Abstract
The influence of copper and its nanoparticles was studied relative to growth and ultrastructure of Aspergillus niger. Laccase production by A. niger using corn cobs as substrate at different concentrations of CuSO4 and copper nanoparticles (CuNPs) is reported. Fungus growth was induced at 100 ppm of CuNPs and CuSO4, while at 300 ppm, the growth inhibition was 65.6% and 86.9%, respectively. Fungus sporulation was reduced to 30.4% and 47.6% at 300 ppm of CuNPs and CuSO4, respectively, compared to the control (100%). Transmission electron microscopy revealed that CuSO4 and CuNPs treatments encouraged the deformed appearance of the fungus at 200 ppm and 300 ppm, particularly CuNPs. The CuNPs and CuSO4 induced laccase production at 1.67 U/mL and 1.51 U/mL at optimum concentrations 0.15 mM and 0.25 mM, respectively. The optimum concentrations of CuNPs and CuSO4 led to reduced incubation periods of 12 d and 14 d, respectively, required to produce the highest amount of laccase (1.66 U/mL and 1.53 U/mL), while without treatments, the incubation period increased to 16 d required for the highest amount of laccase production (1.36 U/mL). Induction of laccase production at acidic pH and at 30 °C was recorded with the addition of CuSO4 and CuNPs, while its effects were slight at pH above 6.
Download PDF
Full Article
Impact of Copper and Its Nanoparticles on Growth, Ultrastructure, and Laccase Production of Aspergillus niger using Corn Cobs Wastes
Tarek M. Abdel Ghany,a,* Marwah M. Bakri,b Aisha M. H. Al-Rajhi,c Mohamed A. Al Abboud,d M. M. Alawlaqi,d and Abdel Rhaman M. Shater d,e
The influence of copper and its nanoparticles was studied relative to growth and ultrastructure of Aspergillus niger. Laccase production by A. niger using corn cobs as substrate at different concentrations of CuSO4 and copper nanoparticles (CuNPs) is reported. Fungus growth was induced at 100 ppm of CuNPs and CuSO4, while at 300 ppm, the growth inhibition was 65.6% and 86.9%, respectively. Fungus sporulation was reduced to 30.4% and 47.6% at 300 ppm of CuNPs and CuSO4, respectively, compared to the control (100%). Transmission electron microscopy revealed that CuSO4 and CuNPs treatments encouraged the deformed appearance of the fungus at 200 ppm and 300 ppm, particularly CuNPs. The CuNPs and CuSO4 induced laccase production at 1.67 U/mL and 1.51 U/mL at optimum concentrations 0.15 mM and 0.25 mM, respectively. The optimum concentrations of CuNPs and CuSO4 led to reduced incubation periods of 12 d and 14 d, respectively, required to produce the highest amount of laccase (1.66 U/mL and 1.53 U/mL), while without treatments, the incubation period increased to 16 d required for the highest amount of laccase production (1.36 U/mL). Induction of laccase production at acidic pH and at 30 °C was recorded with the addition of CuSO4 and CuNPs, while its effects were slight at pH above 6.
Keywords: Copper nanoparticles; Ultrastructure; Laccase; A. niger; Corn cobs
Contact information: a: Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt11725; b: University College, Al-Ardah, Jazan University, Jazan, Saudi Arabia; c: Biology Department, Faculty of Science, Princess Nora Bent Abdularahman University, Riyadh, Saudi Arabia; d: Biology Department, Faculty of Science, Jazan University, Jazan, Saudi Arabia; e: Biology Department, Faculty of Science, Thamar University, Yemen; * Corresponding author: tabdelghany.201@azhar.edu.eg
INTRODUCTION
Several studies have shown the biocidal activity of copper with broad-spectrum effectiveness against bacteria and fungi. Metal nanoparticles (NPs) are mainly used as fungicidal agents against phyto- and human-pathogens (Abdel Ghany 2013; Abdel Ghany et al. 2013, 2018a,b; Ganash et al. 2018). Among the metal NPs, copper nanoparticles (CuNPs) have been used increasingly in numerous applications in the current decade due to their cost-effectiveness; however, their impact on agricultural soil microorganisms has been poorly investigated (Rajput et al. 2019). Copper at the nanoscale demonstrates major antimicrobial activity (Gamalero et al. 2009; Raffi et al. 2010; Durán and Seabra 2012; Jia et al. 2012). Banik and Pérez-de-Luque (2017) recommend that CuNPs be applied as an alternative to chemical fungicides or non-nanoform copper, or at minimum scale used as an additive for increasing and enhancing the impact of commercial products of fungicides. Therefore, the antifungal properties of CuNPs alone or with other chemical fungicides have been studied (Banik and Pérez-de-Luque 2017; Chalandar et al. 2017). Moreover, the antibacterial activity of CuNPs was also investigated against Gram –ve and Gram +ve bacteria (Gopalakrishnan et al. 2012; Amatya and Joshi 2020).
Incorporation of 50 mg/L CuNPs with copper oxychloride (non-nano copper) enhances the inhibition of Phytophthora cinnamomi growth compared to the inhibition at copper oxychloride application alone. Banik and Pérez-de-Luque (2017) recorded synergistic action of CuNPs with copper oxychloride against growth development and sporulation of Alternaria alternata. Recently, results of Sarkar et al. (2020) detected highest activity of defense enzymes and total phenolic compounds in the roots of Lens culinaris treated by 0.025 mgmL−1 CuNPs; therefore, CuNPs may be used as a potent plant defense booster. An increase in lignification of soybean root cells was recorded after CuNPs treatment (Nair and Chung 2014).
Microbial cell walls have a greater capability to accumulate metals from the surrounding environment. Penicillium ochrochloron accumulates Cu2+ from the environment in cell walls (Fukami et al. 1983). High levels of potentially toxic elements create side effects on microbial growth. Besides the toxicity, the potentially toxic elements can cause changes in or block enzyme action, inhibit proteins, carbohydrates, lipids, and nucleic acids synthesis, as well as induce disturbance of internal organelles in the cell (Denkhaus and Salnikow 2002). Changes in fatty acids of cell membrane as well as lowered polyunsaturated fatty acid content also have been reported (García et al. 2005). Hefnawy et al. (2009) investigated the rate of fungal sporulation under effects of copper in growth medium, where perithecia and spores numbers were markedly reduced with injure of seta, conidiophores, and phialides. Stohs and Bagchi (1995) declared that the elevated levels of Cu (II) and Zn cause a fast decline in membrane integrity, which is generally manifested by leakage of mobile cellular solutes and cell death.
The relationship between laccases and copper is due to structural properties, where several studies have identified laccases as glycosylated polyphenol oxidases that include four copper ions for each molecule. Fernandes et al. (2008) report that laccases are broadly spread among microorganisms as well as plants. The importance of laccases for various applications includes degradation of a large range of synthetic dyes, delignification of lignocellulosics, detoxification of waste, conversion of textile dye, food technological applications, and biosensor as well as analytical uses (Mayer and Staples 2002; Patel et al. 2019). Laccases catalyze oxidation of organic compounds, such as aromatic amines, polyphenols, methoxyphenols, and ascorbate (Yaropolov et al. 1994; Leonowicz et al. 2001).
Several species of rot fungi, such as Trametes versicolor, T. villosa, Pleurotus ostreatus, Ganoderma lucidum, Panus tigrinus, and Agaricus bisporus have been reported as sources for laccases (Ko et al. 2001). However, other fungi such as Aspergillus spp. have been reported to produce laccase, including A. nidulans (Aramayo and Timberlake 1993; Ko et al. 2001), A. niger (Abu and Ado 2004; Vandana et al. 2014), A. flavus (Kumar et al. 2016; Ghosh and Ghosh 2017), and Aspergillus sp. (Bhamare et al. 2018). Also, Penicillium chrysogenum (Senthivelan et al. 2019) and Alternaria solani (Abdel Ghany and Bakri 2019) were recorded as a producer of cellulases as well as laccase.
According to Revankar and Lele (2006), numerous conditions such as constituents of media, carbon and nitrogen sources, carbon-to-nitrogen ratio, pH, temperature, and aeration rate play a critical role in fungal laccases secretion. Bhamare et al. (2018) reported the vital role of toxic elements ions, mainly Cu2+, for induction or inhibition of laccases production by fungi. Many studies report that laccases are cupro-proteins; therefore, the addition of copper salts as a growth medium constituent at appropriate conditions lead to improved enzyme production (Fonseca et al. 2010; Nakade et al. 2013; Gomaa and Momtaz 2015). Enhancement of laccase secretion from Fusarium solani, Pleurotus ostreatus, and Agaricus bisporus have been observed by Kumar et al. (2007, 2011) at 1 mM of copper sulfate. In another study, Mann et al. (2015) reported that the medium growth supplemented by 0.4 mM and 0.75 mM copper increased laccase secretion in Ganoderma lucidum and Cerrena consors, respectively. Bhamare et al. (2018) attributed the role of copper sulfate in enhancement of laccase production to the fact that copper acts as a strong inducer of laccase in fungi. An earlier study reported that the promoting role of copper is due to regulation of laccase gene transcription (Collins and Dobson 1997). Currently, minimization of the potentially toxic elements using its nano-forms are required for reduce its biohazards. Therefore the objective of this research was to investigate the influence of copper and its NPs on growth and ultrastructure of A. niger, as well as study its role on laccase production by A. niger using corn cobs.
EXPERIMENTAL
Materials and Methods
Fungal isolation and identification
Fifteen samples of corn cobs that showed fungal infection were collected from storage in Monufia Governorate (Lat. 30° 31′ 12″ N, Long. 30° 59′ 24″ E), Egypt. The infected samples were kept in sterile plastic bags and transferred to a microbiology lab for fungal isolation. Parts of infected corn cobs ranged from 3 mm to 5 mm in diameter were placed on the surface of potato dextrose agar (PDA) medium, then incubated at 30 °C (similar to temperature of storage place) for 7 d, and the developed fungal colonies were purified and sub-cultured. The colonies that appeared on all samples were similar in all morphological and microscopical characterizations. According to available criteria by Raper and Fennell (1973) and Samson et al. (1981), the fungal isolate was identified with regards to texture and color of fungal colony, reverse color, pigmentation, and colony diameter measured after 7 d of inoculation on different media, including PDA and Czapek’s dox agar (CDA) media, where the diameter (μm) of conidiophores, conidial heads, hyphae, conidiospores, and vesicles as well as phialide length, were measured.
Copper nanoparticles
By chemical reduction method of CuCl2.2H2O; CuNPs were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used in the current study. Their properties included nano-powder composition, 40 nm to 60 nm particle size, and assay with more than 99.5% trace metals basis according to producer pamphlet.
Antifungal assay of CuSO4 and CuNPs using well diffusion and poisonous food methods
The well diffusion method was applied to determine the antifungal activity CuSO4 and CuNPs against A. niger. Wells by sterile cork borer (5 mm) were made on CDA surface and seeded by 0.1 mL of fungus spores suspension. The CuSO4 and CuNPs at different concentrations (100, 200, 300, 400, and 500 ppm) were added separately in each well. The plates were incubated at 30 °C for 10 d; then the inhibition zone was observed and recorded (Roy et al. 2013). The poisonous food technique was also applied to determine the antifungal activity of CuSO4 and CuNPs against A. niger (Singh et al. 2010), and studying the morphological and sporogenesis of the fungus. For studying the morphological properties, a transmission electron microscopy investigation of A. niger under different separately concentrations of CuSO4 and CuNPs (100, 200 ppm and 300 ppm) was done. With using 5 mm of cork borer, colony margin of the growing isolated fungus (5 d old) was cut, followed by inoculation at the middle of the Petri dish containing growth medium CDA supplemented with different concentrations of CuSO4 and CuNPs, and then incubated for 7 d at 30 °C. Fungus sporulation was recorded at different concentrations of CuSO4 and CuNPs using hemocytometer slide, regarding the control as 100%. Moreover, the inhibition of growth (%) was estimated based on Kumar et al. (2007) using the following equation,
(1)
where medium without any treatment was used as control.
Morphological and transmission electron microscopy investigation of A. niger
The PDA medium containing different concentrations of CuSO4 and CuNPs as mentioned in poisoned food method was autoclaved and poured, and then A. niger spores were seeded. Under aseptic conditions the sterilized cover slips were dipped obliquely in the seeded agar layer along the line where the medium meets the upper surface of the cover slip; then they were incubated for 7 d at 30 °C. Using a tiny drop of Canada balsam (Carl Roth, Karlsruhe, Germany), the cover slips were fixed on glass slides and examined under a microscope. For electron microscopy studies, hyphal tips were cut from the margin of growing colony and fixed with 5% gluteraldehyde as a primary fixative for 24 h. The specimens were washed three times with phosphate buffer (pH 7.2), followed by buffer removal, then covered for 2 h by osmium tetraoxide (1%), followed by removal of the osmium tetraoxide. The specimens were dehydrated by passage through a sequence of ethanol levels, which ranged from 50% to 96%, followed by alcohol removal and substitution by propylene oxide for 1 h. The specimens were placed in propylene oxide and Epon 812 resin (2:1) (Carl Roth, Karlsruhe, Germany), then placed in a pure resin overnight, and then placed in an oven for 48 h at 60 °C. Small blocks were sectioned (50 nm) via ultramicrotomy, stained using uranyl acetate-lead citrate 500A (Agar Scientific, London, UK), and then examined using the transmission electron microscope (C JEOL Jem-1200 EX II. Acc. Voltage 120 KV. MAG-medium, JEOL, Tokyo, Japan) in the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt.
Induction of laccase production by A. niger
Five g of non-fungal infected ground corn cobs (size less than 2 mm) were moistened with 20 mL of water as moistening agent, placed in a 250 mL conical flask, sterilized at 121 °C for 30 min, then inoculated by 5 discs (5 mm) of growing colony, each disc containing 5 x 106 spores of A. niger, and incubated at 30 °C for 12 d. Under aseptic conditions, different concentrations of CuSO4 and CuNPs (0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45, and 0.50 mM) were added at the second day of incubation period as inducer for laccase production. The control was without any concentrations of CuSO4 or CuNPs. Distilled water (50 mL) was added after incubation period to each flask and incubated for 60min on shaking incubator (150 rpm). Then, the metabolized substrate of each flask was filtered through muslin cloth, and the filtrates were centrifuged at 80,000 rpm (35,000 RCF) for 10 min by cooling centrifuge (Minispin; Eppendorf AG, Hamburg, Germany). The enzyme in the supernatants was assayed as described by Garzillo et al. (2001).
Laccase assay
The supernatant (100 µL) was added to the reaction mixture of 1 mL containing 2 mM of 22,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate (ABTS) (Sigma Aldrich, St. Louis, MO, USA), in citrate-phosphate buffer (pH 5.0). The enzymatic activity was estimated in IU by monitoring the absorbance change at 420 nm, Є=36 mM-1cm-1 by spectrophotometer (Model 6300, EU, JENWAY, Stone, UK) at 30 °C.
Effect of temperature, pH, and incubation period on enzyme production
In order to study the optimal period of incubation for maximum laccase production, the production medium was adjusted at pH 6 and inoculated with spore suspension of A. niger (5 x 106 spores/mL), then incubated at 30 °C for different incubation periods from 2 d to18 d. The metabolized medium was withdrawn at different periods for measuring enzyme activity. The prepared productive medium was adjusted at p
H 6 and inoculated by 5 discs (5 mm) of growing colony and incubated at different temperatures viz. 10 °C, 20 °C, 30 °C, 40 °C, and 50 °C for 12 d. The effect of pH on laccase production was carried out by adjusting the growth media at different initial pH values viz. 3, 4, 5, 6, 7, 8, and 9, then inoculated and incubated at 30 °C for 12 d. At all conditions the growth medium was supplemented with the optimum concentrations of CuNPs and CuSO4 at the second day of incubation period under aseptic conditions. The enzyme was estimated as mentioned earlier.
Statistical analysis
The mean ± SD (standard deviation) was calculated using three independent replicates of each treatment. The SPSS ver. 22.0 computer software was applied for statistical analyses of data.
RESULTS AND DISCUSSION
One fungus that was isolated from all collected corn cobs samples showed black fungal infection (Fig. 1), and the isolate fungus was identified as A. niger according to the mentioned criteria in material and methods. Therefore, corn cobs wastes were used as a low-cost substrate by using CuSO4 and CuNPs as inducers of laccase production by A. niger. The inhibitor effect of copper ions and its NPs has already been established in various reports. For this reason, in the current study different concentrations of CuSO4 and CuNPs were tested against A. niger growth as well as their ultrastructure to determine the noninhibitor concentration for laccase production.
The well diffusion agar technique revealed the antifungal activity of copper ion represented by CuSO4 and its NPs on A. niger growth. However, CuNPs showed stronger antifungal activity than CuSO4 (Fig. 2) at the same used concentrations (100 ppm to 500 ppm). Surprisingly, the resistance to low concentrations of CuSO4 (100 to 400 ppm) appeared after 8 d of incubation period, where the inhibition zone was covered with new growth of A. niger, while the inhibition zone with CuNPs was still clear until 8 d after inoculation (Fig. 2). The lowest concentration of CuNPs (100ppm) showed no antifungal activity. The present results agreed with the obtained results of Banik and Pérez-de-Luque (2017), who reported that Trichoderma harzianum growth was completely inhibited at 1000 ppm of CuNPs but not inhibited at low concentrations.
Through the poisonous food technique and measuring the mycelial radial growth, A. niger growth was promoted at low concentrations (100 ppm), but the growth was inhibited at 200 and 300 ppm of CuSO4 and CuNPs, where the growth inhibition was 58.1% and 86.9% using CuNPs and 30.3% and 65.6% using CuSO4, respectively. At the same time, sporulation decreased at 300 ppm of CuNPs and CuSO4, reaching 30.4% and 47.6%, respectively, compared to control at 100% (Table 1). Decreasing of Alternaria alternata and Botrytis cinerea sporulation was observed as a result of CuNPs treatment at 15mg L-1 (Sahar 2014). Not only did A. niger tolerate the low concentration of copper ion and its NPs, but other fungi were reported. Hefnawy et al. (2009) established that Chaetomium globosum and Stachybotrys chartarum were able to grow at 800 mg/L, while they failed to grow at 1000 mg/L of copper. In a prior study (Viet et al. 2016), Fusarium sp. growth was inhibited at concentrations more than 200 ppm of CuNPs, where its inhibition reached to 94.0% at 450 ppm after 9d of incubation period. Recently Banik and Pérez-de-Luque (2017) observed that the growth of Alternaria alternata, Botrytis fabae, Fusarium oxysporum f. sp. ciceris, and F. oxysporum f. sp. melonis was promoted at low concentrations (100 ppm) of CuNPs. Fungal growth enhancement at low concentrations of CuSO4 and CuNPs might be due to biological activity of copper ion as a cofactor of enzymes. Similar results have been reported for antifungal activity of CuNPs (Pariona et al. 2019), where Neofusicoccum sp. exhibited great tolerance to CuNPs at low concentrations, but its growth was inhibited at concentration greater than 500 ppm.
The morphological changes of A. niger at control and CuNPs treatments are reported in Table 2 and shown in Fig. 3. Microscopic examination showed normal structural characteristics of A. niger cultivated in medium without treatments. In contrast, CuSO4 and CuNPs treatments promoted deformation of fungus. Conidial heads, vesicles, conidiospores, and hyphae diameters, as well as phialides length, were reduced at high concentrations (200 ppm and 300 ppm) of CuSO4 and CuNPs (Tables 2 and 3). The CuNPs were more effective than CuSO4 with respect to morphological changes of A. niger.
Transmission electron microscope (TEM) revealed deformation of A. niger mycelia at high concentrations (200 ppm and 300 ppm) of CuSO4 and CuNPs (Fig. 4). In contrast, at control and 100 ppm of CuSO4 and CuNPs, no changes appeared in the cell wall and cell membrane, with clearing nucleus inside the cell. The cytoplasmic membrane was collapsed outside the cell wall at 200 ppm of CuNPs. Furthermore, numerous granules were aggregated inside the cytoplasm at 300 ppm of CuNPs, and these might be due to accumulation of copper within fungal cell.
Little is known regarding the impact of CuNPs on ultrastructure of fungi, because most of scientific papers have focused on the growth inhibition only. Using TEM, Sahar (2014) revealed collapse of Alternaria alternata and Botrytis cinerea hyphae treated CuNPs, beside hyphae damage and precipitations of NPs on walls of cells.
Many large vacuoles appeared as a response to high concentrations of CuSO4 (200 ppm and 300 ppm) and CuNPs (300 ppm). Under stress conditions of potentially toxic elements, Gamalero et al. (2009) reported that the potentially toxic elements were accumulated in Glomus intraradices cell wall and vacuoles, but slight changes in the potentially toxic elements levels were detected in the cytoplasm. Recently, Pariona et al. (2019) observed strong morphological changes in the mycelium and cell membranes of Fusarium oxysporum, F. solani, and Neofusicoccum sp. exposed to CuNPs. Salvadori et al. (2013) investigated the dead biomass of Hypocrea lixii treated by CuNPs. They found NPs inside cell wall but not in cytoplasm and cytoplasmic membrane.
Fig. 1. Corn cobs samples as a source of A. niger isolate
Fig. 2. Impact of different concentrations of CuSO4 and CuNPs on A. niger growth: (1) 100 ppm, (2) 200 ppm, (3) 300 ppm, (4) 400 ppm, (5) 500 ppm
Table 1. Growth of A. niger at Different Concentrations of CuNPs
±, Standard deviation
Table 2. Morphological Characterization of A. niger at Different Concentrations of CuNPs
±, Standard deviation
Table 3. Morphological Characterization of A. niger at Different Concentrations of CuSO4
±, Standard deviation
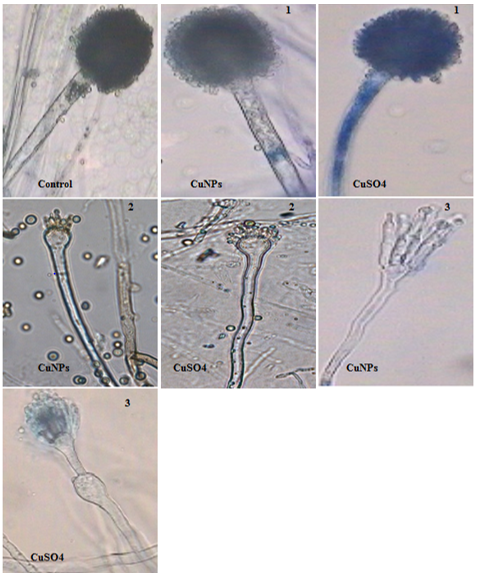
Fig. 3. Morphological changes of A. niger at different concentrations of CuNPs and CuSO4: (1) 100ppm, (2) 200ppm, (3) 300ppm. Mag=400x
Fig. 4. TEM of A. niger mycelia at different concentrations of CuNPs and CuSO4: (1) 100 ppm, (2) 200 ppm, (3) 300 ppm; N: nucleus, CW: cell wall, CM: cytoplasmic membrane, V: vacules, GICY: granules inside cytoplasm. TEM mag=6000X
Metallic ions play an essential role in regulation of laccases secretion by fungi (Piscitelli et al. 2011), particularly copper (Palmieri et al. 2000; Vrsanska et al. 2016). However, there are very few studies on the impact of metal NPs on production of laccase (Maurya et al. 2017). Therefore, the present study focused on the effect of CuNPs on laccase production compared to its non-nanoparticle form.
In the current results, CuNPs and CuSO4 at concentrations of 0.15 and 0.25 mM, respectively, induced maximum amounts of laccase at 1.67 and 1.51 U/mL, respectively. At concentrations greater than those, enzyme production decreased (Fig. 5). However, a previous study demonstrated that an increase in copper concentration causes a raise in laccases production in Trametes versicolor (Collins and Dobson 1997). Increased laccase production by T. versicolor was observed with increasing CuSO4, but over concentrations of 80 mM, the activity decreased (Lorenzo et al. 2005). In another study, 25 μM of CuSO4 was shown to inhibit the activity of laccases in F. oxysporum. f. sp. lycopersici (Hernández-Monjaraz et al. 2018).
A similar situation to the current results has been observed in numerous studies. Saparrat (2004) has observed that the highest activity of Grammotheles subargentea laccase occurs at level range of 0.6 mM to 1.2 mM CuSO4, whereas at higher levels of the metal (1.5 mM and 1.8 mM), the activity decreases. Copper acts as a cofactor in the catalytic center of laccase, but at high concentrations it might induce oxidative stress and result in damage to proteins, and therefore decreases in laccase production at high concentrations of CuNPs and CuSO4 were observed. Four copper atoms in the catalytic center of laccases were detected (Pannu and Kapoor 2014), which had various characteristics, and therefore copper at low concentrations could stimulate laccase production. Fillat et al. (2016) used CuSO4 as an inducer to increase laccase production up to 85%. In contrast, Bhamare et al. (2018) discovered that 0.025 mg/L CuSO4 was the best concentration for laccase production via Aspergillus sp. The CuNPs were more efficient than CuSO4 at lower concentrations as an inducer for laccase production by A. niger, while at higher concentrations CuNPs were also more an inhibitor to enzyme activity (Fig.5). From this point of view, CuNPs in general were potent inhibitors of fungal growth as well as enzymatic reactions. CuNPs had nano-size to large surface area which caused the highest adsorptions of enzymatic protein on the surface of NPs and therefore could amplify activity of enzymes (Galhaup and Haltrich 2001). Recently, Singh et al. (2018) observed that microparticles enhanced production of Aspergillus oryzae enzymes up to ten-fold.
Fig. 5. Effect of different concentrations of CuSO4 and CuNPs (mM) on laccase production by A. niger
Further studying was achieved for optimization of laccase production. An increase in incubation time resulted in increased laccase secretion from A. niger cultivated in medium without CuSO4 and CuNPs up to 14 d, while addition of optimum concentrations of CuNPs and CuSO4 (0.15 mM and 0.25 mM, respectively) reduced the appropriate time to 12 d using CuNPs and to 14 d using CuSO4 for highest level of laccase (1.66 U/mL and 1.53U/mL, respectively) compared with untreated (1.36 U/mL at 16 d) (Fig. 6). These validated that production of laccase was dependent on fungal growth and the presence of metallic ions. Singh et al. (2018) recorded that the highest production of amylase, cellulose, phytase, and xylanase by Aspergillus oryzae was on the fourth day of fermentation, but addition of microparticle to medium growth resulted in production of enzymes at the third day. At the second day, the enzyme was not detected, but at the fourth day it appeared in low quantity; hence the laccase production began at the third day of incubation period (Fig. 6). The induction impact of CuSO4 and CuNPs on laccase production was observed at the starting process of production. Optimum incubation period for laccase production might differ according to fungal species, substrate type, presence of inducers, and environmental conditions. Earlier studies reported maximum laccase production by Ganoderma lucidum at 14 d (Songulashvili et al. 2007), by Aspergillus fumigatus at 6 d using banana peel (Vivekanand et al. 2011), by Trametes hirsuta at 20 d (Bakkiyaraj et al. 2013) using wheat bran, by A. niger at 5 d (Priyam et al. 2014), by A. flavus at 7 d using various agro-wastes, including heat bran and apple peel (Ghosh and Ghosh 2017), and by Aspergillus sp. at 9 d (Bhamare et al. 2018).
Fig. 6. Impact of different incubation periods on laccase production by A. niger at optimum concentrations of CuSO4 and CuNPs (mM)
Laccase productivity varied with the different pH of growth medium, but optimum production was mainly obtained at pH 5 (Fig. 7). Acidic pH was more favorable than alkaline for enzyme production. Therefore, productivity at pH 3 and pH 4 were better than productivity at pH 8 and pH 9. A similar behavior has been observed in numerous studies (Ravikumar et al. 2012; Patel and Gupte 2016), with the highest enzyme production at pH 5.0. Laccase production by A. niger was reported at pH 5 (Priyam et al. 2014). When the copper level was less than optimal, laccase production improved with the increase in copper supplementation, but when the copper concentration was above the optimal concentration, the laccase production was induced with a reduction in copper supplementation (Tavares et al. 2005; Zhao et al. 2017). For this reason, the laccase production was studied at different pH ranging from 3 to 9 and temperature ranged from 10 °C to 50 °C (Figs. 7 and 8). Addition of CuSO4 and CuNPs induced laccase production at acidic conditions, while its effects were negligible at pH above 6. At pH 9, laccase productivity in medium growth without CuSO4 and CuNPs was higher than medium supported by them. In the present result, maximal laccase production was obtained at 30 °C, and further increases in temperature showed decreased enzyme production (Fig. 8).
The current results were similar to the obtained results of Priyam et al. (2014), who noted that the optimum temperature was 30 °C for laccase production by A. niger. Laccase was not detected at low temperatures such as 10 °C, and this might be attributed to the failure of the fungus to grow at these temperatures. The current results agreed with the results obtained by Ghosh and Ghosh (2017), who revealed that the optimal values of laccase production by A. flavus were at 25 °C and pH 4. Recently, Senthivelan et al. (2019) recorded that maximum laccase activity from Penicillium chrysogenum was at pH 5.5 using 1.3 g/L of yeast extract. Addition of copper in the NPs form or non-nanoform supported enzyme production at different temperatures, particularly at optimum temperature 30 °C. Unsuitable pH or temperature might restrict fungal growth and inhibit metabolic rate and therefore restrict fungus activity as well as laccase production. From the current results, CuNPs played a greater role for laccase induction than CuSO4, and this might be due to unique properties of NPs related to their size. In contrast, Bhamare et al. (2018) revealed that 34 °C and pH 6.0 were optimal for laccase production by Aspergillus sp. Additionally, Abd El Monssef et al. (2016) reported that the maximum yield of laccase enzyme by Trichoderma harzianum was at pH 5 and 35 °C after 6 d.
Fig. 7. Impact of different pH on laccase production by A. niger at optimum concentrations of CuSO4 and CuNPs (mM)
Fig. 8. Impact of different temperatures on laccase production by A. niger at optimum concentrations of CuSO4 and CuNPs (mM)
CONCLUSIONS
- The results showed the fungistatic effects of CuSO4 and CuNPs on A. niger contaminated corn cobs and their grains.
- CuNPs resulted in higher yields of laccase, but their higher concentrations negatively affected the production of laccase by A. niger.
- Production of laccase by A. niger was controlled via numerous cultural conditions such as pH, temperature, incubation periods, and composition of culture medium.
- The addition of CuSO4 and CuNPs minimized the incubation periods required for laccase production by A. niger.
REFERENCES CITED
Abdel Ghany, T. M. (2013). “Stachybotrys chartarum: A novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity,” Indones. J. Biotechnol. 18(2), 75-82. DOI: 10.22146/ijbiotech.7871
Abdel Ghany, T. M., Shater, A. R. M., Al Abboud, M.A., and Alawlaqi, M. M. (2013). “Silver nanoparticles biosynthesis by Fusarium moniliforme and their antimicrobial activity against some food-borne bacteria,” Mycopath 11(1), 1-7.
Abdel Ghany, T. M., Al-Rajhi, A. M. H., Al Abboud, M. A., Alawlaqi, M. M., Magdah, A.G., Helmy, E. A. M., and Mabrouk, A. S. (2018a). “Recent advances in green synthesis of silver nanoparticles and their applications: About future directions. A review,” BioNanoScience 8(1), 5-16. DOI: 10.1007/s12668-017-0413-3
Abdel Ghany, T. M., Magdah, G., Bakry, M., and Al-Rajhi, A. M. H. (2018b). “Molecular characterization of Trichoderma asperellum and lignocellulolytic activity on barley straw treated with silver nanoparticles,” BioResources 13(1) 1729-1744. DOI: 10.15376/biores.13.1.1729-1744
Abdel Ghany, T. M., and Bakri, M. M. (2019). “Effectiveness of a biological agent (Trichoderma harzianum and its culture filtrate) and a fungicide (methyl benzimacold-2-ylcarbamate) on the tomato rotting activity (growth, celluloytic, and pectinolytic activities) of Alternaria solani,” BioResources 14(1), 1591-1602. DOI: 10.15376/biores.14.1.1591-1602
Abd El Monssef, R A., Hassan, E. A., and Ramadan, E. M. (2016). “Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment,” Ann. Agric. Sci. 61(1), 145-154. DOI: 10.1016/j.aoas.2015.11.007
Abu, E. A., and Ado, S. A. (2004). “Comparative studies on the effect of organic and inorganic nitrogen supplementation of millet and sorghum pomace on the production of three industrial enzymes by Aspergillus niger SL.,” Biokemistri 16(2), 64-70. DOI: 10.4314/biokem.v16i2.32572
Amatya, S., and Joshi, L. (2020). “Bio-Synthesis of copper nanoparticles (CuNPs) using Garlic extract to investigate antibacterial activity,” Bibechana 17, 13-19. DOI: 10.3126/bibechana.v17i0.23485
Aramayo, R., and Timberlake W. E. (1993). “The Aspergillus nidulans yA gene is regulated by abaA,” EMBO J. 12(5), 2039-2048.
Bakkiyaraj, S., Aravindan, R., Arrivukkarasan, S., and Viruthagiri, T. (2013). “Enhanced laccase production by Trametes hirusta using wheat bran under submerged fermentation,” Int. J. ChemTech Res. 5(3), 1224-1238.
Banik, S., and Pérez-de-Luque, A. (2017). “In vitro effects of copper nanoparticles on plant pathogens, beneficial microbes and crop plants,” Span. J. Agric. Res. 15(2), e1005. DOI: 10.5424/sjar/2017152-10305
Bhamare, H. M., Jadhav, H. P., and Sayyed, R. Z. (2018). “Statistical optimization for enhanced production of extracellular laccase from Aspergillus sp. HB_RZ4 isolated from bark scrapping,” Environ. Sustainability 1(2), 159-166. DOI: 10.1007/s42398-018-0015-1
Chalandar, H. E., Ghorbani, H. R., Attar, H., and Alavi, S. A. (2017). “Antifungal effect of copper and copper oxide nanoparticles against Penicillium on orange fruit,” Biosci. Biotechnol. Res. Asia 14(1), 279-284. DOI: 10.13005/bbra/2445
Collins, P. J., and Dobson, A. (1997). “Regulation of laccase gene transcription in Trametes versicolor,” Appl. Environ. Microbiol. 63(9), 3444-3450.
Denkhaus, E., and Salnikow, K. (2002). “Nickel essentiality, toxicity, and carcinogenicity,” Crit. Rev. Oncol./Hematol. 42(1), 35-56. DOI: 10.1016/s1040-8428(01)00214-1
Durán, N., and Seabra, A. B. (2012). “Metallic oxide nanoparticles: State of the art in biogenic syntheses and their mechanisms,” Appl. Microbiol. Biotechnol. 95(2), 275-288. DOI: 10.1007/s00253-012-4118-9
Fernandes, S. C., de Oliveira, I. R. W. Z., Fatibello-Filho, O., Spinelli, A., and Vieira, I. C. (2008). “Biosensor based on laccase immobilized on microspheres of chitosan crosslinked with tripolyphosphate,” Sens. Actuat. B: Chem. 133(1), 202-207.DOI: 10.1016/j.snb.2008.02.023
Fillat, Ú., Martín-Sampedro, R., Macaya-Sanz, D., Martín, J. A., Ibarra, D., Martínez, M. J., and Eugenio, M. E. (2016). “Screening of eucalyptus wood endophytes for laccase activity,” Process Biochem. 51(5), 589-598. DOI: 10.1016/j.procbio.2016.02.006
Fonseca, M. I., Shimizu, E., Zapata, P. D., and Villalba, L. L. (2010). “Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina),” Enzyme Microb. Technol. 46(6), 534-539. DOI: 10.1016/j.enzmictec.2009.12.017
Fukami, M. Yamazaki, S., and Todo, S. (1983). “Distribution of copper in the cells of heavy metal tolerant fungus, Penicillium ochrochloron, cultured in concentrated copper medium,” Agric. Biol. Chem. 47(6), 1367-1369. DOI: 10.1080/00021369.1983.10863892
Galhaup, C., and Haltrich, D. (2001). “Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper,” Appl. Microbiol. Biotechnol. 56(1-2), 225-232. DOI: 10.1007/s002530100636
Gamalero, E., Lingua, G., Berta, G., and Glick, B. R. (2009). “Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress,” Can. J. Microbiol. 55(5), 501-514.DOI: 10.1139/w09-010
Ganash, M., Abdel Ghany, T. M., and Omar, A. M. (2018). “Morphological and biomolecules dynamics of phytopathogenic fungi under stress of silver nanoparticles,” BioNanoScience 8(2), 566-573. DOI: 10.1007/s12668-018-0510-y
García, J. J., Martínez-Ballarín, E., Millán-Plano, S., Allué, J. L., Albendea, C., Fuentes, L., and Escanero, J. F. (2005). “Effects of trace elements on membrane fluidity,” J. Trace Elem. Med. Biol. 19(1), 19-22. DOI: 10.1016/j.jtemb.2005.07.007
Garzillo, A. M., Colao, M. C., Buonocore, V., Oliva, R., Falcigno, L., Saviano, M., Santoro, A. M., Zappala, R., Bonomo, R. P., Bianco, C., et al. (2001). “Structural and kinetic characterization of native laccases from Pleurotus ostreatus, Rigidoporus lignosus, and Trametes trogii,” J. Protein Chem. 20(3), 191-201.DOI: 10.1023/A:1010954812955
Ghosh, P., and Ghosh, U. (2017). “Statistical optimization of laccase production by Aspergillus flavus PUF5 through submerged fermentation using agro-waste as cheap substrate,” Acta Biologica Szegediensis 61(1), 25-33.
Gomaa, O. M., and Momtaz, O. A. (2015). “Copper induction and differential expression of laccase in Aspergillus flavus,” Braz. J. Microbiol. 46(1), 285-292. DOI: 10.1590/s1517-838246120120118
Gopalakrishnan, K., Ramesh, C., Ragunathan, V., and Thamilselvan, M. (2012). “Antibacterial activity of copperoxide nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline,” Dig. J. Nanomater. Bios. 7, 2012, 833-839.
Hefnawy, M. A., Ali, M. I., and Abdul-Ghanay, S. A. (2009). “Influence of copper and cobalt stress on morphology and ultra-structure of Chaetomium globosum and Stachybotrys chartarum,” Aust. J. Basic Appl. Sci. 3(4), 3158-3165.
Hernández-Monjaraz, W. S., Caudillo-Pérez, C., Salazar-Sánchez, P. U., and Macías-Sánchez, K. L. (2018). “Influence of iron and copper on the activity of laccases in Fusarium oxysporum f. sp. lycopersici,” Braz. J. Microbiol. 49(Supl. 1), 269-275. DOI: 10.1016/j.bjm.2018.06.002
Jia, B., Mei, Y., Cheng, L., Zhou, J., and Zhang, L. (2012). “Preparation of copper nanoparticles coated cellulose films with antibacterial properties through one-step reduction,” ACS Appl. Mater. Interfaces 4(6), 2897-2902. DOI: 10.1021/am3007609
Ko, E.-M., Leem, Y.-E., and Choi, H. T. (2001). “Purification and characterization of laccase from the white-rot fungi basidiomycete Ganoderma lucidum,” Appl. Microbiol. Biotechnol. 57(1-2), 98-102. DOI: 10.1007/s002530100727
Kumar, R., Kaur, J., Jain, S., and Kumar, A. (2016). “Optimization of laccase production from Aspergillus flavus by design of experiment technique: Partial purification and characterization,” J. Genet. Eng. Biotechnol. 14(1), 125-131. DOI: 10.1016/j.jgeb.2016.05.006
Kumar, R., Mishra, A. K., Dubey, N. K., and Tripathi, Y. B. (2007). “Evaluation of Chenopodiumam brosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity,” Int. J. Food Microbiol. 115(2), 159-164. DOI: 10.1016/j.ijfoodmicro.2006.10.017
Kumar, V. V., Kirupha, S. D., Periyaraman P., and Sivanesan, S. (2011). “Screening and induction of laccase activity in fungal species and its application in dye decolorization,” African Journal of Microbiology Research. 5(11), 1261-1267. DOI: 10.5897/AJMR10.894
Leonowicz, A., Cho, N. S., Luterek, J., Wilkolazka, A., Wojtas-Wasilewska, M., Matuszewska, A., Hofrichter, M., Wesenberg, D., and Rogalski, J. (2001). “Fungal laccase: Properties and activity on lignin,” J. Basic Microbiol. 41(3-4), 185-227. DOI: 10.1002/1521-4028(200107)41:3/4<185::aid-jobm185>3.0.co;2-t
Lorenzo, M., Moldes, D., Rodríguez Couto, S., and Sanromán, M. A. (2005). “Inhibition of laccase activity from Trametes versicolor by heavy metals and organic compounds,” Chemosphere 60(8), 1124-1128. DOI: 10.1016/j.chemosphere.2004.12.051
Mann, J., Markham, J. L., Peiris, P., Spooner-Hart, R. N., Holford, P., and Nair, N. G. (2015). “Use of olive mill wastewater as a suitable substrate for the production of laccase by Cerrenaconsors,” Int. Biodeter. Biodegr. 99,138-145. DOI: 10.1016/j.ibiod.2015.01.010
Maurya, S., Chaurasia, P. K., Gupta, K. K., Kushwaha, A., Bhardwaj, A. K., and Singh, M. P. (2017). “The effects of nanoparticles on laccase production and its activity,” in: Incredible World of Biotechnology, M. P. Singh, V. Verma, A. K. Singh, (eds.), Nova Science Publisher, Hauppauge, NY, USA, pp. 59-66.
Mayer, A. M., and Staples, R. C. (2002). “Laccase: New functions for an old enzyme,” Phytochemistry 60(6), 551-565.DOI: 10.1016/S0031-9422(02)00171-1
Nair, P. M. G., and Chung, I. M. (2014). “Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification, and molecular level changes,” Environ. Sci. Pol. Res., 21, 12709-12722.
Nakade, K., Nakagawa, Y., Yano, A., Konno, N., Sato, T., and Sakamoto Y. (2013). “Effective induction of pblac1 laccase by copper ion in Polyporus brumalis ibrc05015,” Fungal Biol. 117(1), 52-61.DOI: 10.1016/j.funbio.2012.11.005
Palmieri, G., Giardina, P., Bianco, C., Fontanella, B., and Sannia, G. (2000). “Copper induction of laccase isozymes in the ligninolytic fungus Pleurotus ostreatus,” Appl. Environ. Microbiol. 66(3), 920-924. DOI: 10.1128/aem.66.3.920-924.2000
Pannu, J. S., and Kapoor, R. K. (2014). “Microbial laccases: A mini-review on their production, purification and applications,” Int. J. Pharm. Biol. Arch. 3,528-536.
Pariona, N., Mtz-Enriquez, A., Sánchez-Rangel, D., Carrión, G., Paraguay-Delgado, F., and Rosas-Saito, G. (2019). “Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens,” RSC Adv. 9(33), 18835-18843. DOI: 10.1039/c9ra03110c
Patel, H., and Gupte, A. (2016). “Optimization of different culture conditions for enhanced laccase production and its purification from Tricholoma giganteum AGHP,” Bioresour. Bioprocess. 3, 11. DOI: 10.1186/s40643-016-0088-6
Patel, N., Shahane, S., Majumdar, R., and Mishra, U. (2019). “Mode of action, properties, production, and application of laccase: A review,” Recent Pat Biotechnol. 13(1), 19-32. DOI: 10.2174/1872208312666180821161015.
Piscitelli, A., Giardina, P., Lettera, V., Pezzella, C., Sannia, G., and Faraco, V. (2011).“Induction and transcriptional regulation of laccases in fungi,” Curr. Genomics 12(2), 104-112. DOI: 10.2174/138920211795564331
Priyam, V., Harison, M, Yashab, K., Kumar S. A., and Kiran P. J. (2014). “Production parameter optimization for laccases by Aspergillus niger,” International Journal of Bioinformatics and Biological Science 2(3-4), 179-188. DOI: 10.5958/2321-7111.2014.00015.8
Raffi, M., Mehrwan, S., Bhatti, T. M., Akhter, J. I., Hameed, A., Yawar, W., and ul Hasan, M. M. (2010). “Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli,” Ann. Microbiol. 60(1), 75-80. DOI: 10.1007/s13213-010-0015-6
Rajput, V., Tatiana Minkina, T., Ahmed B., Sushkova, S., Singh, R., Soldatov M., Laratte B., Fedorenko A., Mandzhieva S., Blicharska E., Musarrat J., Saquib Q., Flieger J., Gorovtsov A. (2019). “Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: critical review of the state of the science and future perspectives,” in: Reviews of Environmental Contamination and Toxicology, P. de Voogt (ed.) 252, 51-96. DOI: 10.1007/398_2019_34
Raper, K. B., and Fennell, D. I. (1973). The Genus Aspergillus, Robert E. Krieger, Huntington, NY, USA.
Ravikumar, G., Gomathi, D., Kalaiselvi, M., and Uma, C. (2012). “Production, purification and partial characterization of laccase from the mushroom Hypsizygus ulmarius,” Int. J. Pharm Bio Sci. 3, 355-365.
Revankar, M. S., and Lele, S. S. (2006). “Increased production of extracellular laccase by the white rot fungus Coriolus versicolor MTCC 138,” World J. Microbiol. Biotechnol. 22(9), 921-926. DOI: 10.1007/s11274-006-9136-2
Roy, S., Mukherjee, T., Chakraborty, S., and Das, T. K. (2013). “Biosynthesis, characterisation and antifungal activity of silver nanoparticles synthesized by the fungus Aspergillus foetidus MTCC8876,” Dig. J. Nanomater. Bios. 8(1), 197-205.
Salvadori, M. R., Lepre, L. F., Ando, R. A., Cláudio A., and Corrêa B. (2013). “Biosynthesis and uptake of copper nanoparticles by dead biomass of Hypocrea lixii isolated from the metal mine in the Brazilian amazon region,” PLoS One 8(11), e80519. DOI: 10.1371/journal.pone.0080519
Samson, R. A., Hoekstra, E. S., and Van Oorschot, C. A. (1981). Introduction to Food-Borne Fungi, Centraalbureauvoor Schimmel cultures, Utrecht, Netherlands.
Saparrat M. C. N. (2004). “Optimizing production of extracellular laccase from Grammotheles ubargentea CLPS no. 436 strain,” World J. Microbiol. Biotechnol. 20(6), 583-586. DOI: 10.1023/B:WIBI.0000043167.39311.0a
Sarkar, J., Chakraborty, N., Chatterjee , A., Bhattacharjee, A., Dasgupta, D., and Acharya K. (2020). “Green synthesized copper oxide nanoparticles ameliorate defense and antioxidant enzymes in Lens culinaris,” Nanomaterials 10, 312. DOI: 10.3390/nano10020312
Senthivelan, T., Kanagaraj, J., Panda, R. C., and Narayani, T. (2019). “Screening and production of a potential extracellular fungal laccase from Penicillium chrysogenum: Media optimization by response surface methodology (RSM) and central composite rotatable design (CCRD),” Biotechnol. Rep. 23, e00344. DOI: 10.1016/j.btre.2019.e00344
Sahar, M. O. (2014). “Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea,” Research Journal of Microbiology 9, 34-42. DOI: 10.3923/jm.2014.34.42
Singh, B. (2018). “Engineering fungal morphology for enhanced production of hydrolytic enzymes by Aspergillus oryzae SBS50 using microparticles,” 3 Biotech 8(6), 283. DOI: 10.1007/s13205-018-1308-x
Singh, P., Shukla, R., Prakash, B., Kumar, A., Singh, S., Mishra, P. K., and Dubey, N. K. (2010). “Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene,” Food Chem. Toxicol. 48(6), 1734-1740. DOI: 10.1016/j.fct.2010.04.001
Songulashvili, G., Elisashvili, V., Wasser, S.P., Nevo, E., and Hadar, Y. (2007). “Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes,” Enzyme Microb. Technol. 41(1-2), 57-61. DOI: 10.1016/j.enzmictec.2006.11.024
Stohs, S. J., and Bagchi, D. (1995). “Oxidative mechanisms in the toxicity of metal ions,” Free Radical Biol. Med. 18(2), 321-336. DOI: 10.1016/0891-5849(94)00159-H
Tavares, A. P. M., Coelho, M. A. Z., Coutinho, J. A. P., and Xavier A. M. R. B. (2005). “Laccase improvement in submerged cultivation: Induced production and kinetic modelling,” J. Chem. Technol. Biotechnol. 80(6), 669-676. DOI: 10.1002/jctb.1246
Viet, P. V., Nguyen, H. T., Cao, T. M., and Hie, L. V. (2016). “Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method,” Journal of Nanomaterials, Volume 2016, 7 p. DOI:10.1155/2016/1957612
Vivekanand, V., Dwivedi, P., Pareek, N., and Singh, R. P. (2011). “Banana peel: A potential substrate for laccase production by Aspergillus fumigatus VkJ2.4.5 in solid-state fermentation,” Appl. Biochem. Biotechnol. 165, 204. DOI: 10.1007/s12010-011-9244-9
Vrsanska, M., Voberkova, S., Langer, V., Palovcikova, D., Moulick, A., Adam, V., and Kopel, P. (2016). “Induction of laccase, lignin peroxidase and manganese peroxidase activities in white-rot fungi using copper complexes,” Molecules 21(11), 1553. DOI: 10.3390/molecules21111553
Yaropolov, A. I., Skorobogat’ko, O. V., Vartanov, S. S., and Varfolomeyev, S. D. (1994). “Laccase: Properties, catalytic mechanism, and applicability,” Appl. Biochem. Biotechnol. 49(3), 257-280. DOI: 10.1007/bf02783061
Zhao, L. H., Chen, W., Wang, L. L., Sun, H. J., and Zhu, Z. (2017). “Improvement of laccase production by Pleurotus ostreatus by means of agroindustrial waste and fermentation kinetics,” Mycosphere 8(1), 147-161. DOI: 10.5943/mycosphere/8/1/14
Article submitted: December 11, 2019; Peer review completed: March 9, 2020; Revised version received: March 18, 2020; Accepted: March 19, 2020; Published: March 23, 2020.
DOI: 10.15376/biores.15.2.3289-3306
