Abstract
Although some engineered S. cerevisiae strains exhibit good xylose utilization ability, the lack of tolerance to inhibitors generated in biomass pretreatment limits the application of such strains in the production of bioethanol from lignocellulosic biomass. By applying a sexual mating method, inhibitor tolerance was developed in xylose-utilizing strains. The final ethanol concentrations in simultaneous scarification and co-fermentation (SScF) process at 38 °C with hybrid strains were 50% higher than the SScF process with the xylose-fermenting parent strain. The strain viability of the hybrid strain E7-12 at 24 h was 282 times higher than the parent strain in the SScF process at 25% solid loading. Due to the improved sugar utilization, the final ethanol concentration reached 69.7 g/L (E7-11) and 70.0 g/L (E7-12), which were 25.3 g/L and 25.6 g/L higher than that of SScF with the xylose-fermenting strain, respectively.
Download PDF
Full Article
Hybridization Improves Inhibitor Tolerance of Xylose-fermenting Saccharomyces cerevisiae
He Liu, † Jia-Qing Zhu, † Xia Li, † Hui-Ze Li, Lei Qin, Hao Li, Xin Wang, Xue Bai, Wen-Chao Li, Bing-Zhi Li,* and Ying-Jin Yuan
Although some engineered S. cerevisiae strains exhibit good xylose utilization ability, the lack of tolerance to inhibitors generated in biomass pretreatment limits the application of such strains in the production of bioethanol from lignocellulosic biomass. By applying a sexual mating method, inhibitor tolerance was developed in xylose-utilizing strains. The final ethanol concentrations in simultaneous scarification and co-fermentation (SScF) process at 38 °C with hybrid strains were 50% higher than the SScF process with the xylose-fermenting parent strain. The strain viability of the hybrid strain E7-12 at 24 h was 282 times higher than the parent strain in the SScF process at 25% solid loading. Due to the improved sugar utilization, the final ethanol concentration reached 69.7 g/L (E7-11) and 70.0 g/L (E7-12), which were 25.3 g/L and 25.6 g/L higher than that of SScF with the xylose-fermenting strain, respectively.
Keywords: Hybridization; UV mutagenesis; Saccharomyces cerevisiae; Xylose utilization; Inhibitor tolerance; High temperature
Contact information: Key Laboratory of Systems Bioengineering (Ministry of Education), School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072, PR China; SynBio Research Platform, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072, PR China; *Corresponding author: bzli@tju.edu.cn; †Equal contribution
INTRODUCTION
An essential need for development of renewable and eco-friendly energy resources is emerging due to the global rise in energy consumption, predicted increase in energy demands in the near future, depletion of fossil fuel reserves with low extraction cost, and climate change (Agbor et al. 2011). Lignocellulosic biomass is regarded as an attractive substrate for a wide range of potential uses because of its renewability, large available quantities, and potential environmental benefits (Miret et al. 2016). However, after pretreatment and hydrolysis, the hydrolysates from lignocellulose, which are mainly composed of glucose and xylose, are accompanied by many inhibitors, such as furfural and acetic acid. Efficient microbial strains for the conversion of lignocellulose should be able to utilize most of sugars and tolerate the inhibitors.
The percentage of hemicellulose in biomass ranges between 20% and 40% depending on the raw materials (Gupta and Verma 2015). Xylose, the main degradation product from hemicellulose, is the second most abundant sugar in lignocellulose (Dussán et al. 2016). Clearly, efficient conversion of xylose, together with cellulose-derived glucose into ethanol is essential to reduce the cost per unit of ethanol produced (Sassner et al. 2008). However, native S. cerevisiae is unable to ferment xylose to ethanol (Batt et al. 1985). Recently, engineered strains for efficient xylose utilization, by introducing a xylose pathway, has attracted extensive attention. A xylose fermenting yeast, which expresses the xylose reductase (XR) and a NADP+-preferring xylitol dehydrogenase (XDH) mutant, was genetically constructed (Zha et al. 2014). Then an adapted strain (E7) with improved xylose fermentation capability (S. cerevisiae CGMCC NO. 6634) was obtained by repeated adaptation using xylose as the sole carbon source. It could completely consume 20 g/L xylose within 48 h in synthetic medium without inhibitor. However, just like many laboratory yeast strains, the mutant strain was found to be vulnerable to the presence of inhibitors (Zhu et al. 2015). In the SScF process with pretreated biomass at 25% solid loading, less than 30% xylose was used, and even glucose utilization was seriously repressed.
Many inhibitors are generated due to the severe conditions during the pretreatment process. These inhibitors, such as FAP (furfural, acetic acid, and phenols), challenge the subsequent fermentation (Wang et al. 2014; Zhu et al. 2015). A robust-fermenting yeast is required to convert all of the sugars released from lignocellulose to ethanol in the presence of inhibitors. However, many laboratory yeast strains are more vulnerable to inhibition in the real hydrolysates of fermentation processes compared with industrial yeast due to the high content of inhibitors (Hou and Yao 2011; Zhu et al. 2015; Dubey et al. 2016). To improve the tolerance of yeast strains to inhibitors, adaptation was widely applied to improve the strain tolerance (Hawkins and Doran-Peterson 2011; Wang et al. 2013; Gu et al. 2014). However, the adapted strains were usually unstable and susceptible to degeneration. Moreover, rational methods for strain improvement have yielded limited success due to the complex mechanism of stress tolerance (Gong et al. 2009; Ling et al. 2014).
Haploid yeast cells exist as one of two mating types, a and α, which are specified by two alternative alleles at the mating-type locus (MAT) (Leu and Murray 2006). Cells of the opposite mating type can be propagated through hybridization, which is one of the simplest and most effective ways to combine the traits of parent haploid strains (Yamada et al. 2010). Therefore, hybridization offers a potential approach to combine xylose utilization ability and inhibitor tolerance into one strain. S. cerevisiae YZ2 was obtained after two rounds of genome shuffling of ultraviolet mutants derived from the original strain S. cerevisiae 308. S. cerevisiae YZ2 showed significantly faster growth and higher cell viability in the presence of acetic acid (Zheng et al. 2011). Kato constructed a hybrid strain S. cerevisiae MN81400 through the mating of two recombinant xylose-fermenting Saccharomyces cerevisiae strains. They found that the hybrid strains exhibited higher ethanol yield under elevated temperatures and acidic conditions compared with their parent strains (Kato et al. 2012). These studies revealed that hybridization of xylose-fermenting Saccharomyces cerevisiae is a simple and effective way to improve ethanol ability.
In the study, we obtained a tolerant haploid yeast that was stemmed from a haploid industrial strain, and it was hybridized with the engineered xylose-utilizing haploid yeast (Fig. 1). This approach led to a rapid improvement in the tolerance to inhibitors in the selected strains, which retained both growth advantage and xylose fermenting capability. The potential of the hybrid strains applied as a workhorse in high temperature and high solid loading simultaneous saccharification and co-fermentation (SScF) was also investigated.
EXPERIMENTAL
Materials and Strains
The industrial S. cerevisiae strain (Product No. 80000012; Angel Yeast Co., Ltd., Yichang, China), a diploid strain, S. cerevisiae 10# (MATa) was derived from an industrial yeast strain (Angel) through sporulation according to the isolation of haploids method (Inoue et al. 2014), which was validated as MAT a. Then the isolation haploid, S. cerevisiae 10# (MATa) was used as the starting strain for UV mutation. Through UV mutation, 30 survival mutants were selected and named from U1 to U30. Four mutants (U1, U11, U12, and U24) were selected from the YPD medium (20 g/L glucose, 20 g/L peptone, 10 g/L yeast extract) with 60% FAP (100% FAP: 1.3 g/L furfural, 5.3 g/L acetic, 0.5 g/L phenol) as parent strains for mating (Wang et al. 2013). Another parent strain was a xylose-fermenting yeast (S. cerevisiae CGMCC NO. 6634), we named as E7. (Zha et al. 2014). The mating selection medium (YNBX) contained 6.7 g/L yeast nitrogen base (YNB), 20 g/L xylose, 20 g/L agar, and 20 g/L of xylose. Four hybrids were constructed and they were named as E7-1, E7-11, E7-12, and E7-24.
Corn stover pretreated via a dry dilute acid method was kindly provided from Professor Jie Bao’s group at the East China University of Science and Technology (Shanghai, China). The pretreatment reactor and process have been described in detail in a previous study (He et al. 2014). In this study, corn stover was pretreated for 3 min at 170 °C using 2.5% sulfuric acid at agitation of 50 rpm. Because dry dilute acid pretreatment (DDAP) is a dry-to-dry process, there is no liquid fraction after pretreatment. The moisture content of the pretreated biomass is up to 51.01%. The compositions of the pretreated corn stover (dry basis) were determined according to Laboratory Analytical Procedure (LAP) methods. The content of glucan, xylan and lignin in dry pretreated biomass is 41.25%, 15.87% and 22.04%, respectively. All prehydrolyzates, including high amount of xylose and inhibitors (acetate 2.76 g/100 g dry matter, furfural 0.28 g/100 g dry matter, 5-HMF 0.16 g/100 g dry matter), are contained in the solid phase of the pretreated corn stover. Then the pretreated corn stover was used as fermentation substrate.
Commercial enzymes Cellic Ctec2 (180 mg protein/mL) and Htec2 (200 mg protein/mL) were used in this study. The enzyme loading used in the SScF process was 10 mg and 5 mg protein/g dry matter for Cellic Ctec2 and Htec2, respectively. To avoid bacterial contamination, 50 mg/L ampicillin was added into the fermentation broth.
Methods
UV mutagenesis
A single colony of yeast was pre-cultivated in 5 mL YPD medium at 30 °C for 24 h. Then, seed cultures with an initial optical density (OD600) of 0.5 were transferred into 250 mL anaerobic vials with 50 mL YPD medium. The cultures were diluted to an OD600 of 1.0 after cultivation for 7 h. Next, 20 μL aliquots of serial dilutions (10-1) were spotted on YPD Agar plates with 30% FAP and exposed to UV light for 7 min at a distance of 56 cm, followed by incubation at 30 °C for 2 days in the dark. The surviving cells were then transferred to YPD-Agar plates with 60% FAP for further characterization.
Evaluation of UV mutagenesis and mating of haploid strains
To analyze the stress tolerance, serial dilution assay was performed. First, a single colony of S. cerevisiae cells from a YPD-Agar (20 g/L glucose, 20 g/L peptone, 10 g/L yeast extract, and 20 g/L agar) plate was cultivated in a glass tube containing 5 mL YPD medium (20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract) at 30 °C and 220 rpm for 8 h. Second, 500 mL seed culture was inoculated into a glass tube flask containing 5 mL YPD medium (20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract) at 30 °C and 220 rpm for 8 h (the growth of the seed was during the logarithmic phase). Then according to the measured OD600, the OD600 of the seed culture was diluted to 1.0. Then 5 μL aliquots of serial dilutions (10-1, 10-2, 10-3, 10-4, 10-5, 10-6, 10-7) were spotted on YPD-Agar plates containing 60% FAP (Wei et al. 2008). Almost all of the mutants grew better than the control strain on the YPD-Agar plate with 60% FAP. Among the 30 yeast strains tested, four colonies were selected, including U1, U11, U12, and U24, under inhibitor stress conditions (Fig. S1). Thus, U1, U11, U12, and U24 were selected as the parent strain for further mating with the xylose-fermenting MATα yeast E7.
Hybridization was conducted according to a modified version of the method as previously described (Inoue et al. 2014). The haploid MATa strains were cultivated in 5 mL YPD medium at 30 °C and 220 rpm. The haploid MATα strains were cultivated in 5 mL YPX medium with the same conditions. MATa and MATα were equally mixed in fresh YPD liquid medium in the tube. The mixture was incubated at 30 °C for 6 h and then diluted and plated on YNBX-Agar plates. Bigger colonies were selected as putative yeast hybrids for determination. A polymerase chain reaction (PCR) was adopted to identify the mating type of the putative yeast hybrids. Primer A (GCACGGAATATGGGAC-TACTTCG) and Primer C (AGTCACATCAAGATCGTTTATGG) were used to determine the mating type α; Primer B (ACTCCACTTCAAGTAAGAGTTTG) and Primer C (AGTCACATCAAGATCGTTTATGG) were used to determine the mating type a (Illuxley et al. 1990). The reaction mixtures of 10 µL contained 0.2 µL primer A and 0.2 µL primer C or 0.2 µL primer B and primer C, 1.0 µL 10×Fast Taq buffer, 0.25 µL dNTP, 0.1 µL Fast Taq, and 7.55 µL Milli-Q water. Fast Taq enzyme was purchased from Thermal (Shanghai, China). A PCR Cloning Kit was purchased from Fermentas (MD, USA). The thermal treatment reaction was 95 °C for 5 min, 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, 72 °C for 5 min, and 4 °C for the suitable time.
Calculation of inhibition degree at the first 12 h under stress conditions
The first seed culture was prepared by inoculating a single colony from a YPX-Agar plate (10 g/L yeast extract, 20 g/L peptone, 20 g/L xylose, and 20 g/L agar) into a tube containing 5 mL YPX medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L xylose). Then it was cultured at 30 ºC and 250 rpm for 24 h. The second seed culture for fermentation was prepared by transferring 5 mL first seed culture into a 250-mL conical flask with 50 mL YPX medium. Next, it was incubated for 12 h at 30 ºC and 250 rpm. The prepared second seed culture was harvested and centrifuged at 4,000 rpm for 5 min. Then, seed cultures with an initial optical density (OD600) of 1.0 were transferred into a 250-mL conical flask with 100 mL of YPX medium. The fermentation experiments were performed at 0% FAP, 30% FAP, 50% FAP, 70% FAP, and 100% FAP and 30 °C, 34 °C, 38 °C, 42 °C. Fermentation samples were collected after fermentation under stress conditions for 12 h and diluted using sterile water. The dilution rate for each sample was varied to obtain 20 to 200 colonies on a single plate. The plates were then cultured at 30 °C for 48 h. Colony forming units (CFU) on the plates were counted and viable cell density was calculated accordingly. The apparent inhibition degree based on the control (agar plate cultured at 30 °C or without inhibitors) was assessed according to the following equation,
Inhibition degree = 1 – N / N0 (1)
where N0 was the number of the viable cell calculated from the agar plate cultured on 30 °C or without inhibitors, and N was the number of viable cell calculated from agar plate cultured under stress conditions (higher temperatures and inhibitor concentrations).
Simultaneous saccharification and co-fermentation (SScF)
The first seed culture was prepared by inoculating a single colony from a YPX-Agar plate into a tube containing 5 mL YPX medium. Then it was cultured at 30 ºC, 250 rpm for 24 h. The second seed culture was prepared by transferring 500 μL first seed culture into a 250 mL conical flask with 100 mL of YPX medium. Then it was incubated for 18 h at 30 ºC and 250 rpm. The prepared second seed culture was harvested and centrifuged at 4,000 rpm for 5 min. Next, it was inoculated into the fermentation broth.
No detoxification process was employed for the pretreated corn stover before the fermentation process, and no extra organic nutrients or inorganic salt were added to the fermentation slurry during the SScF process. A rubber stopper with a syringe needle was used to seal the flasks as well as release the carbon dioxide produced during fermentation. All SScF experiments were performed in duplicates under anaerobic conditions using shaking flasks in an orbital incubator at 150 rpm for 120 h. Unless otherwise specified, the pretreated biomass was pre-hydrolyzed by enzymes at 50 ºC and 200 rpm for 12 h before the inoculation of yeast. Citrate buffers of 0.05 M and KOH of 3 M were used to regulate the pH of fermentation broth to 5.5.
Analyses of sugars, ethanol, cell growth, and measurement of viable cell density
Samples taken from the SScF process were centrifuged at 12,000 rpm for 5 min. The supernatant was filtered using a 0.22 µm filter. Ethanol, glucose, and xylose in the samples were analyzed using high performance liquid chromatography (HPLC) with an Aminex HP-87H column (Bio-Rad, Hercules, CA, USA) at 65 °C. The detection system used for HPLC analysis was a refractive index detector (Model 500, LabAlliance, Tianjin, China). The eluent was 5 mM H2SO4 with a flow rate of 0.6 mL/min (Qin et al. 2013).
The absorbance of the culture at 600 nm was measured with a spectrophotometer (Model 75-6 spectrophotometer; Tianjin Kanas Optical Analytical Instruments Co., Ltd., Tianjin, China) to monitor the cell growth. Fermentation samples were collected and diluted using sterile water. Then, 100 µL of each diluted sample was plated on a YPX agar medium. The dilution rate for each sample was varied to obtain 20 to 200 colonies on a single plate. The plates were then cultured at 30 °C for 48 h. Single colonies formed on the plates were counted and viable cell density was calculated accordingly.
RESULTS AND DISCUSSION
Tolerance to Inhibitors of the Hybrid Strains
To get robust hybrid strains, hybridization was performed between U1, U11, U12, U24, and the other parent xylose fermenting yeast strain (Fig. 1). The PCR was performed to identify the mating type of the putative yeast hybrids. When these three oligo-nucleotides were used in a single PCR, DNA at MATα generated a 404 bp product, whereas DNA at MATa generated a 544 bp product, which was consistent with the previous study (Illuxley et al. 1990).
We identified the mating type of hybrid strains (E7-1, E7-11, E7-12, E7-24). The MATa S. cerevisiae U11 and MATα S. cerevisiae E7 were used as the reference mating type strains. As shown in Fig. 2, the hybrid strains (E7-1, E7-11, E7-12, and E7-24) had two DNA products, and the MATα haploid strain (E7) and MATa haploid strain U11 had only one DNA product. These results indicated that hybrid strains were successfully constructed.
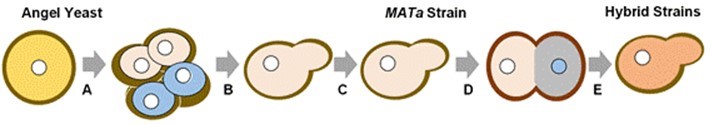
Fig. 1. Construction strategy of robust hybrid strains with xylose fermenting and inhibitor tolerance ability by sexual mating; A: Sporulation and selection; B: Selection; C: UV-mutation and selection; D: Mating with xylose-fermenting MATα Strain; and E: Selection and characterization
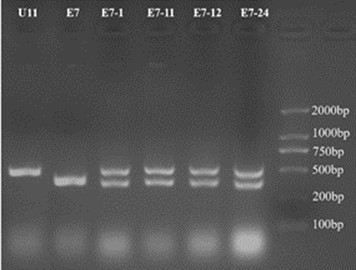
Fig. 2. Determination of mating type of six yeast strains by PCR
To evaluate the stress tolerance of the hybrid strains, two more stable hybrid strains, E7-11 and E7-12 were selected for characterization. The inhibition degree of high temperature and composite inhibitors were assessed according to Eq. 1. Our previous study revealed that high temperature could amplify the adverse effect of inhibitors on strain growth and fermentation (Zhu et al. 2015). Here, high temperature stress elicited a much higher inhibition degree to the haploid strain than the hybrid strains (Fig. 3A). For example, at 42 °C, the inhibition degree of E7 was 82.85%, while the inhibition degrees of E7-11 and E7-12 were 53.42% and 39.49%, respectively. This result demonstrated that the strain viability of hybrid strains was much higher than the parent xylose-fermenting strain under high temperature. In the presence of 30% FAP, E7-11 and E7-12 were less inhibited (inhibition degrees were 12.74% and 15.11%, respectively) compared with E7 (55.13%) (Fig. 3B). Growth of the strains at other FAP concentrations (50%, 70%, and 100% FAP) showed the same trend, which suggested that the hybrid strains inherited the inhibitor tolerance from the industrial strain. The hybridization of two different strains proved to be feasible in this study as well as in a previous study, in which a hybrid yeast strain with improved inhibitor tolerance was obtained by protoplast fusion of Spathaspora passalidarum M7 and a robust yeast Saccharomyces cerevisiae ATCC 96581 (Hou and Yao 2011).
Effect of Inhibitors on the Hybrid and Haploid Strain in Synthetic Medium
The S. cerevisiae used as the parent strain is an industrial yeast, Angel Yeast, which could grow and ferment well under high temperatures. Moreover, it also has good inhibitor tolerance. Therefore, the selected hybrid strains, E7-11 and E7-12, were expected to convert glucose and xylose to ethanol under stress conditions (high temperature and inhibitors). The fermentation performance at 40 °C and combined inhibitors using a synthetic medium was investigated, respectively (Fig. 4).
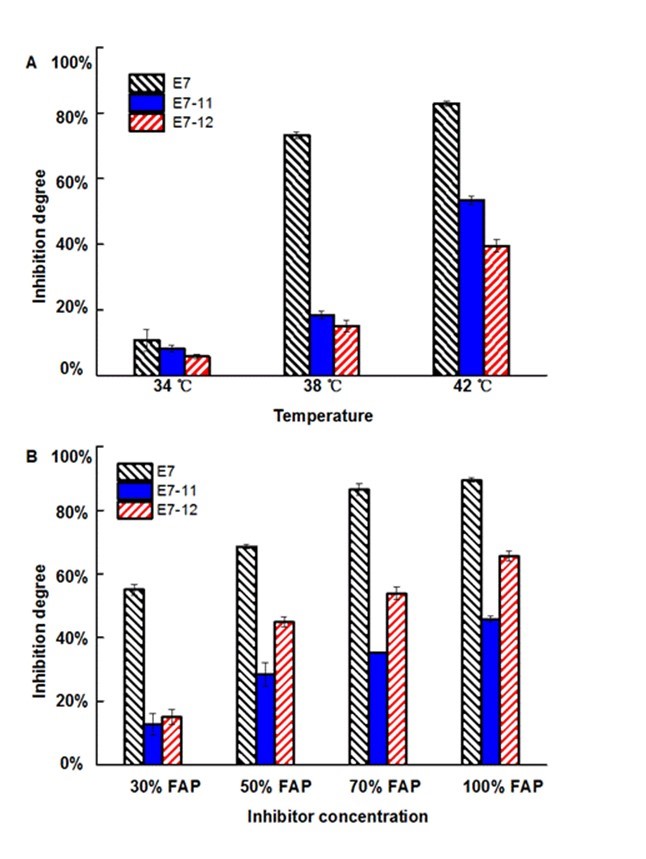
Fig. 3. Inhibition degrees to hybrid strains (E7-11 and E7-12) and haploid strain (E7) under different temperatures (A) and different inhibitor concentrations (100% FAP: 1.3 g/L furfural, 5.3 g/L acetic, 0.5 g/L phenol) (B); the values are means ± S.D of triplicate analyses
The parent xylose-fermenting strain showed poor fermentation performance at 40 °C. Only minimal monosaccharide was consumed, and less than 10 g/L ethanol was produced (Fig. 4A and B). The cell density was almost constant during the fermentation process (Fig. 4B). This result indicated that the parent strain could not grow and ferment well at elevated temperatures. In contrast, fermentation performances of the hybrid strains were much better. When the hybrid strain E7-11 was used, sugars were quickly consumed, and glucose and xylose conversion increased 60% and 15%, respectively, compared with the parent strain. The final ethanol concentration by E7-11 reached 28.2 g/L, which was 287% higher than the parent strain. Fermentation with the hybrid strain E7-12 showed better performance than E7-11: the glucose consumption rate reached 1.2 g/L/h in the first 12 h, there was less than 1 g/L glucose left in the fermentation broth, and 32.4 g/L ethanol was produced (corresponding to the 73.8% ethanol yield). The ethanol yield of parent xylose-fermenting strain E7 was 16.7%, while the ethanol yield of hybrid strains E7-11 and E7-12 were 64.6% and 73.8%, the conversion of both glucose and xylose of hybrid strains were higher than the parent xylose-fermenting stain. Compared with the parent xylose-fermenting strain E7, the increased xylose consumption of hybrid strains E7-11 and E7-12 were 4.8 g/L and 7.6 g/L, the increased glucose consumption of hybrid strains E7-11 and E7-12 were 34.4 g/L and 42.1 g/L. OD600 of hybrid strain E7-12 reached 7.32 at 24 h. These results suggested that the hybrid stain E7-12 was thermal tolerant and had the potential to be applied in SScF at higher temperatures. The increased tolerance of the hybrid strains to temperature can significantly decrease the fuel ethanol productions costs (Abdel-Banat et al. 2010).
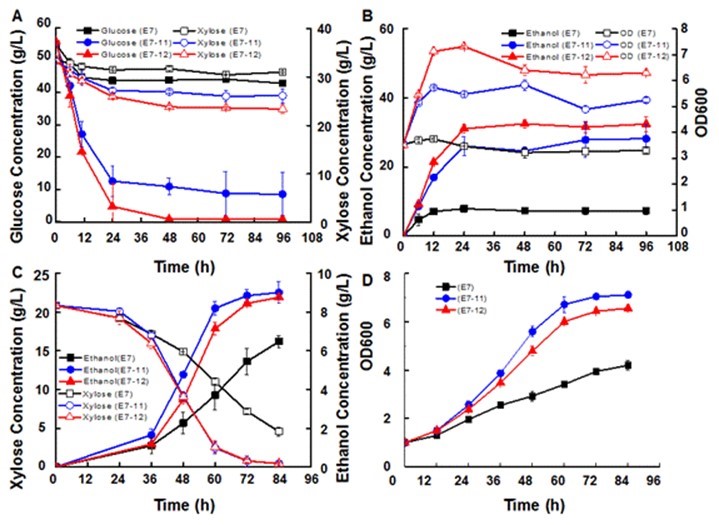
Fig. 4. Effect of temperature of 40 ºC (A and B) and 30% FAP (C and D) on the sugar utilization, ethanol production, and cell growth of hybrid strains and the haploid strain in synthetic medium; the values are means ± standard deviation of triplicate analyses
Regardless of the pretreatment technology, toxic compounds, such as furfural, acetic acid, and phenol are produced during pretreatment, and they inhibit microbial growth, metabolism, and ethanol yield (Klinke et al. 2004). In previous study, FAP were added into the YPX medium to investigate the inhibitor tolerance of the hybrid strains (Wang et al. 2013). Therefore, in our study, FAP were also added to YPX medium. When no inhibitors were added into the medium, there was no substantial difference in xylose consumption and ethanol production between the xylose-fermenting strain and hybrid strains. However, the hybrid strains showed improved growth. The OD600 reached 10.5 (E7-11) and 9.8 (E7-12) at 24 h, which was 54% and 44% higher than that of the parent xylose-fermenting strain (Fig. S2). When 30% FAP was added into the medium, there was 4.6 g/L xylose left with the parent xylose-fermenting strain, while all of the xylose was consumed with the hybrid strains. So, compared with the parent xylose-fermenting strain, the xylose-fermenting consumption of hybrid strains were increased by 23% in the presence of inhibitors. Correspondingly, the final ethanol concentrations for the hybrid strains were 39% and 35% higher than the xylose-fermenting strain. These results indicated that hybridization increased the inhibitor tolerance of the hybrid strains. Previous researchers have obtained a hybrid xylose-fermenting yeast strain with improved inhibitor tolerance via protoplast fusion (Hou and Yao 2011) or meiotic recombination with diploid strains (Demeke et al. 2013). In the present study, a hybrid xylose-fermenting yeast strain was obtained by hybridization of two haploid strains, which showed improved inhibitor tolerance and temperature tolerance.
Performances of the Hybrid Strains and Haploid Strain in High Solids Loading SScF
A previous report demonstrated that only when the concentration exceeds 40 g/L would the production of lignocellulosic ethanol become economically feasible (Zacchi and Axelsson 1989). To increase ethanol concentration, a high solids loading is necessary for fermentation (Koppram et al. 2014). However, high solids loading increases the inhibitor concentration, which could severely repress the fermentation. In the present study, SScF at 25% solid loading was performed to investigate the effect of high solid loading on the hybrid strains.
SScF with the hybrid strains at 25% solid loading at 34 °C and 150 rpm showed an improved fermentation performance compared to the parent xylose-fermenting strain. Glucose in SScF with the hybrid strains was almost completely consumed in the initial 48 h and then maintained at a low level, while it gradually accumulated to 45.8 g/L in SScF with the xylose-fermenting strain (Fig. 5). Glucose utilization in SScF processes with the parent xylose-fermenting strain was severely repressed. Though xylose utilization in all SScF were inhibited, the xylose utilization of hybrid strains was slightly better than the parent xylose-fermenting strain. Because of the significantly improved glucose utilization, the final ethanol concentration reached 69.7 g/L (E7-11) and 70.0 g/L (E7-12), which were 56.95% and 57.66% higher than SScF with the parent xylose-fermenting strain, respectively (Fig. 5). The enhanced glucose consumption of the hybrid strains might be attributed to the improved strain viability. Therefore, the strain viability was monitored during the fermentation process. Strain viabilities decreased during the SScF process (Fig. 5C), which indicated that the strains were affected by the absence of nutrients. Strain viability of the hybrid strains during the entire fermentation process was much higher than the parent xylose-fermenting strain. The strain viability of the hybrid strains at 24 h were 179 (E7-11) and 282 (E7-12) times higher than the parent xylose-fermenting strain. These results were consistent with the author’s previous study and suggested that the improved strain viability led to the increased bioethanol production (Zhu et al. 2015).
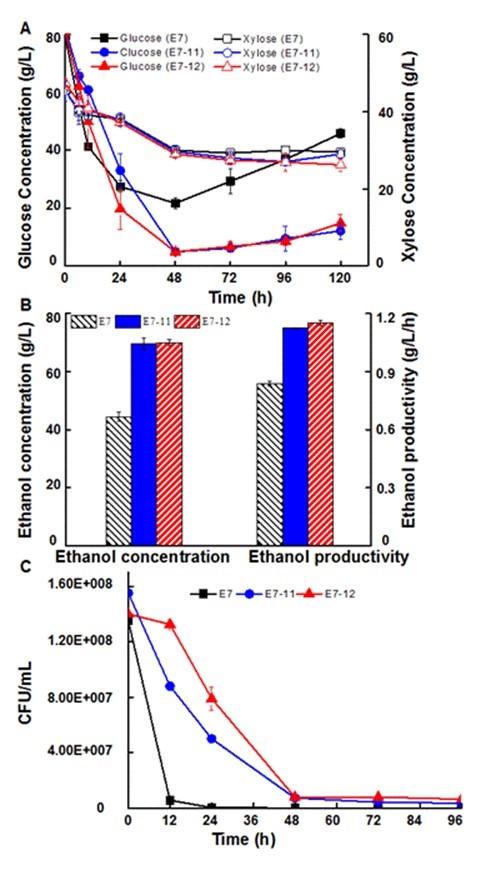
Fig. 5. Effect of inhibitors on sugar consumption (A), ethanol production and ethanol productivity (B), and the strain viability (C) during the SScF process of the hybrid strains and haploid strain; Ethanol productivity was calculated at 48 h and the values are means ± S.D of triplicate analyses
To deal with the inhibitors issue, previous researchers have discovered many strategies. Detoxification processes, such as washing, chemical detoxification, or biological detoxification, prior to the fermentation are an effective approach to removing the inhibitory and toxic compounds (Qin et al. 2013; Wang et al. 2013). Fed-batch fermentation mode was also applied to reduce the repression effect of inhibitors (Zhu et al. 2015). However, these extra operations might increase the cost of biorefinery. With in situ removal of ethanol, a monomeric sugar conversion as high as about 93% was achieved at 9% glucan loading (24.9 wt% solids loading) (Jin et al. 2017). In SScF with 9% (w/w) glucan loading of AFEX pretreated corn stover at 35 °C, a wild S. cerevisiae strain obtained by phenotypic selection exhibited higher cellular viabilities and produced 51.3 g/L ethanol (Jin et al. 2013). In this study, the hybrid strains allowed the direct fermentation of pretreated biomass without detoxification processes, which might have effectively reduced the cost of bioethanol production.
Previous study showed that xylose utilization was much more sensitive to inhibitors (Zhu et al. 2015). In present study, Fig. 4 showed that xylose utilization increased by 23% when hybrid strains were used in the synthetic medium in the presence of 30% FAP. However, xylose utilization increased less compared with the significant improvement of glucose utilization in high solid loading SScF. There was still a lot of residual xylose at the end of fermentation process. From the above results, it could be inferred that it is much more difficult to improve xylose utilization in the presence of inhibitors. The hybrid strains showed much potential to improve xylose utilization, which would be the focus for the future work.
Effect of Inhibitors on the Hybrid Strains and Haploid Strain in SScF Process at High Temperature
The previous report demonstrated the synergistic effect of high temperature and the inhibitors on yeast in SScF of DDAP pretreated corn stover (Zhu et al. 2015). Simultaneous saccharification and co-fermentation at high temperatures has a lot of advantages, such as minimizing the risk of ethanol toxicity for the culture and reducing the cost required for cooling (Balat 2011; Kuhad et al. 2011). Furthermore, cellulolytic enzymes require high temperatures for biomass hydrolysis. Saccharomyces cerevisiae is naturally mesophilic, but fermentation is an exothermic process. As a result, the viability of the cells will be severely repressed by the increased temperature in the reaction vessel during fermentation (Edgardo et al. 2008). Thermotolerant yeast was needed for SScF at high temperatures. Therefore, SScF of DDAP pretreated corn stover (20% solid loading) at 34 °C, 36 °C, and 38 °C were conducted to test the thermotolerance of the hybrid strains (Fig. 6).
No major differences in monosaccharide (glucose and xylose) consumption and ethanol production were observed between the parent xylose-fermenting strain and the hybrid strains in SScF at 34 °C. However, glucose consumption rates with hybrid strains reached 7.3 g/L/h and 7.6 g/L/h (during the first 12 h), which were much higher than SScF with the parent xylose-fermenting strain (5.4 g/L/h). Correspondingly, the ethanol productivities of the two hybrid strains during the first 12 h were both 1.3 g/L/h higher than the parent strain. This may have been attributed to the improved inhibitor tolerance of the hybrid strains, which could result in shorter time lag phases and higher glucose consumption rates (Hou and Yao 2011; Wang et al. 2013). When higher temperatures (36 °C and 38 °C) were applied in the SScF processes, all of the xylose-fermenting strains showed declined fermentation performance: although glucose was quickly consumed in the first 24 h, less than half of the released glucose in 12 h pre-hydrolysis was utilized. In addition, glucose accumulated to 60.5 and 76.9 g/L in the late fermentation phase (in 120 h) (Fig. 6), which indicated that the strain activity was very low and the glucose release via enzymatic hydrolysis was faster than the glucose consumption by the strains. Xylose consumption was even worse, and the xylose concentration remained almost the same during the entire fermentation process at 38 °C. Previous studies have reported that xylose utilization was more prone to be repressed by inhibitors than glucose utilization (Wang et al. 2013; Zhu et al. 2015). From these results, it can be inferred that the xylose consumption was more sensitive to inhibitors under higher temperature. As the fermentation temperature increased, the final ethanol concentration decreased rapidly. The final ethanol concentration was 60.4 g/L in SScF at 34 °C. When 36 °C was applied, it decreased by more than 50% (to 29.2 g/L). When the fermentation temperature increased to 38 °C, the final ethanol concentration decreased 73.4%. There was only 15.8 g/L ethanol in the fermentation broth. Clearly, the parent strain could not be used as a workhorse to conduct high temperature SScF.
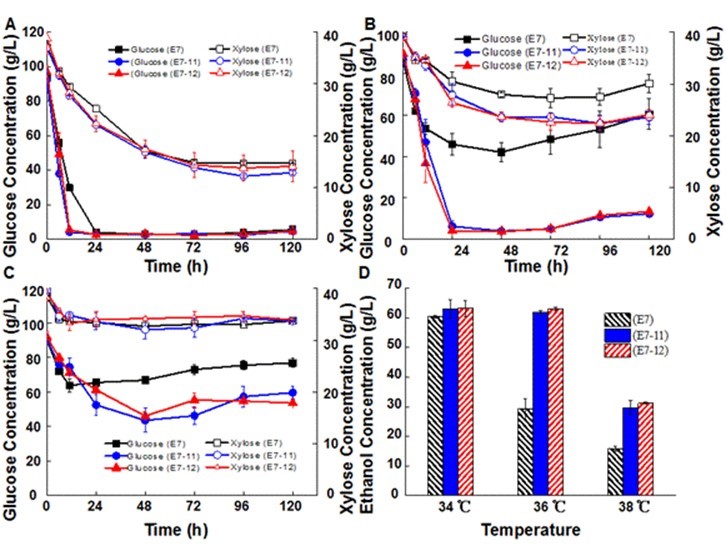
Fig. 6. Effect of inhibitors on the hybrid strains and haploid strain in SScF process at high temperature, A: 34 °C, B: 36 °C, and C: 38 °C
Compared with the parent xylose-fermenting strain, SScF with the hybrid strains at a higher temperature (36 °C) showed an improved fermentation performance. Glucose was almost completely consumed in the initial 24 h and maintained at a low level during the entire fermentation process, which was considered to be beneficial for xylose consumption (Pitkänen et al. 2003). Thus, compared to the parent xylose-fermenting strain, xylose utilization increased 12.5% and 12.3% when the hybrid strains E7-11 and E7-12 were used in SScF. The final ethanol concentration (at 120 h) at 36 °C stayed almost the same with SScF at 34 °C, which indicated that the hybrid strains could grow and ferment well in real lignocellulosic hydrolysates at higher temperatures. There was 61.9 g/L and 63.0 g/L ethanol produced in the fermentation by strains E7-11 and E7-12, respectively, which were 32.7 g/L and 33.8 g/L higher than SScF with the parent xylose-fermenting strain, respectively. These results suggested that the hybrid strains inherited the ability to resist high temperature from their industrial parent strain. When the fermentation temperature further increased to 38 °C, the hybrid strains were severely repressed. Less than half of the initial glucose was consumed and the xylose concentration almost maintained the same during the entire fermentation process. However, it still showed much better fermentation performance than SScF with the xylose-fermenting parent strain. The final ethanol concentration was 50% higher than the SScF with the parent xylose-fermenting strain.
The SScF at high temperature and high solid loading was preferred due to lower cooling costs (Balat 2011; Kuhad et al. 2011), lower distillation costs (Sassner et al. 2008), and so on. Therefore, strain development for inhibitor and temperature tolerance is attractive. In the present study, strains acquired by hybridization showed increased inhibitor and temperature tolerance compared with their parent xylose-fermenting strain. The results confirmed that hybridization is a simple way to improve the tolerance of xylose-fermenting S. cerevisiae, the hybrid strains have the potential to be applied as a workhorse in high solid loading SScF under high temperature conditions.
CONCLUSIONS
- Hybrid S. cerevisiae strains were successfully constructed through hybridization between an engineered xylose-fermenting strain and an industrial inhibitor-tolerant strain. The viability of the hybrid strains E7-11 and E7-12 at 24 h were 179 and 282 times higher than the parent xylose-fermenting strain in the SScF process, which resulted in the improvement of sugar utilization. The final ethanol concentration reached 69.7 g/L (E7-11) and 70.0 g/L (E7-12) in SScF with 25% solid loading of pretreated corn stover, which were 25.3 g/L and 25.6 g/L higher than that with the parent xylose-fermenting strain, respectively.
- It is much more difficult to improve xylose utilization in the presence of inhibitors. In the synthetic medium fermentation with 30%FAP, the xylose-fermenting consumption were improved by 23% using hybrid strains, while in high solid loading SScF processes, xylose utilization increased less compared with the significant improvement of glucose utilization
- Results herein exhibited the potential application of the hybrid strains as a workhorse in SScF at high temperatures and high solid loading.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technology of China (2013CB733601 and 2015DFA00960), the National Natural Science Foundation of China (21576198 and 21622605), and the Science and Technology Program of Tianjin (13RCGFSY19800).
REFERENCES CITED
Abdel-Banat, B.M., Hoshida, H., Ano, A., Nonklang, S., and Akada, R. (2010). “High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast, ” Appl. Microbiol. Biot. 85(4), 861-867. DOI: 10.1007/s00253-009-2248-5
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., and Levin, D. B. (2011). “Biomass pretreatment: Fundamentals toward application,” Biotechnol. Adv. 29(6), 675-85. DOI: 10.1016/j.biotechadv.2011.05.005
Balat, M. (2011). “Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review,” Energ. Convers. Manage. 52(2), 858-875. DOI: 10.1016/j.enconman.2010.08.013
Batt, C. A., Carvallo, S., Easson, D. D., Akedo, M., and Sinskey, A. J. (1985). “Direct evidence for xylose metabolic pathway in Saccharomyces cerevisiae,” Biotechnol. Bioeng. 28(4), 549-553. DOI: 10.1002/bit.260280411
Demeke, M. M., Dumortier, F., Li, Y. Y., Broeckx, T, Foulquié-Moreno, R. M.,
and Thevelein, J. M. (2013). “Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production,” Biotechnol. Biofuel. 6(1), 120. DOI: 10.1186/1754-6834-6-120
Dubey, R., Jakeer, S., and Gaur, N. A. (2016). “Screening of natural yeast isolates under the effects of stresses associated with second-generation biofuel production,” J. Biosci. Bioeng. 121(5), 509-516. DOI: 10.1016/j.jbiosc.2015.09.006
Dussán, K. J., Silva, D. D. V., Perez, V. H., and da Silva, S. S. (2016). “Evaluation of oxygen availability on ethanol production from sugarcane bagasse hydrolysate in a batch bioreactor using two strains of xylose-fermenting yeast,” Renew. Energ. 87, 703-710. DOI: 10.1016/j.renene.2015.10.065
Edgardo, A., Carolina, P., Manuel, R., Juanita, F., and Baeza, J. (2008). “Selection of thermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production,” Enzyme Microb. Tech. 43(2), 120-123. DOI: 10.1016/j.enzmictec.2008.02.007
Gong, J., Zheng, H., Wu, Z., Chen, T., and Zhao, X. (2009). “Genome shuffling: Progress and applications for phenotype improvement,” Biotechnol. Adv. 27(6), 996-1005. DOI: 10.1016/j.biotechadv.2009.05.016
Gu, H., Zhang, J., and Bao, J. (2014). “Inhibitor analysis and adaptive evolution of Saccharomyces cerevisiae for simultaneous saccharification and ethanol fermentation from industrial waste corncob residues,” Bioresource Technol. 157(2), 6-13. DOI: 10.1016/j.biortech.2014.01.060
Gupta, A., and Verma, J. P. (2015). “Sustainable bio-ethanol production from agro-residues: A review,” Renew. Sust. Energ. Rev. 41(41), 550-567. DOI: 10.1016/j.rser.2014.08.032
Hawkins, G. M., and Doran-Peterson, J. (2011). “A strain of Saccharomyces cerevisiae evolved for fermentation of lignocellulosic biomass displays improved growth and fermentative ability in high solids concentrations and in the presence of inhibitory compounds,” Biotechnol. Biofuel. 4(1). DOI: 10.1186/1754-6834-4-49
He, Y., Zhang, J., and Bao, J. (2014). “Dry dilute acid pretreatment by co-currently feeding of corn stover feedstock and dilute acid solution without impregnation,” Bioresource Technol. 158(4), 360-364. DOI: 10.1016/j.biortech.2014.02.074
Hou, X., and Yao, S. (2011). “Improved inhibitor tolerance in xylose-fermenting yeast Spathaspora passalidarum by mutagenesis and protoplast fusion,” Appl. Microbiol. Biot. 93(6), 2591-2601. DOI: 10.1007/s00253-011-3693-5
Illuxley, C., Green, E. D., and Dunbam, I. (1990). “Rapid assessment of S. cerevisiae mating type by PCR,” Trends Genet. 6(8), 236-236. DOI: 10.1016/0168-9525(90)90190-H
Inoue, H., Hashimoto, S., Matsushika, A., Watanabe, S., and Sawayama, S. (2014). “Breeding of a xylose-fermenting hybrid strain by mating genetically engineered haploid strains derived from industrial Saccharomyces cerevisiae,” J. Ind. Microbiol. Biot. 41(12), 1773-1781. DOI: 10.1007/s10295-014-1531-3
Jin, M., Sarks, C., Bals, B. D., Posawatz, N., Gunawan, C., Dale, B. E., and Balan, V. (2017). “Toward high solids loading process for lignocellulosic biofuel production at a low cost,”Biotechnol. Bioeng. 114(5), 980-989. DOI: 10.1002/bit.26229
Jin, M., Sarks, C., Gunawan, C., Bice, B. D., Simonett, S. P., Narasimhan, R.A., Willis, L. B., Dale, B. E., Balan, V., and Sato, T. K.(2013). “Phenotypic selection of a wild Saccharomyces cerevisiae strain for simultaneous saccharification and co-fermentation of AFEX™ pretreated corn stover,” Biotechnol. Biofuel. 6(1), 1-14. DOI: 10.1186/1754-6834-6-108
Kato, H., Suyama, H., Yamada, R., Hasunuma, T., and Kondo, A. (2012). “Improvements in ethanol production from xylose by mating recombinant xylose-fermenting Saccharomyces cerevisiae strains,” Appl. Microbiol. Biot. 94(6), 1585-1592. DOI: 10.1007/s00253-012-3914-6
Klinke, H. B., Thomsen, A. B., and Ahring, B. K. (2004). “Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass,” Appl. Microbiol. Biot. 66(1), 10-26. DOI: 10.1007/s00253-004-1642-2
Koppram, R., Tomas-Pejo, E., Xiros, C., and Olsson, L. (2014). “Lignocellulosic ethanol production at high-gravity: Challenges and perspectives,” Trends Biotechnol. 32(1), 46-53. DOI: 10.1016/j.tibtech.2013.10.003
Kuhad, R. C., Gupta, R., Khasa, Y. P., Singh, A., and Zhang, Y. H. P. (2011). “Bioethanol production from pentose sugars: Current status and future prospects,” Renew. Sust. Energ. Rev. 15(9), 4950-4962. DOI: 10.1016/j.rser.2011.07.058
Leu, J. Y., and Murray, A. W. (2006). “Experimental evolution of mating discrimination in budding yeast,” Curr. Biol. 16(3), 280-286. DOI: 10.1016/j.cub.2005.12.028
Ling, H., Teo, W., Chen, B., Leong, S. S., and Chang, M. W. (2014). “Microbial tolerance engineering toward biochemical production: From lignocellulose to products,” Curr. Opin. Biotech. 29, 99-106. DOI: 10.1016/j.copbio.2014.03.005
Miret, C., Chazara, P., Montastruc, L., Negny, S., and Domenech, S. (2016). “Design of bioethanol green supply chain: Comparison between first and second generation biomass concerning economic, environmental and social criteria,” Comput. Chem. Eng. 85, 16-35. DOI: 10.1016/j.compchemeng.2015.10.008
Pitkänen, J. P., Aristidou, A., Salusjärvi, L., Ruohonen, L., and Penttilä, M. (2003). “Metabolic flux analysis of xylose metabolism in recombinant Saccharomyces cerevisiae using continuous culture,” Metab. Eng. 5(1),16-31. DOI: 10.1016/S1096-7176(02)00012-5
Qin, L., Liu, Z. H., Jin, M., Li, B. Z., and Yuan, Y. J. (2013). “High temperature aqueous ammonia pretreatment and post-washing enhance the high solids enzymatic hydrolysis of corn stover,” Bioresource Technol. 146(10), 504-511. DOI: 10.1016/j.biortech.2013.07.099
Sassner, P., Galbe, M., and Zacchi, G. (2008). “Techno-economic evaluation of bioethanol production from three different lignocellulosic materials,” Biomass. Bioenerg. 32(5), 422-430. DOI: 10.1016/j.biombioe.2007.10.014
Wang, X., Jin, M., Balan, V., Jones, A. D., Li, X., Li, B. Z., Dale, B. E., and Yuan, Y. J. (2014). “Comparative metabolic profiling revealed limitations in xylose-fermenting yeast during co-fermentation of glucose and xylose in the presence of inhibitors,” Biotechnol. Bioeng. 111(1), 152-164. DOI: 10.1002/bit.24992
Wang, X., Li, B. Z., Ding, M. Z., Zhang, W. W., and Yuan, Y. J. (2013). “Metabolomic analysis reveals key metabolites related to the rapid adaptation of Saccharomyce cerevisiae to multiple inhibitors of furfural, acetic acid, and phenol,” OMICS Int. 17(3), 150-159. DOI: 10.1089/omi.2012.0093
Wei, P. Y., Li, Z., He, P., Lin, Y. P., and Jiang, N. (2008). “Genome shuffling in the ethanologenic yeast Candida krusei to improve acetic acid tolerance,” Biotechnol. Appl. Bioc. 49(2), 113-120. DOI: 10.1042/BA20070072
Yamada, R., Tanaka, T., Ogino, C., and Kondo, A. (2010). “Gene copy number and polyploidy on products formation in yeast,” Appl. Microbiol. Biot. 88(4), 849-857. DOI: 10.1007/s00253-010-2850-6
Zacchi, G., and Axelsson, A. (1989). “Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions,” Biotechnol. Bioeng. 34(2), 223-233. DOI: 10.1002/bit.260340211
Zha, J., Shen, M., Hu, M., Song, H., and Yuan, Y. (2014). “Enhanced expression of genes involved in initial xylose metabolism and the oxidative pentose phosphate pathway in the improved xylose-utilizing Saccharomyces cerevisiae through evolutionary engineering,” J. Ind. Microbiol. Biot. 41, 27-39. DOI: 10.1007/s10295-013-1350-y
Zheng, D.Q., Wu, X.C., Wang, P. M. (2011). “Drug resistance marker-aided genome shuffling to improve acetic acid tolerance in Saccharomyces cerevisiae,” J. Ind. Microbial. Biot. 38, 415-422. DOI: 10.1007/s10295-010-0784-8
Zhu, J. Q., Qin, L., Li, W. C., Zhang, J., Bao, J., Huang, Y. D., Li, B. Z., and Yuan, Y. J. (2015). “Simultaneous saccharification and co-fermentation of dry diluted acid pretreated corn stover at high dry matter loading: Overcoming the inhibitors by non-tolerant yeast,” Bioresource Technol. 198, 39-46. DOI: 10.1016/j.biortech.2015.08.140
Article submitted: January 2, 2017; Peer review completed: May 2, 2017; Revised version received and accepted: May 9, 2017; Published: May 15, 2017.
DOI: 10.15376/biores.12.3.4737-4753
