Abstract
Poplar wood fibers (WFs) were pretreated with ionic liquid (IL) 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) at 125 °C for 1 h, and the influence of the wood loadings (5%, 15%, 30%, and 50%) on the pretreatment efficiency and downstream acetylation was studied. The crystallinity and lignin content of the WFs decreased after IL pretreatment at low wood loadings, especially at 5%. Wood fiber acetylation was performed under a molar ratio (acetic anhydride/–OH in the WFs) of 2:1, with toluene as the reaction medium and pyridine as the acid capturer. Ionic liquid pretreatment at 5% and 15% loading greatly contributed to the increase of weight percent gain (WPG) after acetylation, leading to much higher reaction efficiency, or lower energy consumption. The acetylated products that underwent pretreatment (mainly at 5% loading) had slightly higher thermal stability than those that did not undergo pretreatment. The crystallinity and moisture sorption ability of the products were determined primarily by the WPG value.
Download PDF
Full Article
Improved Acetylation Efficacy of Wood Fibers by Ionic Liquid Pretreatment
Xiaoping Shen,a Yanjun Xie,a,* and Qingwen Wang b,*
Poplar wood fibers (WFs) were pretreated with ionic liquid (IL) 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) at 125 °C for 1 h, and the influence of the wood loadings (5%, 15%, 30%, and 50%) on the pretreatment efficiency and downstream acetylation was studied. The crystallinity and lignin content of the WFs decreased after IL pretreatment at low wood loadings, especially at 5%. Wood fiber acetylation was performed under a molar ratio (acetic anhydride/–OH in the WFs) of 2:1, with toluene as the reaction medium and pyridine as the acid capturer. Ionic liquid pretreatment at 5% and 15% loading greatly contributed to the increase of weight percent gain (WPG) after acetylation, leading to much higher reaction efficiency, or lower energy consumption. The acetylated products that underwent pretreatment (mainly at 5% loading) had slightly higher thermal stability than those that did not undergo pretreatment. The crystallinity and moisture sorption ability of the products were determined primarily by the WPG value.
Keywords: Poplar wood; Ionic liquid; Pretreatment; Wood loading; Acetylation
Contact information: a: Key Laboratory of Bio-based Material Science and Technology (Ministry of Education), College of Material Science and Engineering, Northeast Forestry University, 26 Hexing Road, Harbin 150040, China; b: College of Materials and Energy, South China Agricultural University, 483 Wushan Road, Guangzhou 510642, China;
* Corresponding authors: yxie@nefu.edu.cn; qwwang2006@126.com
INTRODUCTION
Wood’s complex cell wall structure, along with the inaccessibility of its semi-crystalline cellulose, results in its high recalcitrance to chemical modification (Zafeiropoulos et al. 2002). Thus, excess amounts of reagent (e.g., acetic anhydride) and catalyst, such as pyridine (Evans et al. 2000), dimethylformamide (Minato and Ito 2004), potassium acetate (Obataya and Minato 2009), etc., are generally required to improve the efficacy of wood acetylation. Without a catalyst, the reaction must be performed at high temperatures from 120 °C to 140 °C (Li et al. 2000), resulting in high energy consumption.
Ionic liquids (ILs; salts with melting points below 100 °C) with strong hydrogen bond-destroying ability have been developed for the homogeneous chemical modification of wood and cellulose after complete dissolution in IL, achieving a weight percent gain (WPG) of up to 50% (Schlufter et al. 2006; Xie et al. 2007). This high WPG is due to the profound destruction of the structure of the cell walls during dissolution, and thus, the significant increase in the amount of accessible –OH groups in the cellulose polymer chains. However, the severe damage to the wood cell walls in this technique inevitably causes grave losses of the stiffness and strength of natural wood, restraining its use in certain applications. Therefore, the development of efficient, economical, and energy-saving alternatives that preserve the mechanical performance of wood is a challenge.
The IL pretreatment of lignocellulosic biomass is the most critical step in its conversion to biofuels, of which the goal is to disrupt and remove lignin and hemicelluloses, and to decrease the crystallinity of cellulose (Weerachanchai et al. 2012). The disruption effect on hydrogen bonding in the wood by the IL pretreatment process may contribute to the release of free –OH groups, offering a potentially promising approach to the promotion of wood modification.
The objective of this study was to explore the effect of the pretreatment of poplar wood fibers (WFs) using the IL 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) on the efficiency of subsequent acetylation. The focus of the study was the parameter of wood loading (relative to IL) during pretreatment, which directly determines the cost of the process. The structure, thermal properties, and moisture sorption ability of the pretreated and acetylated WFs were also investigated.
EXPERIMENTAL
Materials and Chemicals
Poplar wood (Populus alba L.) supplied by Harbin Yongxu Company (Harbin, China) was ground and sieved into fibers with particle sizes in the range of 40 mesh to 60 mesh. The WFs were refluxed in a Soxhlet extractor with a mixture of toluene and ethanol (2:1, v/v) for 6 h to remove most extractives (Teramoto et al. 2008), followed by drying at 103 °C overnight. 1-butyl-3-methylimidazolium chloride ([C4mim]Cl, purity 99%, melting point 73 °C) was provided by Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences (Lanzhou, China). Its residual water content was about 3000 ppm, as determined by Karl Fisher titration. The other chemicals, including acetic anhydride, toluene, and pyridine, were all of reagent grade and used without further purification.
IL Pretreatment of WFs
The WFs were added to molten [C4mim]Cl at different loadings (5, 15, 30, or 50 g of WFs in 100 g of IL) in a 100-mL beaker, and the mixture was heated at 125 °C for 1 h under magnetic stirring. After cooling, the mixture was transferred to a 250-mL beaker containing 100 mL of deionized (DI) water and stirred for 1 h. The pretreated WFs were then filtered and rinsed thoroughly with DI water using a ceramic funnel with Whatman filter paper (Grade 4, 20 µm to 25 µm), followed by drying at 103 °C overnight. The products were annotated as P5, P15, P30, and P50, respectively. The IL was recycled by evaporating water using a rotary evaporator, and the yield was approximately 94%.
Acetylation of WFs
Pretreated or original WFs were added to 10 mL of toluene per gram as the reaction medium in a 250-mL flask equipped with a condenser and a calcium chloride drying tube. Assuming that each gram of wood possessed 14.6 mmol –OH groups (Yuan et al. 2010), acetic anhydride and pyridine were then added at a molar ratio of 2:1 to the hydroxyl groups, respectively. Acetylation was performed at 90 °C for a specific time period (1 h to 30 h), or at 70 °C to 90 °C for 30 h, obtaining “AP” (from pretreated WFs) or “Ctrl” samples (from original WFs) with different WPG values. After the reaction, all of the samples were alternately rinsed with DI water and ethanol, and then oven dried at 103 °C overnight.
Characterization
Lignin and hemicellulose contents of pretreated WFs
The lignin content of the original and pretreated WFs was determined by a scaled process of TAPPI method T222 om-98 (1998). Approximately 0.1 g of dried WFs were placed in a Pyrex culture tube, and 1.5 mL of 72% H2SO4 aqueous solution was gradually added. The tube was sealed with Parafilm, and the mixture was magnetically stirred at room temperature for 2 h. The mixture was then transferred to a 250-mL flask, diluted with 56 mL of DI water, and refluxed for 4 h. After filtration, the acid-insoluble lignin content was determined gravimetrically. The filtrate was diluted to 250 mL with DI water, and the acid-soluble lignin content was determined with a UV-Vis spectrophotometer (TU-1901, Beijing Persee, China) at 205 nm, the absorbance of which was in the range of 0.8 to 0.9. Triplicates were recorded for each sample throughout the article, and average and standard deviation values provided. The delignification (Delig., %) was determined as the reduction of the lignin content in the pretreated WFs compared to the lignin content in the original WFs using Eq. 1,
![]() (1)
(1)
where LWF and LPWF represent the lignin content (%) in the original and pretreated WFs, respectively.
The determination of hemicellulose content was based on NaClO2 delignification and NaOH treatment (Teramoto et al. 2008; Li et al. 2011). One gram dried biomass and 37.5 mL DI water were transferred to a 100 mL round-bottom flask equipped with a 25 mL Erlenmeyer flask inverted in the neck of the reaction flask; 0.25 mL glacial acetic acid and 0.3 g sodium chlorite were added successively into the mixture. The flask was placed in an oil bath and heated at 75 °C for 1 h with magnetic stirring. This addition of chemicals and 1-h heating were repeated 5 times. After reaction, the flask was placed in a refrigerator to cool the reactants below 5 °C. The product was filtered using nylon filter paper and washed with DI water and acetone successively. The resulting holocellulose (i.e. hemicellulose and cellulose) was oven-dried at 103 °C overnight and weighed; 12.5 mL NaOH aqueous solution (17.5 wt%) was added to a 100-mL beaker containing 0.5 g holocellulose obtained as above. The mixture was stirred at room temperature for 40 min, then 12.5 mL DI water was added to it. After 5 min, the residue was filtered, followed by adding 20 mL acetic acid aqueous solution (10 wt%). The residue was filtered again and washed with 500 mL boiling water during filtration. The resulting cellulose was oven-dried at 103 °C overnight and weighed. The hemicellulose content (%) was determined as the difference between the contents of holocellulose and cellulose using Eq. 2,
![]() (2)
(2)
where Holocellulose and Cellulose are the contents (%) of holocellulose and cellulose, respectively.
Weight percent gain (WPG) of acetylated WFs
The WPG of the acetylated WFs was calculated as the increase of the dry weight of the material after acetylation using Eq. 3,
![]() (3)
(3)
where Macetylated and Mun are the dry mass (g) of the acetylated and unacetylated WFs, respectively.
X-Ray diffraction (XRD) analysis
The XRD patterns of both the pretreated and acetylated WFs were obtained using an X-ray diffractometer (D/max 2200, Rigaku, Tokyo, Japan) with Ni-filtered Cu-Kα radiation (λ = 0.15406 nm) at 40 kV, 30 mA, and a step size of 0.02°. Scattered radiation was detected in the range (2θ) of 5° to 40° at a scan rate of 3° min-1. The crystallinity index (CrI) was calculated using a simple approach (Roncero et al. 2005), according to Eq. 4,
![]() (4)
(4)
where I002 is the intensity (a.u.) of the maximum crystalline peak at 2θ between 22° and 23°, and Iam is the minimum intensity (a.u.) at 2θ between 18° and 19°.
Fourier transform infrared spectroscopy (FT-IR) analysis
Fourier transform infrared spectroscopy was conducted using a Nicolet 6700 spectrometer (Thermo Nicolet, Madison, WI, USA) by acquiring 32 scans at 4-cm-1 resolution over the range between 500 cm-1 and 4000 cm-1 in a nitrogen atmosphere. The measurements were performed using an attenuated total reflection (ATR) technique cell (GladiATR, Pike Technologies, Fitchburg, WI, USA).
Laser scanning fluorescence microscopy
Transverse sections of poplar wood of a thickness of 40 µm were cut on a cryostat (Microm HM 560, Thermo Scientific, Madison, WI, USA). The sections were dried in an oven at 60 °C for 1 h between two glass slides to prevent curling. Samples were immersed in the IL (15% wood/IL) and heated at 125 °C for 1 h on a glass slide to simulate pretreatment. After rinsing in DI water, fluorescence of the samples was visualized at 405 nm using a confocal laser scanning microscope (LSM 700, Zeiss, Oberkochen, Germany).
Thermal analysis
The thermal properties of the pretreated and acetylated WFs were detected on a Perkin-Elmer TGA-7 thermogravimetric analyzer (Waltham, MA, USA) in the temperature range of 30 °C to 800 °C at a heating rate of 10 °C min-1 under a nitrogen atmosphere.
Moisture sorption tests
The moisture sorption values of the pretreated and acetylated WFs were obtained at 20 °C in desiccators, where the relative humidity was controlled with various saturated salt solutions, i.e., 12% (LiCl), 23% (CH3COOK), 33% (MgCl2), 44% (K2CO3), 59% (NaBr), 76% (NaCl), and 97% (K2SO4). The equilibrium moisture content (EMC) of the samples was measured using the gravimetric method.
RESULTS AND DISCUSSION
IL Pretreatment of WFs
The original WFs displayed a strong diffraction peak at 22.3°, corresponding to the (002) crystal plane of cellulose I, as well as a composite peak corresponding to the (101) and (10) crystal planes at about 14.75° and 16.5°, respectively (Fig. 1a). After pretreating in IL at 125 °C for 1 h, the intensity of the peaks decreased at low wood loadings (5% and 15%). The decrease of crystallinity after IL pretreatment was calculated, and P5 and P15 had CrI values of 49.6% and 53.9%, respectively, which were lower than that of the original WFs (57.5%; Table 1). However, at high loadings (30% and 50%), the IL was only able to wet the surface of the WFs during pretreatment. In other words, the IL behaved as a surface modifier rather than a solvent for cellulose, leading to the slightly increased crystallinity, likely caused by the tiny removal of lignin or hemicellulose on the surface of the WFs.
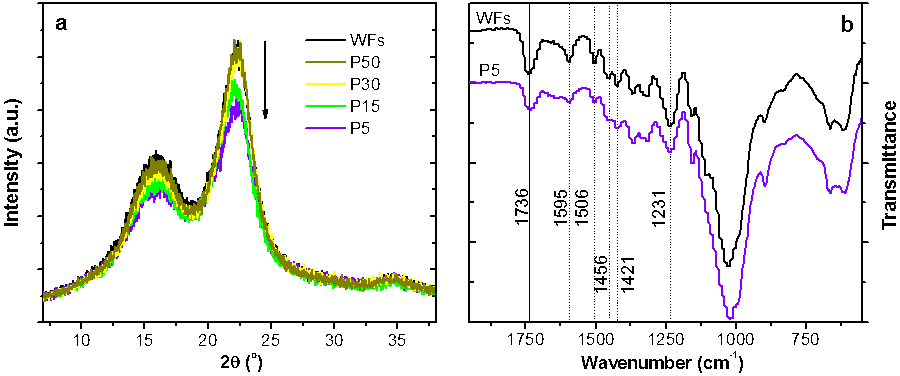
Fig. 1. XRD (a) and FT-IR (b) spectra of original and IL-pretreated wood fibers. The arrow indicates the order of the sample codes.
During IL pretreatment, the IL first interacts with lignin and then deconstructs the cellulose crystalline structure, after removing some fraction of lignin (Wu et al. 2011). As expected, a low wood loading appeared to benefit the removal of lignin in the WFs during IL pretreatment. Delignification reached 34.3% and 9.9% at loadings of 5% and 15%, respectively, while the extraction of lignin at higher loadings was negligible (Table 1). The delignification effect by IL pretreatment at low wood loadings was confirmed by the FT-IR spectra (Fig. 1b). Specifically, the lignin characteristic peaks, C=C stretching vibration at 1595/1506 cm-1, CH3 asymmetric bending at 1456 cm-1, C–H deformation at 1421 cm-1, and the syringyl ring at 1231 cm-1 (Labbe et al. 2005) were less intense in the spectrum of P5 (P15 less noticeable, not shown) than those in the original WFs. Additionally, part of hemicelluloses were also removed during pretreatment at 5% and 15% loadings (Table 1), as confirmed by the decrease of the band at 1736 cm-1 (Fig. 1b) assigned to the C=O stretching vibration in the acetyl groups of hemicelluloses (Sun et al. 2009).
Table 1. Crystallinity Index (CrI), Delignification, and Hemicellulose Content of IL-Pretreated Wood Fibers
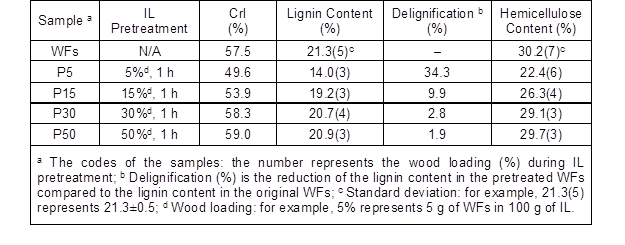
Acetylation of WFs
For the conventional acetylation of wood in an excess of acetic anhydride-pyridine mixture (v/v 4:1), pyridine accelerates the reaction through not only its swelling ability but also its catalytic function as an acid acceptor (Minato and Ogura 2003; Minato and Ito 2004). In this study, a much smaller amount of reagent and catalyst was added to the toluene medium, and the WFs were subjected to IL pretreatment at different wood loadings prior to the reaction, attempting to improve the acetylation.
On the basis of WPG, it appears that IL pretreatment contributed to the modification efficiency of the wood fibers, more so for the P5 and P15 samples (Fig. 2). After reacting at 90 °C for 30 h, the ultimate WPG value increased to 26.9% for AP5 and 24.7% for AP15, compared to 20.8% for the Ctrl sample (Fig. 2a). To reach a 20% WPG, P5 and P15 needed only 6 h to 7 h of reaction at 90 °C, whereas the original WFs required 24 h. Acetylation could produce a 20% WPG at much lower temperatures (around 75 °C) after 30 h for P5 and P15, whereas for original WFs, as well as P30 and P50 (not shown), a temperature near 90 °C was needed (Fig. 2b). Therefore, IL pretreatment, even at a higher wood loading (15%), seems to be a promising measure to substantially improve the reaction efficiency, or energy conservation, during wood modification.
Lignified structures exhibited intense autofluorescence at 405 nm, and thus the unequivocal identification of the xylem and phloem, earlywood, and latewood areas of poplar was achieved using fluorescence microscopy (Fig. 3). More importantly, autofluorescence helps reveal the mechanism behind the improved acetylation of the pretreated WFs, which was most likely due to the immediate and simultaneous disintegration of the lignin-rich, middle lamella in the plant tissues, as the primary and secondary cell walls started separating from the middle lamella even at higher wood loadings during pretreatment, thus exposing free hydroxyl groups (reaction sites) in both the lignin and cellulose. This result is consistent with the observation previously reported by Singh et al. (2009). These –OH groups in the lignin and amorphous cellulose may have reacted rapidly with the acetic anhydride in the early stage of acetylation (within 9 h), leading to the higher acetylation efficiency of AP5, AP15, and AP30 (Fig. 2a). With increasing reaction time, cellulose in both the amorphous and opening crystal regions, which has lower reactivity than lignin (Rowell et al. 1994), continued to be substituted. Therefore, the disruption of the crystalline structure of cellulose seems to be the most critical factor in improving the ultimate WPG.
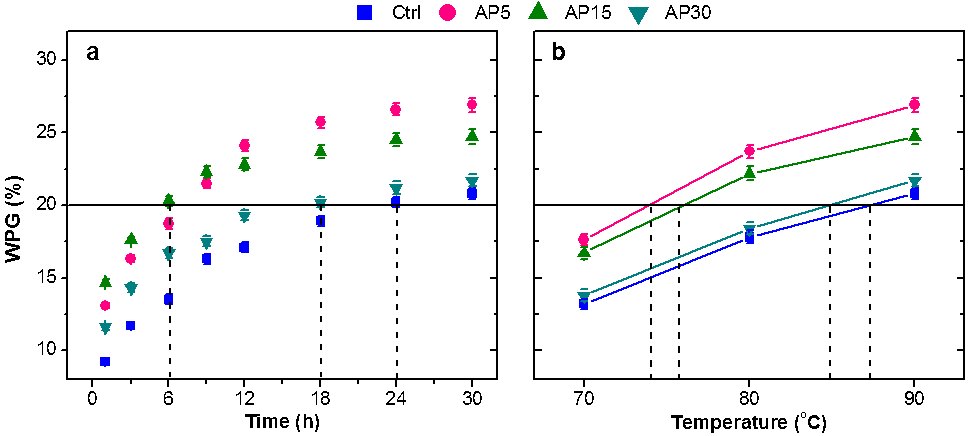
Fig. 2. Effects of reaction time (a; at 90 °C) and reaction temperature (b; for 30 h) on the weight percent gain (WPG) of original or pretreated wood fibers after acetylation. The products were annotated as “Ctrl” and “AP”, respectively, where the numbers represent the wood loading during pretreatment.
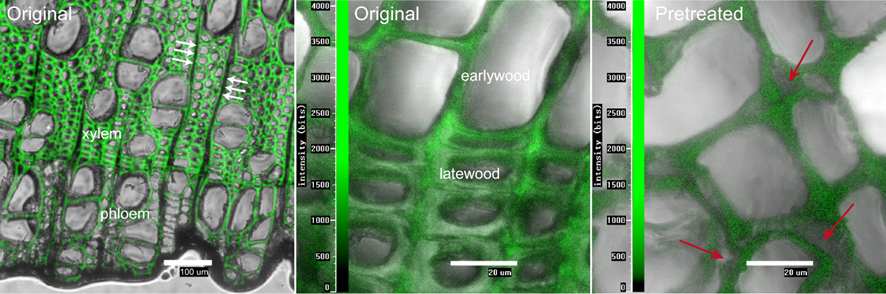
Fig. 3. Autofluorescence images of the original poplar wood (xylem radial cells are indicated with white arrows) and the sample pretreated in IL (15% wood/IL) at 125 °C for 1 h. The image of the pretreated sample was collected after regeneration with DI water.
Structure of Acetylated WFs
After acetylation, a decrease in the intensity of the O–H absorption band at 3370 cm-1 was observed (Fig. 4a), indicating the occurrence of esterification. The enhanced carbonyl stretching (C=O ester) at 1738 cm-1 (Barud et al. 2008), C–H bending (–CH3) at 1364 cm-1, and C–O–C stretching at 1220 cm-1 further confirmed the formation of ester bonds. Additionally, no bands of unreacted acetic anhydride in the range of 1840 cm-1 to 1760 cm-1 were observed in the spectra, indicating that the products were completely rinsed.
As expected, the crystallinity of the WFs was gradually reduced as the WPG increased (Fig. 4b), because of the substitution of hydroxyl groups for larger-sized acetyl groups capable of breaking the inter- and intra-molecular hydrogen bonds of the wood. The CrI of the AP5 (90 °C, 18 h) sample decreased to 37.1%. Interestingly, a new diffraction peak emerged at about 8.3° in this sample, which is cited as the principal characteristic of crystalline acetylated cellulose, caused by the projection of the substituting acetyl groups along the axes, and thus, an increase in the interplanar distance (Rodrigues et al. 2000).
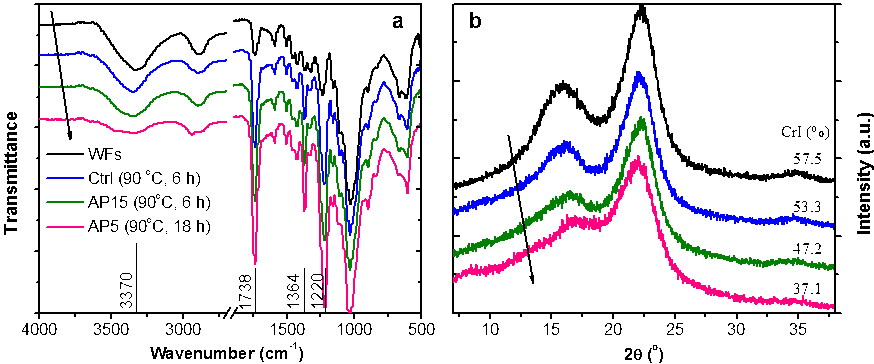
Fig. 4. FT-IR (a) and XRD (b) spectra of original and acetylated wood fibers. The arrows indicate the order of the sample codes.
Thermal Properties of Acetylated WFs
The thermal properties of the WFs were primarily influenced by IL pretreatment, specifically, the wood loading during pretreatment, while acetylation had little effect (Fig. 5).
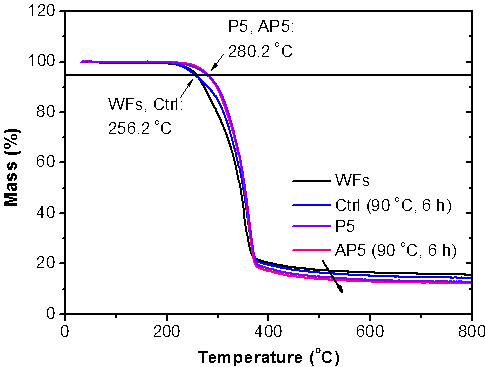
Fig. 5. Comparison of the thermogravimetric analysis data for original, IL-pretreated (P5), and acetylated (Ctrl and AP5: 90 °C, 6 h) wood fibers. The arrow indicates the order of the sample codes.
The starting decomposition temperature of P5 and AP5 was slightly higher than that of the original WFs and the Ctrl (280.2 °C vs. 256.2 °C for a 5% weight loss). This result can most likely be attributed to the decreased hemicellulose content after pretreating at 5% loading. With increased wood loading (P15, P30, and P50), the thermal properties of both the pretreated and acetylated WFs remained mostly unchanged (not shown).
EMC of Acetylated WFs
To compare the effects of IL pretreatment and acetylation on the water sorption behavior of WFs, the moisture sorption isotherms were determined based on the equilibrium moisture content (EMC) over the relative humidity (RH). The sorption curves of all of the samples displayed a typical sigmoidal shape (Fig. 6a), as found in cellulose-based materials (Xie et al. 2011a, b). With the stepwise increase in RH, the EMC of the WFs increased (Fig. 6a) because of the abundant hydroxyl groups and elastic swelling of the cell walls by water molecules. Ionic liquid pretreatment at lower wood loadings (5% and 15%) increased the EMC of the WFs in the high RH range because of the increase of free –OH groups in the cell walls. After acetylation, the EMC values of all of the samples were remarkably reduced (Fig. 6b). Compared to the Ctrl sample, the moisture sorption abilities of the AP samples were further decreased as their WPG values increased.
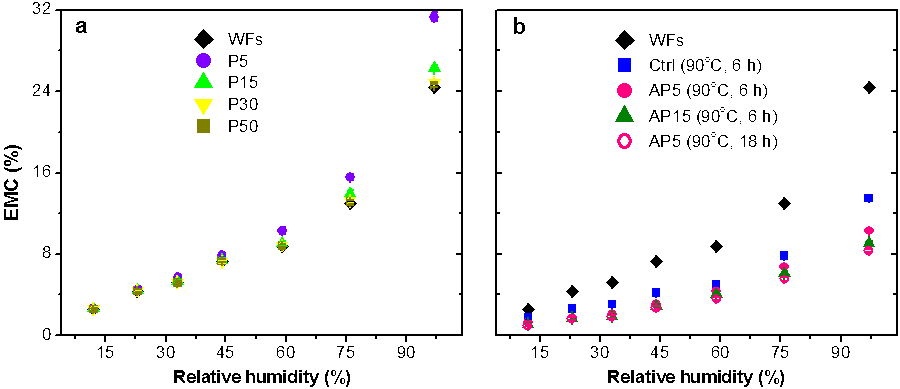
Fig. 6. Equilibrium moisture content (EMC) of IL-pretreated (a) and acetylated (b) wood fibers
CONCLUSIONS
- Pretreatment of poplar wood with 1-butyl-3-methylimidizolium chloride ([C4mim]Cl) at 5% wood/IL and 125 °C for 1 h resulted in a dramatic reduction of cellulose crystallinity (up to 14%) and the extraction of lignin (up to 34%). As the wood loading increased up to 15%, the crystallinity reduction and delignification were decreased to 6% and 10%, respectively.
- Ionic liquid (IL) pretreatment, even at a high wood loading (15%), substantially increased the reaction efficiency or energy conservation in wood modification. While the destruction of lignin-cellulose bonds by IL pretreatment played an important role in the acetylation efficiency in the early stage of the reaction, the decrease of cellulose crystallinity seemed to be the most critical factor for improving the ultimate weight percent gain (WPG) of the products.
- Ionic liquid pretreatment at 5% and 15% wood loadings increased the ultimate WPG of the acetylated AP5 and AP15 products and, thus, lowered their crystallinity and moisture sorption ability compared to the control samples. The thermal stability of the AP5 samples was improved because of the decreased hemicellulose content.
ACKNOWLEDGMENTS
The authors are grateful for the support of the National Natural Science Foundation of China (31470585) and the Natural Science Foundation of Heilongjiang Province, China (JC2015006).
REFERENCES CITED
Barud, H. S., de Araujo, A. M., Santos, D. B., de Assuncao, R. M. N., Meireles, C. S., Cerqueira, D. A., Rodrigues, G., Ribeiro, C. A., Messaddeq, Y., and Ribeiro, S. J. L. (2008). “Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose,” Thermochimica Acta 471(1-2), 61-69. DOI: 10.1016/j.tca.2008.02.009
Evans, P. D., Wallis, A. F. A., and Owen, N. L. (2000). “Weathering of chemically modified wood surfaces – Natural weathering of Scots pine acetylated to different weight gains,” Wood Science and Technology 34(2), 151-165. DOI: 10.1007/s002260000039
Labbe, N., Rials, T. G., Kelley, S. S., Cheng, Z. M., Kim, J. Y., and Li, Y. (2005). “FT-IR imaging and pyrolysis-molecular beam mass spectrometry: New tools to investigate wood tissues,” Wood Science and Technology 39(1), 61-U19. DOI: 10.1007/s00226-004-0274-0
Li, J. Z., Furuno, T., Katoh, S., and Uehara, T. (2000). “Chemical modification of wood by anhydrides without solvents or catalysts,” Journal of Wood Science 46(3), 215-221. DOI: 10.1007/BF00776452
Li, W., Sun, N., Stoner, B., Jiang, X., Lu, X., and Rogers, R. D. (2011). “Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin,” Green Chemistry 13(8), 2038-2047. DOI: 10.1039/C1GC15522A
Minato, K., and Ito, Y. (2004). “Analysis of the factors influencing the acetylation rate of wood,” Journal of Wood Science 50(6), 519-523. DOI: 10.1007/s10086-003-0598-7
Minato, K., and Ogura, K. (2003). “Dependence of reaction kinetics and physical and mechanical properties on the reaction systems of acetylation I: Reaction kinetic aspects,” Journal of Wood Science 49(5), 418-422. DOI: 10.1007/s10086-002-0514-6
Obataya, E., and Minato, K. (2009). “Potassium acetate-catalyzed acetylation of wood: Reaction rates at low temperatures,” Wood Science and Technology 43(5-6), 405-413. DOI: 10.1007/s00226-008-0212-7
Rodrigues, G., da Cruz, S. F., Pasquini, D., Cerqueira, D. A., Prado, V. D., and de Assuncao, R. M. N. (2000). “Water flux through cellulose triacetate films produced from heterogeneous acetylation of sugar cane bagasse,” Journal of Membrane Science 177(1-2), 225-231. DOI: 10.1016/S0376-7388(00)00469-5
Roncero, M. B., Torres, A. L., Colom, J. F., and Vidal, T. (2005). “The effect of xylanase on lignocellulosic components during the bleaching of wood pulps,” Bioresource Technology 96(1), 21-30. DOI: 10.1016/j.biortech.2004.03.003
Rowell, R. M., Simonson, R., Hess, S., Plackett, D. V., Cronshaw, D., and Dunningham, E. (1994). “Acetyl distribution in acetylated whole wood and reactivity of isolated wood cell-wall components to acetic anhydride,” Wood and Fiber Science 26(1), 11-18.
Schlufter, K., Schmauder, H. P., Dorn, S., and Heinze, T. (2006). “Efficient homogeneous chemical modification of bacterial cellulose in the ionic liquid 1-N-butyl-3-methylimidazolium chloride,” Macromolecular Rapid Communications 27(19), 1670-1676. DOI: 10.1002/marc.200600463
Singh, S., Simmons, B. A., and Vogel, K. P. (2009). “Visualization of biomass solubilization and cellulose regeneration during ionic liquid pretreatment of switchgrass,” Biotechnology and Bioengineering 104(1), 68-75. DOI: 10.1002/bit.22386
Sun, N., Rahman, M., Qin, Y., Maxim, M. L., Rodriguez, H., and Rogers, R. D. (2009). “Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate,” Green Chemistry 11(5), 646-655. DOI: 10.1039/B822702K
TAPPI T222 om-98 (1998). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
Teramoto, Y., Lee, S. H., and Endo, T. (2008). “Pretreatment of woody and herbaceous biomass for enzymatic saccharification using sulfuric acid-free ethanol cooking,” Bioresource Technology 99, 8856-8863. DOI: 10.1016/j.biortech.2008.04.049
Weerachanchai, P., Leong, S. S. J., Chang, M. W., Ching, C. B., and Lee, J. M. (2012). “Improvement of biomass properties by pretreatment with ionic liquids for bioconversion process,” Bioresource Technology 111, 453-459. DOI: 10.1016/j.biortech.2012.02.023
Wu, H., Mora-Pale, M., Miao, J., Doherty, T. V., Linhardt, R. J., and Dordick, J. S. (2011). “Facile pretreatment of lignocellulosic biomass at high loadings in room temperature ionic liquids,” Biotechnology and Bioengineering 108(12), 2865-2875. DOI: 10.1002/bit.23266
Xie, H., King, A., Kilpelainen, I., Granstrom, M., and Argyropoulos, D. S. (2007). “Thorough chemical modification of wood-based lignocellulosic materials in ionic liquids,” Biomacromolecules 8(12), 3740-3748. DOI: 10.1021/bm700679s
Xie, Y., Hill, C. A. S., Xiao, Z. F., Mai, C., and Militz, H. (2011a). “Dynamic water vapour sorption properties of wood treated with glutaraldehyde,” Wood Science and Technology 45(1), 49-61. DOI: 10.1007/s00226-010-0311-0
Xie, Y., Hill, C. A. S., Jalaludin, Z., Curling, S. F., Anandjiwala, R. D., Norton, A. J., and Newman, G. (2011b). “The dynamic water vapour sorption behaviour of natural fibres and kinetic analysis using the parallel exponential kinetics model,” Journal of Materials Science 46(2), 479-489. DOI: 10.1007/s10853-010-4935-0
Yuan, T., Sun, S., Xu, F., and Sun, R. (2010). “Homogeneous esterification of poplar wood in an ionic liquid under mild conditions: Characterization and properties,” Journal of Agricultural and Food Chemistry 58(21), 11302-11310. DOI: 10.1021/jf103050t
Zafeiropoulos, N. E., Williams, D. R., Baillie, C. A., and Matthews, F. L. (2002). “Engineering and characterisation of the interface in flax fibre/polypropylene composite materials. Part I. Development and investigation of surface treatments,” Composites Part A: Applied Science and Manufacturing 33(8), 1083-1093. DOI: 10.1016/S1359-835X(02)00082-9
Article submitted: August 13, 2016; Peer review completed: November 12, 2016; Revised version received and accepted: November 17, 2016; Published: November 30, 2016.
DOI: 10.15376/biores.12.1.684-695
