Abstract
Industrial production of biogas offers a way to manage distillery leachate. The waste is usually subjected to anaerobic digestion for producing biogas. However, the effluent from anaerobic processes has high chemical oxygen demand (COD) and is harmful to the environment. An effective method of lowering COD is ozonation. Effluent from biogas plants after ozonation has the potential for use in breeding grounds for plants of the Lemnaceae family. Thus, they can provide a valuable additional source of biomass for the production of bioethanol. Lemna minor L. and Spirodela polyrhiza cultures were grown in media with the addition of 2.5% PFE, which had been treated by ozonation for between 6 and 50 min. Using ozonated effluent was an effective cultivation technique in all variants. The analyzed parameters were plant growth, chlorophyll index, fresh plant weight and photosynthetic traits (net photosynthesis, stomatal conductance, transpiration and concentration of intercellular CO2). The best growth of Lemna minor L. was observed in the media with PFE treated for 12 min. Similar effects were obtained for S. polyrhiza, with ozone treatment for 12 and 25 min. The results show the potential of using ozone-treated post-fermentation leachate as a supplement in culture media.
Download PDF
Full Article
Improving Biorefinery Sustainability and Profitability by Cultivating Aquatic Plants on Ozonized Distillery Effluents
Piotr Dziugan,a Zdzisława Romanowska-Duda,b Krzysztof Piotrowski,b Weronika Cieciura-Wloch,a Hubert Antolak,c Krzysztof Smigielski,a Michał Binczarski,d Izabela Witonska,d and Jarosław Domański a,*
Industrial production of biogas offers a way to manage distillery leachate. The waste is usually subjected to anaerobic digestion for producing biogas. However, the effluent from anaerobic processes has high chemical oxygen demand (COD) and is harmful to the environment. An effective method of lowering COD is ozonation. Effluent from biogas plants after ozonation has the potential for use in breeding grounds for plants of the Lemnaceae family. Thus, they can provide a valuable additional source of biomass for the production of bioethanol. Lemna minor L. and Spirodela polyrhiza cultures were grown in media with the addition of 2.5% PFE, which had been treated by ozonation for between 6 and 50 min. Using ozonated effluent was an effective cultivation technique in all variants. The analyzed parameters were plant growth, chlorophyll index, fresh plant weight and photosynthetic traits (net photosynthesis, stomatal conductance, transpiration and concentration of intercellular CO2). The best growth of Lemna minor L. was observed in the media with PFE treated for 12 min. Similar effects were obtained for S. polyrhiza, with ozone treatment for 12 and 25 min. The results show the potential of using ozone-treated post-fermentation leachate as a supplement in culture media.
DOI: 10.15376/biores.18.1.317-336
Keywords: Biorefinery; Post-fermentation effluent; Ozone treatment; Lemnaceae
Contact information: a: Department of Environmental Biotechnology, Faculty of Biotechnology and Food Science, Lodz University of Technology, Wolczanska 171/173, 90-924 Lodz, Poland; b: Department of Plant Ecophysiology, Faculty of Biology and Environmental Protection, University of Lodz, Banacha 12/16, 92-237 Lodz, Poland; c: Institute of Fermentation Technology and Microbiology, Faculty of Biotechnology and Food Science, Lodz University of Technology, Wolczanska 171/173, 90-924 Lodz, Poland; d: Institute of General and Ecological Chemistry, Faculty of Chemistry, Lodz University of Technology, Zeromskiego 116, 90-924 Lodz, Poland;
* Corresponding author: jaroslaw.domanski@p.lodz.pl
GRAPHICAL ABSTRACT

INTRODUCTION
Biorefineries that use biomass to produce energy, fuels, and chemicals are seen as a promising alternative to petrochemical technologies based on non-renewable raw materials (Zaman 2015). Biomass, in comparison to petrochemical raw materials, contains more oxygen, less hydrogen, and less carbon. Therefore, a larger group of chemical products can be obtained from lignocellulose than from petrochemical raw materials. However, the processing of biomass into fuels and valuable chemical compounds requires the use of more complex technologies, including pretreatment processes necessary to obtain sugars or their derivatives, and further processes for obtaining final products. At present, the majority of lignocellulosic biomass processing technologies are in the pre-commercial phase and are not implemented in industrial installations (Cherubini and Strømman 2011). Among the possible methods of processing biomass into chemical products, only two technologies can be considered as fully commercial technologies: the production of bioethanol from lignocellulosic biomass (sawdust, corn straw) (Nguyen et al. 2017) and the production of lactic acid from glucose obtained from starchy raw materials (rye, triticale, maize, potatoes, etc.) (Altaf et al. 2007). For example, DuPont launched a plant in 2013 that uses corn straw (stems, leaves, and cob devoid of seeds) as the raw material. This biorefinery is the world’s largest producer of cellulosic ethanol. Its production capacity exceeds 113 million liters of bioethanol annually, with greenhouse gas emissions reduced by 90% compared to the conventional gasoline production process (Rosen 2015). It may also be observed that most installations produce first generation fuels: biodiesel or bioethanol from sugar plants (sugar beet, sugar cane), starchy materials (e.g., cereals, manioc), or oil (e.g., rape, soy) (Rabaçal et al. 2017). An example of a modern biorefinery is the Vivergo plant founded by the companies AB Sugar, BP, and DuPont. Built in Hull (UK), the biorefinery produces 420 million liters of bio-ethanol per year from grain (Nichols 2013). An example of biofuel production based on oilseeds is provided by the Brazilian company Petrobras Biofuels. In their biodiesel production plant, oils are produced from soybean, castor, sunflower, cotton, as well as animal fats. The biorefinery achieves annual productivity of 170 million liters of biodiesel (Zonin et al. 2014). However, it should be emphasized that, regardless of the technology used to produce them, biofuels are still more expensive than conventional petroleum fuels, as a result of the large amount of energy required to produce them.
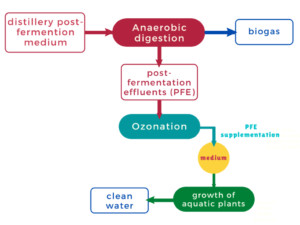
To improve the energy efficiency of biorefineries, partnerships have been set up between distilleries and biogas plants, which use the post-fermentation wastewater, which is still rich in nutrients and useful to biogas production (Fig. 1A). Following anaerobic fermentation, the biogas is burnt in cogeneration installations. The heat is used for the needs of the plant, and the electrical current is sold to electricity suppliers. However, biogas plants generate large amounts of leachates with high organic load, which are harmful to the environment and the surrounding population. In the authors’ previous work, the use of sugar beet pulp for the production of ethanol (Berlowska et al. 2017), biogas (Ziemiński and Kowalska-Wentel 2017), lactic acid (Joanna Berlowska et al. 2018), and biodegradable polymers (Tomaszewska et al. 2018) was investigated. However, the authors did not consider waste disposal methods in the proposed biological-chemical technologies, and their ecological use in plant production as fertilizers (Romanowska-Duda et al. 2018, 2019; Dȩbowski et al. 2018, 2020; Kisielewska et al. 2020; Szufa et al. 2020).
The novelty of the current study is cultivation of aquatic plants on ozonized effluents from biogas plants. This is the first study in which ozonized effluents were used to produce biomass from aquatic plants of the family Lemnaceae. The process provides phytoremediation, as well as a supplement to the biogas plant charge or a valuable feed for animals. In the solution proposed in this work, the effluents are ozonated to reduce their organic charge and then used as a medium for the aquatic plants Lemna minor L. and Spirodela polyrhiza (Fig. 1B). Lemnaceae are a family of simple, fast-growing, floating aquatic plants. They are a suitable choice for wastewater treatment because of their high nutrient-uptake capabilities and resilience to severe environmental conditions. The Lemnaceae family consists of five genera (Landoltia, Lemna, Spirodela, Wolfa, and Wolfella), and 38 species have been classified to date (Cui and Cheng 2015; Xu et al. 2015). The rapid growth rates of these aquatic plants, their high starch content, and low lignin content make them a popular feedstock for bioethanol production (Calicioglu et al. 2018). The biochemical conversion of duckweed starch and cellulose into simple sugars for fermentation into alcohols has been described at both the laboratory and pilot scale (Kazemi et al. 2020; Su et al. 2014).
Fig. 1. A – Diagram of a bio-refinery producing ethanol from lignocellulosic raw materials from the sugar industry; B – Diagram of bio-refinery producing ethanol from lignocellulosic raw materials from the sugar industry supplemented with wastewater treatment processes and production of biomass from aquatic plants. Green squares show the raw materials used and blue squares are the final products.
The plants considered in this study are Lemna minor L. and Spirodela polyrhiza, which belong to the family Lemnaceae – the world’s smallest angiosperms (Basiglini et al. 2018). Because of the ease with which they can be cultivated, their rapid growth rate, and the wide range of their possible uses, they are commonly used in biotechnological processes (Iatrou et al. 2015; Pena et al. 2017; Tang et al. 2017; Ma et al. 2018). Previous studies have explored their application in wastewater treatment (Zhao et al. 2015; Bokhari et al. 2016; Tang et al. 2017;); biomass generation (Gaur and Suthar 2017), which can be used in the production of biogas (Toyama et al. 2018), ethanol (Cui and Cheng 2015), and liquid fuel (Xu et al. 2018); or as a source of nutrients for humans and animals (Appenroth et al. 2017; Chakrabarti et al. 2018).
However, chemicals present in the culture media can significantly inhibit the growth of these plants (Bourioug et al. 2018). Therefore, to ensure effective and economically efficient cultivation of Lemna minor and Spirodela polyrhiza in media containing wastewater materials, it is essential to reduce the concentration of chemicals that are the growth inhibitors in the media. Anaerobic digestion of effluent from biogas plants is characterized by high chemical oxygen demand (COD) between 12,000 and 20,000 mg/L. One of the ways to lower the chemical load of sewage, as a preliminary stage in biotechnological processes, is ozonation (Muradov et al. 2014; Dziugan et al. 2016).
The use of ozone to purify leachate from biogas plants is one of the most effective methods of reducing the COD in sewage. Tests conducted at the Institute of Fermentation and Microbiology, Lodz University of Technology (Lodz, Poland), showed a strong decline in the COD of 200 mL of sewage during the initial ozonation period, from almost 14,000 mg/L to about 4000 mg/L. However, raising the ozone dose increases the COD again, to a value close to the initial level, as shown in Fig. 2. Ozone was also an effective disinfectant and fragrance corrector of sewage. Untreated wastewater had a strong and unpleasant odor, whereas the post-ozonated wastewater was odorless, after ozonation for 35 min, corresponding to an ozone dose of 0.42 g/1000 L. Therefore, if ozonation of sewage is to be conducted on an industrial scale, the oxidizer dose should be optimized, as a critical initial step.
Fig. 2. COD changes depending on the ozone dose delivered to wastewater from a biogas plant
Fig. 3. Installation for the treatment of leachate digestates with ozone operating in a Polish distillery (A – general view; B – ozone generator; C – reactor for sewage ozonation)
The use of ozone in second-generation ethanol production technology from sugar beet can bring many technological and economic benefits. One advantage of this method is that ozone can be produced from oxygen in situ, thereby avoiding storage and transportation costs. Moreover, by generating their own heat and electricity, modern distilleries (or biorefineries) can considerably reduce the cost of producing bioethanol. In Poland, there is growing interest in the use of ozone as part of industrial biofuel production processes. For example, a treatment station for post-digestion leachate has been installed in one of the Polish distilleries (Fig. 3).
This paper set out to study the influence of post-fermentation effluents (PFEs) from a biogas plant treated with ozone on the growth of aquatic plants belonging to the Lemnaceae family. Lemna minor and Spirodela polyrhiza cultures were grown in water with PFE additives previously treated with ozone for a duration between 6 and 60 min. The analyzed parameters were as follows: plant growth, fresh plant weight, chlorophyll index, and photosynthesis traits (net photosynthesis, stomatal conductance, transpiration, and concentration of intercellular CO2). The parameters were monitored to see whether the ozonated effluents had a positive effect on the growth of Lemna minor and Spirodela polyrhiza. Increasing the yields of aquatic plants by adding ozonated leachates from biogas plants could reduce the ecological footprint of the biorefinery, while at the same time increasing its profitability, because the additional biomass could itself be used for biofuel production.
EXPERIMENTAL
Post-fermentation Effluents
Effluent produced following anaerobic fermentation of a distillery post-fermentation medium was used to supplement cultivation media for Lemna minor L. and Spirodela polyrhiza (Fig. 4).
Fig. 4. Diagram of a utilization of PFEs
The PFEs were subjected to basic characterization in terms of COD, as well as total ammonium nitrogen (TAN), and phosphates, which were determined using a HACH-Lange DR6000 spectrophotometer (Hach Lange, Loveland, CO, USA) and HACH-Lange tests No. 8000, 8038, and 8048 (Loveland, CO, USA), respectively. The ozonation process was then performed. For this purpose, 20% (w/v) solution of the effluent in demineralized water was placed into a reactor, through which a mixture of oxygen and ozone was passed containing 80 g of ozone in 1000 L of gas. The flow of gaseous was 0.4 dm3/min. Ozonation was performed for periods of 6, 12, 25, or 60 min. After ozonation, tests for COD, TAN, and phosphates were performed.
Plant Material
Two aquatic plants belonging to the Lemnaceae family were used in the study. Spirodela polyrhiza and Lemna minor (Fig. 5) were isolated from water reservoirs in the Lodz voivodeship (Poland). The plants were grown in in vitro cultures at the Laboratory of Plant Ecophysiology, Faculty of Biology and Environmental Protection, University of Lodz (Lodz, Poland).
Fig. 5. Cultivation of (A) Lemna minor L. and (B) Spirodela polyrhiza plants used in the study
Culture Conditions
Spirodela polyrhiza and L. minor were cultivated in medium Z (Pádrová et al. 2015) (pH 6.4) as a control sample and the same medium was supplemented with different variants of ozonated PFEs: (I) Medium Z; (II) Medium Z + 2.5% non-ozonated PFE; (III) Medium Z + 2.5% PFE ozonated for 6 min; (IV) Medium Z + 2.5% PFE ozonated for 12 min; (V) Medium Z + 2.5% PFE ozonated for 25 min; (VI) Medium Z + 2.5% PFE ozonated for 60 min.
Erlenmeyer flasks were filled with 250 mL of different variants of the medium inoculated with 20 separate individuals (fronds) of S. polyrhiza or L. minor. The plants were cultivated in a phytotron at 24 °C, with continuous lighting supplied by 2 × 18 W/840 Philips Master TL-D lamps (Amsterdam, Netherlands). The culture was grown for 14 days. The fronds of the individual plants were counted on each day of the experiment. After 14 days of cultivation, the chlorophyll index and fresh weight of the plants as well as parameters of the photosynthesis process (net photosynthesis, stomatal conductance, transpiration, and concentration of intercellular CO2) were determined.
Chlorophyll Index
Index of chlorophyll content in the plants after 14 days of cultivation in the tested media was evaluated using a Konica Minolta SPAD-502 chlorophyll meter (Tokyo, Japan). The results were expressed in SPAD units (Grzesik and Romanowska-Duda 2014; Grzesik et al. 2017).
Fresh Weight of Plants
All fronds were transferred into pre-weighed polystyrene round bottom tubes with 1.0-mm holes. Next, the samples were centrifuged using an Eppendorf 5417R (Hamburg, Germany) at 3000 rpm for 10 min at 25 °C. The remaining supernatant was discarded and the tubes containing the tested plant biomass were weighed. Finally, the fresh weight was calculated by subtracting the weight of the tube from the weight of the tube with biomass (Oecd 2006).
Photosynthesis
Gas exchange parameters (net photosynthesis Pn [µmol CO2 m-2s-1], transpiration Tr [mmol H2O m-2s-10], stomatal conductance Gs [mol H2O m-2s-1], and intercellular CO2 concentration Ci [µmol mol-1] for the tested S. polyrhiza and L. minor plants were measured using an Amesbury TPS-2 apparatus from PP Systems (Amesbury, MA, USA), in accordance with the methodology described above. All parameters were evaluated after 14 days of cultivation.
Statistics
Statistical differences in plant growth were compared using a one-way repeated measures analysis of variance (ANOVA; OriginPro 9.2.214, OriginLab Corp., Northampton, MA, USA). Statistical significance was set at the conventional level of 5% (p < 0.05). The profiles of the parameters for the plant cultures in different media were compared by hierarchical clustering using Clustvis (https://biit.cs.ut.ee/clustvis/), a web tool for visualizing clustering of multivariate data (Metsalu and Vilo 2015).
RESULTS AND DISCUSSION
Ozonation was investigated as a potential post-treatment step for post-fermentation effluent to enhance biodegradability and observe the influence of the initial organic matter concentration. At low concentrations, ozone mainly influences soluble COD compounds. Longer exposure time also affected particulate compounds, resulting in the solubilization of the COD fractions. Ozonation, despite its distinct technical advantages, may prove costly when applied to the entire wastewater volume. Ozonation efficiency was tested in terms of contact time. It seems that application of ozonation should be economically viable and not generate toxic intermediates affecting the process, which is consistent with the findings of Chiavola and co-workers (2021) and Mainardis and co-workers (2020).
In general, the PFEs ozonation time affected the number of plant fronds cultivated in supplemented media. The number of fronds of L. minor and S. polyrhiza after cultivation in different media are presented in Fig. 5. The largest numbers of fronds after 14 days of cultivation of L. minor and S. polyrhiza were observed in Medium 4 (PFEs with 12 min of ozonation), at 430 fronds and 279 fronds, respectively. Compared to the non-ozonated medium, these values were 99% higher for L. minor and 36% higher for S. polyrhiza. It is worth emphasizing that further ozonation led to smaller plant populations. In the medium supplemented with PFEs that had been ozonated for the longest time (60 min), the numbers of fronds of both plants were lower than for both the medium with PFEs without ozonation and the control medium (L. minor). Therefore, in terms of the increase in the number of individual fronds, the most favorable variant was Medium 4, supplemented with PFEs subjected to ozonation for 12 min.
Fig. 6. Influence of the tested media on the number of fronds of L. minor L. (A); and S. polyrhiza (B). Results for media supplemented with effluent after ozonation over time 6, 12, 25, or 60 min (Media 3, 4, 5, and 6, respectively) are compared to the results for medium without ozone treatment (Medium 2) using one-way repeated measures ANOVA. Values with different letters are statistically different (p < 0.05): a— p ≥ 0.05; b— 0.005 < p < 0.05; c— p < 0.005.
The results of the growth parameters for the tested plants in media supplemented with PFEs after different times of ozonation are presented in Table 1. The results indicated that the best medium for the cultivation of L. minor was Medium 4, which showed the highest values for the analyzed parameters, photosynthesis (3.54 ± 0.11 µmol CO2 m-2s-1), the chlorophyll index (25.31 ± 2.84 SPAD units), transpiration rate (3.32 ± 0.19 mmol H2O m-2s-1), and stomatal conductance (693 ± 16 mmol H2O m-2s-1). At the same time, the intercellular CO2 concentration (389 ± 40 µmol CO2 mol-1) was the lowest in this medium. Medium 4 was second only in terms of fresh weight (2.15 ± 0.13 mg), which was slightly lower than in the case of Medium 5 (2.40 ± 0.04 mg) (Fig. 7).
Table 1. Influence of Ozonation on Photosynthesis Parameters for L. minor and S. polyrhiza. The Highest Results for Each Feature for a Given Plant are Underlined and Denoted in Bold
The results for S. polyrhiza were more varied. Medium 4 showed the highest values for fresh weight (2.86 ± 0.13 mg), net photosynthesis (3.14 ± 0.01 µmol CO2 m-2s-1), and transpiration (4.05 ± 0.04 mmol H2O m-2s-1), but also the highest intercellular CO2 concentration (468 ± 7 µmol CO2 mol-1). However, the highest results for chlorophyll index (4.05 ± 0.04 SPAD units) and stomatal conductance (981 ± 8 mmol H2O m-2s-1) were obtained with Medium 5. Despite differences in the results, both Medium 4 and Medium 5 stimulated the growth of L. minor and S. polyrhiza. The differences between Medium 2 (2.5 PFE without ozonation) and Mediums 4 and 5 were also statistically significant (p < 0.05). Thus, the process of ozonation positively affected the growth of S. polyrhiza. This observation was further confirmed by hierarchical clustering of the tested media in terms of the analyzed parameters (Fig. 8). For L. minor, Medium 4 was a separate clade, while for S. polyrhiza the separate clade included Medium 4 and Medium 5. The strongest determining factors were photosynthesis and stomatal conductance (Fig. 8A) as well as number of fronds after 7 days of incubation and fresh weight (Fig. 8B).
Fig. 7. Influence of the tested media on the fresh weight of L. minor L. (A); and S. polyrhiza (B). Results obtained for media supplemented with effluent after ozonation in proper time (Medium 3, 4, 5, 6) were compared to the results for medium without ozone treatment (Medium 2) using one-way repeated measures ANOVA. Values with different letters are statistically different (p < 0.05): a— p ≥ 0.05; b— 0.005 < p < 0.05; c— p < 0.005.
Ozone treatment has a wide range of possible applications across many industries. It has been used for the treatment of drinking water (Siddiqui et al. 1997), to remove organic matter (Zanacic et al. 2016), for inactivation of microorganisms in the food industry (Brodowska et al. 2017, 2018), and as an active agent for the removal of contaminants and pollutants from wastewater (Rosal et al. 2010; Schindler Wildhaber et al. 2015; Ashauer 2016; Bourgin et al. 2018). Ozonation has been employed in combination with specific organisms for the treatment of pulp and paper mill effluents (Bijan and Mohseni 2005; Remondino and Valdenassi 2018); in combination with biofilm for the removal of micro-contaminants from municipal influents (Gunnarsson et al. 2009); as well as in combination with anaerobic digestion in the treatment of wastewater containing pharmaceuticals (Carballa et al. 2007). In this study, the application of ozone (80 g in 1000 L) in the treatment of the PFE had a beneficial effect on the growth of L. minor and S. polyrhiza.
The results of basic chemical analysis of the medium after ozonation (Table 2) show that the process resulted in the increased availability of phosphate and nitrogen compounds. The concentration of phosphate increased from 2.73 ± 0.02 mg PO43-/mL before ozonation to 3.39 ± 0.02 mg PO43-/mL after ozonation. Similar trends were noted for ammonia nitrogen and free nitrogen. Moreover, a significant decrease in the analyzed parameters was observed after cultivation of the plants. Slightly lower values were obtained for medium after the cultivation of S. polyrhiza compared to L. minor. Ozonation decreased the COD from 397 ± 1 mg/L (Medium 2) to 235 ± 1 (Medium 4). The COD values after the cultivation of L. minor and S. polyrhiza were 35 ± 1 and 45 ± 1 mg/L, respectively. The COD values after cultivation of the tested plants were lowest for Medium 4 and Medium 5. The results show that the use of ozonation alone is insufficient, as it only decreases COD slightly (41%). This is in line with results reported by Hadavifar and others (2016), who noted a 25% reduction of COD with ozonation of vinasse from alcohol distilleries (Gunnarsson et al. 2009).
Table 2. Influence of Medium Ozonation and Plant Cultivation on Free Phosphate and Nitrogen
In general, the mechanism for removing contamination by ozonation is based on direct reaction with ozone or hydroxyl radicals formed during the decomposition process (Schindler Wildhaber et al. 2015). While electron-rich moieties (olefins, tertiary amines, thioethers, and activated aromatics) react easily and quickly with ozone, the hydroxyl groups that form during the decomposition process react with alkanes and amides (Bourgin et al. 2018). As a result of the oxidative transformation of organic matter, the content of assimilable organic carbon or biodegradable organic carbon also increases. Consequently, the resulting compounds can be utilized by organisms introduced in subsequent biological stages of wastewater treatment (Zimmermann et al. 2011; Schindler Wildhaber et al. 2015). In the context of the present study, in which the content of easily digestible forms of nitrogen and phosphorus increased, this can explain the improved growth of the tested plants in media supplemented with 2.5% PFEs ozonated for 12 min.
The effect of nitrogen and phosphate on the growth of Lemnaceae plants has been studied in the literature (Ge et al. 2012; Zhao et al. 2014; Iatrou et al. 2015). In studies conducted by Paolacci and co-workers (2016), the highest number of fronds of L. minor. and the highest biomass were noted for 0.03 g/L of nitrate. The lowest biomass was associated with the medium without nitrate. The optimum concentration of nitrogen for L. minor appears to lie in the range from 2.8 mg/L to 350 mg/L, depending on the tested plant. For good plant growth, the presence of phosphate in the water medium is also necessary. The best results of growth of L. minor were obtained when the control medium contained 93 mg/L of P.
Fig. 8. Hierarchical clustering of analyzed parameters for L. minor (A); and S. polyrhiza (B) according to the tested media. Manhattan distance was used hierarchical clustering.
Another study found that number of L. minor fronds increased with higher phosphate concentrations and increases in temperature (up to 25 °C) (Appenroth 2002). In this study, the maximum growth rate of L. minor and S. polyrhiza was achieved in the medium with 1.08 mg/mL of P and 7.88 mg/mL of N. It should be emphasized that the variation in these results may stem from the different nutritional requirements and metabolic activity of the plants used in this study, as well as from the other compounds present in the tested media (Njambuya et al. 2011; Appenroth and Adamec 2014).
As well as the effects of nutrient components on plant population and fresh weight, the growth environment has a major impact on parameters such as chlorophyll content. It is widely known that chlorophyll plays a major part in the energetic metabolism of green plants, and therefore any significant change in its levels will have a marked effect on the entire metabolism. Lemna minor has been used as a test organism for ecotoxicological assessment of industrial effluents by Radić et al. (2010). They found that the tested wastewaters inhibited growth rates, based on frond numbers and biomass, and also decreased chlorophyll content. The same authors concluded in a separate study that L. minor can be used as a tool for testing the toxicity of surface waters, and that one of the parameters that change under the influence of water parameters is the concentration of chlorophyll (Radić et al. 2011). In a study conducted by Chaudhuri et al. (2014), cadmium at concentrations of 3 mg/L reduced the chlorophyll content of L. minor to 0.7 mg/g, while for S. polyrhiza the value was 0.13 mg/g. These authors concluded that Spirodela plants were more susceptible to cadmium than Lemna plants (Chaudhuri et al. 2014). The effect of copper exposure on photosynthetic pigments in L. minor and S. polyrhiza was evaluated in a study by Kanoun-Boulé et al. (2009). They found that the content of a-type chlorophyll and carotenoids decreased as the concentration of copper rose. At the same time, b-type chlorophyll seemed less affected by exposure to the toxic agent. It should be mentioned that the studies discussed here varied in terms of temperature, pH, type of wastewater, and the composition of the resulting medium, as well as in terms of the tested plant species. Therefore, it is difficult to compare their results with the present work. Moreover, most research has focused on determining the impact of certain factors on the number of fronds, fresh weight, and chlorophyll content. In this study, additional photosynthesis parameters were examined (transpiration, stomatal conductance, and intracellular CO2 content). The authors’ results indicate that ozonation of PFEs before addition to a culture medium can have a beneficial effect on biomass yields.
Wastewater containing ozonated PFEs from a biogas plant can be considered for use in the cultivation of aquatic plants to improve the profitability and sustainability of biorefineries (Fig. 1B). Polish law allows the water discharged into watercourses to have parameters not exceeding 0.25 mg/L for phosphorous and 5 mg/L for nitrogen. Ozonated effluents with Lemna minor and Spirodela polyrhiza did not exceed these values for nitrogen and only second microorganisms led to normative results for phosphorus. The use of reclaimed wastewater as a source of process water reduces the ecological footprint of such installations and cost of energy used for fuel production. Lowering the costs of bioethanol production can be achieved by recycling the biomass of the aquatic plants through the fermentation process. After partial chemical, physical, or biological degradation, the biomass obtained from the process of wastewater treatment is a good fermentation medium, which is rich in fermentable carbohydrates. It may be used successfully as an additional substratum for bio-ethanol production. The amount of Lemnaceae and duckweed biomass that can be produced on an area of water rich in nutrients is 39.2 to 44.0 t/ha⋅year. This is comparable to or even higher than the yield of biomass that can be obtained from bioenergy grass such as miscanthus (5.0 to 44.0 t/ha⋅year) (Miranda et al. 2016). Because of their high growth rates, these water plants have already been used as a feedstock for bioethanol production (Cheng and Stomp 2009; Xu et al. 2011). To increase the yield of bioethanol from lignocellulosic biomass, it is widely recommended to perform simultaneous saccharification and fermentation (Olofsson et al. 2008; Berłowska et al. 2016). Further enhancement of ethanol fermentation efficiency in media derived from aquatic plants can be achieved by using the ozonization method to stabilize the broth (Berłowska et al. 2018). Further research is necessary to increase the scale of cultivation of L. minor and S. polyrhiza in waters enriched by ozonized biogas plant effluent. The collected biomass will be used as a fermentation medium in ethanol production processes coupled with biogas production processes.
CONCLUSIONS
- Post-fermentation effluents can be used as a raw material to supplement conventional culture media for L. minor and S. polyrhiza. Prior ozonation of the PFEs significantly improved the growth efficiently of these plants.
- The most preferred variant was the medium supplemented with 2.5% of PFEs subjected to ozonation for 12 min. The plant growth parameters for this variant were the highest of the supplemented media, but also significantly higher than the results obtained for conventional medium (Medium Z). Thus, ozonation prior to basic biomass cultivation of aquatic plants leads not only to more effective wastewater purification but also to higher yields of biomass.
ACKNOWLEDGMENTS
The authors are grateful for the support of the National Centre for Research and Development Grant No. BIOSTRATEG 2/296369/5/NCBR/2016.
REFERENCES CITED
Altaf, M., Venkateshwar, M., Srijana, M., and Reddy, G. (2007). “An economic approach for l-(+) lactic acid fermentation by Lactobacillus amylophilus GV6 using inexpensive carbon and nitrogen sources,” J. Appl. Microbiol. 103, 372-380. DOI: 10.1111/j.1365-2672.2006.03254.x
Appenroth, K.-J., and Adamec, L. (2014). “Specific turion yields of different clones of Spirodela polyrhiza depend on external phosphate thresholds,” Pant. Biotech. 17, 125-129. DOI: 10.1111/plb.12154
Appenroth, K.-J. (2002). “Co-action of temperature and phosphate in inducing turion formation in Spirodela polyrhiza (Great duckweed),” Plant. Cell Environ. 25, 1079-1085. DOI: 10.1046/j.1365-3040.2002.00885.x
Appenroth, K.-J., Sree, K. S., Böhm, V., Hammann, S., Vetter, W., Leiterer, M., and Jahreis, G. (2017). “Nutritional value of duckweeds (Lemnaceae) as human food,” Food Chem. 217, 266-273. DOI: 10.1016/j.foodchem.2016.08.116
Ashauer, R. (2016). “Post-ozonation in a municipal wastewater treatment plant improves water quality in the receiving stream,” Environ. Sci. Eur. 28(1), article 1. DOI: 10.1186/s12302-015-0068-z
Basiglini, E., Pintore, M., and Forni, C. (2018). “Effects of treated industrial wastewaters and temperatures on growth and enzymatic activities of duckweed (Lemna minor L.),” Ecotoxicol. Environ. Saf. 153, 54-59. DOI:10.1016/j.ecoenv.2018.01.053
Berlowska, J., Binczarski, M., Dziugan, P., Wilkowska, A., Kregiel, D., and Witonska, I. (2018). “Sugar beet pulp as a source of valuable biotechnological products,” in: Advances in Biotechnology for Food Industry, A. M. Holban and A. M. Grumezescu (eds.), Academic Press, USA, pp 359-392. DOI:10.1016/B978-0-12-811443-8.00013-X
Berlowska, J., Cieciura-Włoch, W., Kalinowska, H., Kregiel, D., Borowski, S., Pawlikowska, E., Binczarski, M., and Witonska, I. (2018). “Enzymatic conversion of sugar beet pulp: A comparison of simultaneous saccharification and fermentation and separate hydrolysis and fermentation for lactic acid production,” Food Technol. Biotechnol. 56(2), 188-196. DOI:10.17113/ftb.56.02.18.5390
Berlowska, J., Pielech-Przybylska, K., Balcerek, M., Cieciura, W., Borowski, S., and Kregiel, D. (2017). “Integrated bioethanol fermentation/anaerobic digestion for valorization of sugar beet pulp,” Energies. 10(9), article 1255. DOI: 10.3390/en10091255
Berłowska, J., Pielech-Przybylska, K., Balcerek, M., Dziekońska-Kubczak, U., Patelski, P., Dziugan, P., and Krȩgiel, D. (2016). “Simultaneous saccharification and fermentation of sugar beet pulp for efficient bioethanol production,” Biomed. Res. Int. DOI:10.1155/2016/3154929
Bijan, L., and Mohseni, M. (2005). “Integrated ozone and biotreatment of pulp mill effluent and changes in biodegradability and molecular weight distribution of organic compounds,” Water Res. 39(16), 3763-3772. DOI:10.1016/j.watres.2005.07.018
Bokhari, S. H., Ahmad, I., Mahmood-Ul-Hassan, M., and Mohammad, A. (2016). “Phytoremediation potential of Lemna minor L. for heavy metals,” Int. J. Phytoremediation. 18, 25-32. DOI:10.1080/15226514.2015.1058331
Bourgin, M., Beck, B., Boehler, M., Borowska, E., Fleiner, J., Salhi, E., Teichler, R., von Gunten, U., Siegrist, H., and McArdell, C. S. (2018). “Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products,” Water Res. 129, 486-498. DOI:10.1016/j.watres.2017.10.036
Bourioug, M., Mazzitelli, J. Y., Marty, P., Budzinski, H., Aleya, L., Bonnafé, E., and Geret, F. (2018). “Assessment of Lemna minor (duckweed) and Corbicula fluminea (freshwater clam) as potential indicators of contaminated aquatic ecosystems: Responses to presence of psychoactive drug mixtures,” Environ. Sci. Pollut. Res. 25, 11192-11204. DOI: 10.1007/s11356-017-8447-1
Brodowska, A. J., Nowak, A., Kondratiuk-Janyska, A., Piątkowski, M., and Śmigielski, K. (2017). “Modelling the ozone-based treatments for inactivation of microorganisms,” Int. J. Environ. Res. Public Health. 14, article 1196. DOI: 10.3390/ijerph14101196
Brodowska, A. J., Nowak, A., and Śmigielski, K. (2018). “Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview,” Crit. Rev. Food Sci. Nutr. 58, 2176-2201. DOI:10.1080/10408398.2017.1308313
Calicioglu, O., Shreve, M. J., Richard, T. L., and Brennan, R. A. (2018). “Effect of pH and temperature on microbial community structure and carboxylic acid yield during the acidogenic digestion of duckweed,” Biotechnol. Biofuels. 11, 275. DOI:10.1186/s13068-018-1278-6
Carballa, M., Manterola, G., Larrea, L., Ternes, T., Omil, F., and Lema, J. M. (2007). “Influence of ozone pre-treatment on sludge anaerobic digestion: Removal of pharmaceutical and personal care products,” Chemosphere 67 (7), 1444-1452. DOI:10.1016/j.chemosphere.2006.10.004
Chakrabarti, R., Clark, W. D., Sharma, J. G., Goswami, R. K., Shrivastav, A. K., and Tocher, D. R. (2018). “Mass production of Lemna minor and its amino acid and fatty acid profiles,” Front. Chem. 6, 479. DOI:10.3389/fchem.2018.00479
Chaudhuri, D., Majumder, A., Misra, A. K., and Bandyopadhyay, K. (2014). “Cadmium removal by Lemna minor and Spirodela polyrhiza,” Int. J. Phytoremediation. 16, 1119-1132. DOI:10.1080/15226514.2013.821446
Cheng, J. J., and Stomp, A. M. (2009). “Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed,” Clean – Soil, Air, Water 37(1), 17-26. DOI:10.1002/clen.200800210
Cherubini, F., and Strømman, A. H. (2011). “Chemicals from lignocellulosic biomass: opportunities, perspectives, and potential of biorefinery systems,” Biofuels, Bioprod. Biorefining. 5, 548-561. DOI:10.1002/bbb.297
Chiavola, A., Salvati, C., Bongirolami, S., Di Marcantonio, C., and Boni, M. R. (2021). “Techno-economic evaluation of ozone-oxidation for sludge reduction at the full-scale. Comparison between the application to the return activated sludge (RAS) and the sludge digestion unit,” J. Water Process Eng. 42, article 102114. DOI: 10.1016/j.jwpe.2021.102114
Cui, W., and Cheng, J. J. (2015). “Growing duckweed for biofuel production: A review,” Plant Biol. 17(1), 16-23. DOI:10.1111/plb.12216
Dȩbowski, M., Kisielewska, M., Kazimierowicz, J., Rudnicka, A., Dudek, M., Romanowska-Duda, Z., and Zielínski, M. (2020). “The effects of microalgae biomass co-substrate on biogas production from the common agricultural biogas plants feedstock,” Energies. 13, article 2186. DOI: 10.3390/en13092186
Dȩbowski, M., Rusanowska, P., Zieliński, M., Dudek, M., and Romanowska-Duda, Z. (2018). “Biomass production and nutrient removal by chlorella vulgaris from anaerobic digestion effluents,” Energies. 11, article 1654. DOI: 10.3390/en11071654
Dziugan, P., Balcerek, M., Binczarski, M. J., Kregiel, D., Kucner, M., Kunicka-Styczynska, A., Pielech-Przybylska, K., Smigielski, K., and Witonska, I. A. (2016). “Ozonation as an effective way to stabilize new kinds of fermentation media used in biotechnological production of liquid fuel additives,” Biotechnol. Biofuels 9, 150. DOI:10.1186/s13068-016-0574-2
Gaur, R. Z., and Suthar, S. (2017). “Nutrient scaling of duckweed (Spirodela polyrhiza) biomass in urban wastewater and its utility in anaerobic co-digestion,” Process Saf. Environ. Prot. 107, 138-146. DOI:10.1016/j.psep.2017.02.005
Ge, X., Zhang, N., Phillips, G. C., and Xu, J. (2012). “Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol,” Bioresour. Technol. 124, 485-488. DOI:10.1016/j.biortech.2012.08.050
Grzesik, M., and Romanowska-Duda, Z. (2014). “Improvements in germination, growth, and metabolic activity of corn seedlings by grain conditioning and root application with Cyanobacteria and microalgae,” Polish J. Environ. Stud. 23(4), 1147-1153
Grzesik, M., Romanowska-Duda, Z., and Kalaji, H. M. (2017). “Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application,” Photosynthetica 55(3), 510-521. DOI:10.1007/s11099-017-0716-1
Gunnarsson, L., Adolfsson-Erici, M., Björlenius, B., Rutgersson, C., Förlin, L., and Larsson, D. G. J. (2009). “Comparison of six different sewage treatment processes-Reduction of estrogenic substances and effects on gene expression in exposed male fish,” Sci. Total Environ. 407(19), 5235-5242. DOI:10.1016/j.scitotenv.2009.06.018
Hadavifar, M., Younesi, H., Zinatizadeh, A. A., Mahdad, F., Lin, Q., and Ghasemi Z. (2016). “Application of integrated ozone and granular activated carbon for decolorization and chemical oxygen demand reduction of vinasse from alcohol distilleries,” J. Environ. Manage. 170, 28-36. DOI: 10.1016/j.jenvman.2016.01.009
Iatrou, E. I., Stasinakis, A. S., and Aloupi, M. (2015). “Cultivating duckweed Lemna minor in urine and treated domestic wastewater for simultaneous biomass production and removal of nutrients and antimicrobials,” Ecol. Eng. 84, 632-639. DOI:10.1016/j.ecoleng.2015.09.071
Kanoun-Boulé, M., Vicente, J. A. F., Nabais, C., Prasad, M. N. V., and Freitas, H. (2009). “Ecophysiological tolerance of duckweeds exposed to copper,” Aquat. Toxicol. 91(1), 1-9. DOI:10.1016/j.aquatox.2008.09.009
Kazemi Shariat Panahi, H., Dekkaghi, M., Aghbashlo, M., Karimi, K., and Tabatabaei, M. (2020). “Conversion of residues from agro-food industry into bioethanol in Iran: An under-valued biofuel additive to phase out MTBE in gasoline,” Renew. Energ. 145, 699-710. DOI: 10.1016/j.renene.2019.06.081
Kisielewska, M., Zielinski, M., Debowski, M., Kazimierowicz, J., Romanowska-Duda, Z., and Dudek, M. (2020). “Effectiveness of Scenedesmus sp. biomass grow and nutrients removal from liquid phase of digestates,” Energies 13(6), 1432. DOI: 10.3390/en13061432
Ma, Y. B., Zhu, M., Yu, C. J., Wang, Y., Liu, Y., Li, M. L., Sun, Y. D., Zhao, J. S., and Zhou, G. K. (2018). “Large-scale screening and characterisation of Lemna aequinoctialis and Spirodela polyrhiza strains for starch production,” Plant Biol. 20(2), 357-364. DOI:10.1111/plb.12679
Mainardis, M., Buttazzoni, M., De Bortoli, N., Mion M., and Goi, D. (2020). “Evaluation of ozonation applicability to pulp and paper streams for a sustainable wastewater treatment,” J. Clean. Prod. 258, article 120781. DOI: 10.1016/j.jclepro.2020.120781
Metsalu, T., and Vilo, J. (2015). “ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap,” Nucleic Acids Res. 43, W566-W570. DOI:10.1093/nar/gkv468
Miranda, A. F., Biswas, B., Ramkumar, N., Singh, R., Kumar, J., James, A., Roddick, F., Lal, B., Subudhi, S., Bhaskar, T., and Mouradov, A. (2016). “Aquatic plant Azolla as the universal feedstock for biofuel production,” Biotechnol. Biofuels. 9, 221. DOI:10.1186/s13068-016-0628-5
Muradov, N., Taha, M., Miranda, A. F., Kadali, K., Gujar, A., Rochfort, S., Stevenson, T., Ball, A. S., and Mouradov, A. (2014). “Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production,” Biotechnol. Biofuels 7, 30. DOI: 10.1186/1754-6834-7-30
Nguyen, Q. A., Yang, J., and Bae, H. J. (2017). “Bioethanol production from individual and mixed agricultural biomass residues,” Ind. Crops Prod. 95, 718-725. DOI:10.1016/j.indcrop.2016.11.040
Nichols, W. (2013). “Vivergo opens UK’s largest biorefinery plant in Hull as biofuel debate heats up,” [WWW Document].
Njambuya, J., Stiers, I., and Triest, L. (2011). “Competition between Lemna minuta and Lemna minor at different nutrient concentrations,” Aquat. Bot. 94 (4), 158-164. DOI:10.1016/j.aquabot.2011.02.001
Oecd. (2006). “Guidelines for the Testing of Chemicals: 221 – Lemna sp. Growth inhibition test,” OECD iLibrary.
Olofsson, K., Bertilsson, M., and Lidén, G. (2008). “A short review on SSF – An interesting process option for ethanol production from lignocellulosic feedstocks,” Biotechnol. Biofuels. 1, 7. DOI:10.1186/1754-6834-1-7
Pádrová, K., Lukavský, J., Nedbalová, L., Čejková, A., Cajthaml, T., Sigler, K., Vítová, M., and Řezanka, T. (2015). “Trace concentrations of iron nanoparticles cause overproduction of biomass and lipids during cultivation of cyanobacteria and microalgae,” J. Appl. Phycol. 27, 1443-1451. DOI:10.1007/s10811-014-0477-1
Paolacci, S., Harrison, S., and Jansen, M. A. K. (2016). “A comparative study of the nutrient responses of the invasive duckweed Lemna minuta, and the native, co-generic species Lemna minor,” Aquat. Bot. 134, 47-53. DOI:10.1016/j.aquabot.2016.07.004
Pena, L., Oliveira, M., Fragoso, R., and Duarte, E. (2017). “Potential of duckweed for swine wastewater nutrient removal and biomass valorisation through anaerobic co-digestion,” J. Sustain. Dev. Energy, Water Environ. Syst. 5 (2), 127-138. DOI:10.13044/j.sdewes.d5.0137
Rabaçal, M., Ferreira, A. F., Silva, C. A. M., and Editors, M. C. (2017). “Biorefineries, Lecture Notes in Energy, Lecture Notes in Energy,” Springer International Publishing, Cham. DOI: 10.1007/978-3-319-48288-0
Radić, S., Stipaničev, D., Cvjetko, P., Marijanović Rajčić, M., Širac, S., Pevalek-Kozlina, B., and Pavlica, M. (2011). “Duckweed Lemna minor as a tool for testing toxicity and genotoxicity of surface waters,” Ecotoxicol. Environ. Saf. 74 (2), 182-187. DOI:10.1016/j.ecoenv.2010.06.011
Radić, S., Stipaničev, D., Cvjetko, P., Mikelić, I. L., Rajčić, M. M., Širac, S., Pevalek-Kozlina, B., and Pavlica, M. (2010). “Ecotoxicological assessment of industrial effluent using duckweed (Lemna minor L.) as a test organism,” Ecotoxicology. 19, 216-222. DOI: 10.1007/s10646-009-0408-0
Remondino, M., and Valdenassi, L. (2018). “Different uses of ozone: Environmental and corporate sustainability. Literature review and case study,” Sustain. 10, 1-18. DOI: 10.3390/su10124783
Romanowska-Duda, Z., Grzesik, M., and Janas, R. (2019). “Maximal efficiency of PSII as a marker of sorghum development fertilized with waste from a biomass biodigestion to methane,” Front. Plant Sci. 9, article 1920. DOI:10.3389/fpls.2018.01920
Romanowska-Duda, Z., Piotrowski, K., and Dziugan, P. (2018). “Utilization of waste from methane fermentation in Lemnaceae plant breeding intended for energy purposes,” in: Renewable Energy Sources: Engineering, Technology, Innovation, K. Mudryk and S. Werle (eds.), Springer Proceedings in Energy, Springer, Cham. DOI:10.1007/978-3-319-72371-6_26
Rosal, R., Rodríguez, A., Perdigón-Melón, J. A., Petre, A., García-Calvo, E., Gómez, M.J., Agüera, A., and Fernández-Alba, A. R. (2010). “Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation,” Water Res. 44(2), 578-588. DOI:10.1016/j.watres.2009.07.004
Rosen, W. (2015). “DuPont Celebrates the Opening of the World’s Largest Cellulosic Ethanol Plant” [WWW Document].
Schindler Wildhaber, Y., Mestankova, H., Schärer, M., Schirmer, K., Salhi, E., and von Gunten, U. (2015). “Novel test procedure to evaluate the treatability of wastewater with ozone,” Water Res. 75, 324-335. DOI:10.1016/j.watres.2015.02.030
Siddiqui, M. S., Amy, G. L., and Murphy, B. D. (1997). “Ozone enhanced removal of natural organic matter from drinking water sources,” Water Res. 31, 3098-3106. DOI:10.1016/S0043-1354(97)00130-9
Su, H., Zhao, Y., Jiang, J., Lu, Q., Li, Q., Luo, Y., Zhao, H., and Wang, M. (2014). “Use of duckweed (Landoltia punctata) as a fermentation substrate for the production of higher alcohols as biofuels,” Energy and Fuels. 28(5), 3206-3216. DOI:10.1021/ef500335h
Szufa, S., Piersa, P., Adrian, Ł., Sielski, J., Grzesik, M., Romanowska-Duda, Z., Piotrowski, K., and Lewandowska, W. (2020). Acquisition of torrefied biomass from Jerusalem artichoke grown in a closed circular system using biogas plant waste,” Molecules 25, article 3862. DOI:10.3390/molecules25173862
Tang, J., Li, Y., Wang, X., and Daroch, M. (2017). “Effective adsorption of aqueous Pb2+ by dried biomass of Landoltia punctata and Spirodela polyrhiza,” J. Clean. Prod. 145, 25-34. DOI:10.1016/j.jclepro.2017.01.038
Tomaszewska, J., Bieliński, D., Binczarski, M., Berlowska, J., Dziugan, P., Piotrowski, J., Stanishevsky, A., and Witońska, I. A. (2018). “Products of sugar beet processing as raw materials for chemicals and biodegradable polymers,” RSC Adv. 8, article 3161. DOI:10.1039/c7ra12782k
Toyama, T., Hanaoka, T., Tanaka, Y., Morikawa, M., and Mori, K. (2018). “Comprehensive evaluation of nitrogen removal rate and biomass, ethanol, and methane production yields by combination of four major duckweeds and three types of wastewater effluent,” Bioresour. Technol. 250, 464-473. DOI:10.1016/j.biortech.2017.11.054
Xu, Y., Ma, S., Huang, M., Peng, M., Bog, M., Sree, K. S., Appenroth, K. -J., and Zhang, J. (2015). “Species distribution, genetic diversity and barcoding in the duckweed family (Lemnaceae),” Hydrobiologia 743, 75-87. DOI:10.1007/s10750-014-2014-2
Xu, Y. P., Duan, P. G., Wang, F., and Guan, Q. Q. (2018). “Liquid fuel generation from algal biomass via a two-step process: Effect of feedstocks,” Biotechnol. Biofuels. 11, 83. DOI: 10.1186/s13068-018-1083-2
Zaman, A.U. (2015). “A comprehensive review of the development of zero waste management: lessons learned and guidelines,” J. Clean. Prod. 91, 12-25. DOI:10.1016/j.jclepro.2014.12.013
Zanacic, E., Stavrinides, J., and McMartin, D. W. (2016). “Field-analysis of potable water quality and ozone efficiency in ozone-assisted biological filtration systems for surface water treatment,” Water. Res. 104, 397-407. DOI: 10.1016/j.watres.2016.08.043
Zhao, Y., Fang, Y., Jin, Y., Huang, J., Bao, S., Fu, T., He, Z., Wang, F., Wang, M., and Zhao, H. (2015). “Pilot-scale comparison of four duckweed strains from different genera for potential application in nutrient recovery from wastewater and valuable biomass production,” Plant Biol. 17(1), 82-90. DOI:10.1111/plb.12204
Zhao, Z., Shi, H., Liu, Y., Zhao, H., Su, H., Wang, M., and Zhao, Y. (2014). “The influence of duckweed species diversity on biomass productivity and nutrient removal efficiency in swine wastewater,” Bioresour. Technol. 167, 383-389. DOI:10.1016/j.biortech.2014.06.031
Ziemiński, K., and Kowalska-Wentel, M. (2017). “Effect of different sugar beet pulp pretreatments on biogas production efficiency,” Appl. Biochem. Biotechnol. 181, 1211-1227. DOI:10.1007/s12010-016-2279-1
Zimmermann, S. G., Wittenwiler, M., Hollender, J., Krauss, M., Ort, C., Siegrist, H., and von Gunten, U. (2011). “Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment: Micropollutant oxidation, by-product formation and disinfection,” Water Res. 45, 605-617. DOI:10.1016/j.watres.2010.07.080
Zonin, V. J., Antunes, J. A. V., and Pinto L. R. (2014). “Multicriteria analysis of agricultural raw materials: A case study of BSBIOS and PETROBRAS BIOFUELS in Brazil,” Energy Policy 67, 255-263. DOI:10.1016/j.enpol.2013.12.029
Article submitted: June 30, 2022; Peer review completed: August 21, 2022; Revised version received: October 26, 2022; Accepted: October 31, 2022; Published: November 11, 2022.
DOI: 10.15376/biores.18.1.317-336
