). “Determination of hemicelluloses and pectins in wood and pulp fibres by acid methanolysis and gas chromatography,” Nordic Pulp & Paper Research Journal 11(4), 216-219.
Van Heiningen, A. (2006). “Converting a kraft pulp mill into an integrated forest biorefinery,” Pulp and Paper Canada 107(6), 38-43.
Van Heiningen, A., Mao, H., Genco, J., Yoon, S.-H., Zou, H., and Pendse, H. (2008). “Near-neural pre-extraction before hardwood kraft pulping: A biorefinery producing pulp, ethanol and acetic acid,” Proc. Intl. Pulp Pap. Biotech. Conf., Nanjing, China 145-156.
Vena, P. F., Görgens, J. F., and Rypstra, T. (2010). “Hemicelluloses extraction from giant bamboo prior to kraft and soda-AQ pulping to produce paper pulps, value-added biopolymers and bioethanol,” Cellulose Chemistry and Technology 44(4-6), 153-163.
Vila, C., Romero, J., Francisco, J. L., Garrote, G., and Parajó, J. C. (2011). “Extracting value from Eucalyptus wood before kraft pulping: Effects of hemicelluloses solubilization on pulp properties,” Bioresource Technology 102(8), 5251-5254.
Willför, S., Sundberg, A., Hemming, J., and Holmbom, B. (2005a). “Polysaccharides in some industrially important softwood species,” Wood Science and Technology 39(4), 245-258.
Willför, S., Sundberg, A., Hemming, J., and Holmbom, B. (2005b). “Polysaccharides in some industrially important hardwood species,” Wood Science and Technology 39(8), 601-617.
Xu, C., Qin, M., Fu, Y., Liu, N., Hemming, J., Holmbom, B., and Willför, S. (2010). “Lipophilic extractives in Populus×euramericana ‘Guariento’ stemwood and bark,” Journal of Wood Chemistry and Technology 30(2), 105-117.
Xu, C., Willför, S. Sundberg, K. Petterson, C., and Holmbom, B. (2007). “Physicochemical characterization of spruce galactoglucomannan solutions: Stability, surface activity, and rheology,” Cellulose Chemistry and Technology 51(1), 51-62.
Yoon, S.-H., Macewan, K., and van Heiningen, A. (2008). “Hot-water pre-extraction from loblolly pine (Pinus taeda) in an integrated forest products biorefinery,” TAPPI Journal 7(6), 27-31.
Article submitted: July 15, 2013; Peer review completed: August 26, 2013; Revised version received: September 5, 2013; Accepted: September 8, 2013; Published: September 24, 2013.
