Abstract
The wet strength of paper is an important property in its various applications. In this paper, chitosan was employed as an additive to improve the wet strength of paper using a dipping process, and maleic anhydride (MA) was used to improve the retention of chitosan. The underlying mechanism was the bridging effect of MA, via the formation of esters between MA and cellulose and amides between MA and chitosan, both of which were supported by the FT-IR results. The temporary and permanent wet strengths of the paper increased significantly, and the key parameters were 1) the concentration of MA and chitosan, 2) dipping duration, and 3) curing temperature. The temporary and permanent wet strengths reached 31.6% and 29.7%, respectively, at a concentration of treating solution of 1%, dipping time of 12 h, and curing temperature of 90 °C. At a curing temperature of 170 °C under otherwise the same conditions, both the temporary and permanent wet strengths were higher than 50%.
Download PDF
Full Article
In situ Grafting of Chitosan onto Cellulosic Fibers Using Maleic Anhydride for Paper Wet Strength Improvement
Zicheng Chen,a,b Zhibin He,b Lanhe Zhang,a,* and Yonghao Ni b,*
The wet strength of paper is an important property in its various applications. In this paper, chitosan was employed as an additive to improve the wet strength of paper using a dipping process, and maleic anhydride (MA) was used to improve the retention of chitosan. The underlying mechanism was the bridging effect of MA, via the formation of esters between MA and cellulose and amides between MA and chitosan, both of which were supported by the FT-IR results. The temporary and permanent wet strengths of the paper increased significantly, and the key parameters were 1) the concentration of MA and chitosan, 2) dipping duration, and 3) curing temperature. The temporary and permanent wet strengths reached 31.6% and 29.7%, respectively, at a concentration of treating solution of 1%, dipping time of 12 h, and curing temperature of 90 °C. At a curing temperature of 170 °C under otherwise the same conditions, both the temporary and permanent wet strengths were higher than 50%.
Keywords: Wet strength; Cellulosic fibers; Chitosan; Maleic anhydride; Ester formation; Amide formation.
Contact information: a: School of Chemical Engineering, Northeast Electric Power University, Jilin, Jilin Province 132012, P. R. China; b: Department of Chemical Engineering, University of New Brunswick, Fredericton, NB E3B 5A3, Canada; *Corresponding author: zhanglanhe@163.com; yonghao@unb.ca
INTRODUCTION
Lignocellulosic feedstocks from natural renewable resources are used for the production of sustainable bio-based products, such as paper and packaging products. The conversion of these renewable materials into various products for diversified applications fits well with the green economy concept (Liu 2010; Luo et al. 2010; Shen et al. 2013; Hubbe et al. 2015; Chen et al. 2016; Villaverde et al. 2016). In many cases, the strength properties of paper products, such as the wet strength, are critical for meeting customer demands (Shen et al. 2014; Liu et al. 2016; Fan et al. 2017).
Chitosan and its derivatives also are natural polymers, and they can be used as naturally sourced wet and dry strength additives for paper products, due to the similar chemistry structure of cellulose, as well as abundant hydroxyl and active primary amino groups in chitosan molecules. These groups ease the formation of hydrogen bonds with hydroxyl group in cellulosic fibers of pulp (Lindström et al. 2005). Their use within various paper products has been reported (Liu and Kim 2012; Chen et al. 2013). However, natural chitosan as a wet strength additive is not as effective as polyamideamine epichlorohydrin (PAE) resin, which is a dominant commercial wet strength agent in the papermaking industry (Niekraszewicz et al. 2001; Yuan et al. 2016).
As a flocculation agent, chitosan and its derivatives have been investigated for applications in wastewater treatment and other related industries (Mourya and Inamdar 2008; Wang et al. 2008; Saeed et al. 2011). In addition, they have uses within various papermaking processes owing to their attractive properties, such as antibacterial behavior (Nada et al. 2006; Qian et al. 2008; Aranaz et al. 2009; Sun et al. 2010; Vallapa et al. 2011). In the last few decades, much effort has been made to develop paper wet strength additives that are environmentally friendly as well as free of formaldehyde and absorbable organic chlorides (AOX) (Zakaria 2004; Saito and Isogai 2006; Hamzeh et al. 2013). For instance, MA-acylated chitosan can serve as both a wet strength and antimicrobial agent for paper products. In the authors’ previous work, a two-step process was developed to use chitosan to increase the wet strength of paper sheet: 1) in the first step, maleic anhydride-acylated chitosan (MAAC) was prepared, 2) then MAAC was added to pulp stock (Chen et al. 2013). The effects of process parameters, such as treatment pH, dosage of the chemicals, and curing temperature were investigated. The results demonstrated that such a process could be an alternative to using PAE, achieving 80% wet strength of that of PAE at the same dosages and similar conditions. In another paper, a dipping treatment process of the prepared MAAC was used to improve the wet strength of paper while providing antimicrobial activity to paper (against the growth of Escherichia coli (E. coli)), the results were very encouraging. In fact, a better antibacterial property than chitosan was observed (Chen et al. 2014).
Different from the use of the prepared maleic anhydride-acylated chitosan (MAAC), either added to the pulp stock (Chen et al. 2013), or impregnated into paper by dipping (Chen et al. 2014), the present study presents another way of using chitosan for improving the paper wet strength by grafting chitosan onto the cellulosic fibers using maleic anhydride (MA) as the grafting agent (bridging effect), and its effect on the overall wet strength properties were investigated. Notably the present approach omitted the MAAC preparation.
It should be noted that maleic anhydride has been used in the paper industry for many years to reinforce rosin sizing, the so-called reinforced rosin sizing technology. In this case, maleic anhydride will not be found in the final paper product, due to the fact that it is readily hydrolyzed in the paper making conditions.
In the present study, the experimental procedure consisted of two sequential steps: 1) the paper samples were dipped in an acetone solution containing MA; and 2), the MA containing paper samples were dipped into an acetic acid solution containing chitosan. The grafting mechanisms of chitosan onto cellulosic fibers were studied via Fourier transform infrared spectroscopy (FT-IR). The effects of process parameters such as the MA and chitosan concentrations, dipping durations, and curing temperature on the wet strength of the treated paper samples were also examined.
EXPERIMENTAL
Materials
Chitosan (with 86% degree of deacetylation) of about 600 kDa was purchased from Shandong Laizhou Highly Bio-Products (Laizhou, China). The filter paper (600 × 600 mm, with a basis weight of 80 g/m2) was provided by Fushun City Civil Affairs Filter Paper Factory (Fushun, China). MA was purchased from BASF Chemistry Industry Co., Ltd. (Tianjin, China). Other chemicals used in this study, such as acetone and acetic acid, were of analytical grade, and were used as received.
Dipping Treatment of Cellulosic Paper
The experimental procedures are shown in Fig. 1. Filter paper was conditioned in an atmosphere of 50.0% ± 2.0% RH and 23.0 ± 1.0 °C for 24 h, and then it was cut into strips of 200 mm (length) × 15 mm (width). The strips were dipped in MA solutions (dissolved in acetone) with various concentrations for a prescribed duration, then air dried at 25 °C. Subsequently, these paper strips were dipped into a 2% (v/v) acetic acid aqueous solution containing chitosan for a prescribed duration. After dipping, to remove excess chitosan solution, the paper strips were placed between two pieces of blotting paper on a flat rigid surface, and a hand roller was moved twice over the pad using only the pressure exerted by the weight of the roller. Finally, the impregnated strips were dried in air and cured in a speed dryer (SD24D, Labtech Instruments Inc., Canada) at prescribed temperatures for 10 min.
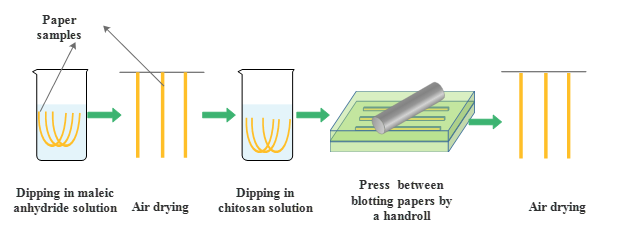
Fig. 1. Experimental procedure of the dipping treatment to graft chitosan onto cellulosic fibers
The weight gain for the paper samples, which were cut into circles with an area of 0.01 m2 prior to the above dipping treatment, was calculated according to Eq. 1,
(1)
where WG was the weight gain of the samples (%), and m and Mwere the weights (o.d.) of the paper samples before and after the dipping treatment, respectively.
Evaluation of Dry Tensile Strength and Wet strength
The dry tensile index of the paper samples was determined according to the TAPPI T494 om (2006) method. The wet tensile strengths of the paper samples were evaluated in accordance with TAPPI T456 om-3 (2005). The paper strip samples for the wet tensile strength measurement were dipped in distilled water for 1 min for the temporary wet strength testing, or 1 h for the permanent wet strength testing, and immediately subjected to tensile strength measurement after the excess water had been lightly wiped off the samples using a piece of blotting paper. The wet strength (W/D ratio) was defined as the ratio of the wet tensile strength of treated paper samples to the dry tensile strength of the control sample. Nine strips were measured for each experiment, and the average wet strength was reported.
FT-IR Analyses
The selected paper samples were analyzed by Fourier transform infrared spectroscopy (FT-IR, Magna-IR 560 E.S.P, Nicolet Corp., Waltham, MA, USA) to investigate if changes in chemical bonds occurred after the dipping treatment.
RESULTS AND DISCUSSION
Grafting of Chitosan onto Cellulosic Fibers
As shown in Table 1, the weight gains (o.d.) of the paper samples increased after they were dipped into solutions containing chitosan, indicating the successful retention of chitosan. Furthermore, the weight gain was increased by increasing the duration of the dipping; however, when the duration exceeded 12 h, the weight gain appeared to reach a plateau. Interestingly, the results demonstrated that the weight gain for the case of MA + chitosan was greater than the sum of the weight gain for the MA or chitosan treatments alone. This indicated that the chemical reactions of MA via grafting increased the retention of chitosan in the paper samples. The weight gain in Table 1 is attributed to the retention of both the grafted chitosan (MA as the grafting agent, shown in Fig 1), and physically- trapped chitosan (unreacted chitosan). These results were similar to what Ranjbar-Mohammadi et al. (2010) found concerning the grafting of chitosan onto wool fibers, based on the acylation mechanism using butanedioic anhydride.
Fig. 2. FT-IR spectra of paper samples: (a) control paper samples; (b) paper samples dipped in 1% (w/w) MA dissolved in acetone for 8 h and dried in air; (c) paper samples dipped in 1% (w/w) chitosan and 2% (v/v) acetic acid and dried in air; (d) paper samples dipped in 1% (w/w) MA in acetone for 8 h, dried in air, then dipped in 1% (w/w) chitosan in 2% (v/v) acetic acid for an additional 8 h, dried in air, and followed by curing for 10 min at 170 °C
Table 1. Weight Gains of Paper Treated by Dipping into Chitosan Containing and MA Containing Solutions
Note: MA: 1% (w/w) MA in acetone; Chitosan: 1% (w/w) chitosan in 2% (v/v) acetic acid; MA + Chitosan: paper samples were dipped first in 1% (w/w) MA in acetone, dried in air, then dipped into 1% (w/w) chitosan in 2% (v/v) acetic acid for an equal time. The weight gain results were carried out three times and the relative standard deviation was acceptable ≤ 5%
FT-IR analyses were conducted in the present study to support the hypothesis that under the conditions studied MA is the bridging chemical connecting chitosan with cellulose fibers. In this case, first, MA reacts with cellulose to form new ester functions, which subsequently reacts with chitosan, resulting in the formation of amide functions. The formation of both esters and amides are supported by the FT-IR results before and after the treatment. As shown in Fig. 2, the FT-IR spectra of the cellulosic fibers were characterized by the strong bands occurring at 3495 cm−1 (O–H stretching vibrations) and at 2900 cm−1 (C–H stretching vibrations). Also evident were the bands at 1650 cm−1, corresponding to the C=O vibrational stretching of the aldehyde or carboxyl groups in cellulose molecules, those at 1730 cm−1, corresponding to the C=O vibrational stretching of ester bonds in parts b and d, and those at 1580 cm−1, corresponding to the N−H vibrational stretching, indicating the presence of amide functions.
Fig. 3. Schematic for grafting of chitosan onto cellulosic fibers
According to the results presented in Table 1, as well as the FT-IR results, the grafting mechanism for the grafting of chitosan onto cellulosic fibers via MA was well supported. The process consisted of two steps, as shown in Fig. 3.
Wet Strength of Paper after Dipping Treatment
In this study, various concentrations of MA solutions were used for the dipping treatment process. As shown in Fig. 4, the temporary wet strength (when immersed in water for 1 min) and the permanent wet strength (when immersed in water for 1 h) of the treated paper samples increased when the concentration of MA was increased from 1% to 2%. In accordance with those in Table 1, the weight gains of the paper samples supported the conclusion that the grafting of chitosan onto the cellulosic fibers via MA grafting was successful. These grafting reactions resulted in the formation of esters between MA and cellulose, and amides between MA and chitosan. Due to these newly formed chemical bonds, the wet strength of the paper samples increased. However, when the concentration of MA increased to 3 to 4%, the wet strength of the paper samples decreased compared to that of an MA concentration of 1 to 2%. This could be attributed to the acid degradation in the amorphous regions of pulp fibers in the paper samples resulting in irreversible supramolecular changes during paper samples drying (Kontturi and Vuorinen 2009).
Fig. 4. Concentration of MA and wet strength of treated paper samples: the paper samples were treated with MA dissolved in acetone solution for 12 h, dried in air, then treated with 1% chitosan in 2% acetic acid solution for an equal time, air dried, and cured at 90 °C for 10 min
The wet strength of the paper samples increased remarkably with the increase in the chitosan concentration in solution (Fig. 5). At a 1% chitosan concentration, the temporary wet strength was 31.6%, while the permanent wet strength was 29.7%. However, the chitosan solutions with chitosan concentrations exceeding 1% became increasingly viscous, causing difficulties in carrying out the experiments for the dipping process. For practical reasons, it was concluded that the concentration of chitosan solutions should not exceed 1%, because the practical operation/handling may become an issue at much higher chitosan concentrations.
Fig. 5. Effect of chitosan solution concentration on the wet strength of treated paper samples: paper samples were treated with 1% MA dissolved in acetone solution for 12 h, dried in air, then treated with a 2% acetic acid solution containing chitosan for an equal time, air dried, and cured at 90 °C for 10 min
Figure 6 shows the effects of curing temperature, which played an important role in the improvement of wet strength for the treated paper samples. For example, at a more practical curing temperature, e.g. 90 °C, a significant increase in the wet strength was evident. At 170 °C curing temperature, the wet strength was higher than 50%. A likely explanation is that the acylation and amidation reactions of the cellulosic fibers require higher temperatures, as proposed in the literature (Zakaria 2004). In practice, 170 °C is not a conventional temperature in the drying section of a paper machine. However, the results in this work provided an approach to achieve wet strength of paper higher than 50% in comparison with our previous work (Chenet al. 2013, 2014), these earlier studies showed that the wet strengths of paper were less than 34% with a two-step process (i.e., the preparation of maleic anhydride-acylated chitosan (MAAC) first, followed by its addition to the pulp stock). Certainly a very high temperature is also harmful to the physical properties of paper sheet.
Mosier et al investigated dicarboxylic acids for cellulose hydrolysis, and the results showed that dilute maleic acid had been shown to hydrolyze cellulose as efficiently as dilute sulfuric acid (Mosier et al. 2001). In the present work, it was inevitable that there was some residual maleic acid in paper samples due to the hydrolysis of maleic anhydride. Included in Fig. 6 are the results on the dry tensile index. A maximum was observed at about 130 °C. A decrease in the dry tensile may be related to the thermal/acid degradation of cellulose, which was reported in the literature (Kontturi and Vuorinen 2009).
Fig. 6. Effect of curing temperature on the wet strength and dry tensile index of treated paper samples: paper samples were treated with 1% MA dissolved in acetone solution for 12 h, dried in air, then treated with 1% chitosan in 2% acetic acid solution for an equal time, air dried, and cured at prescribed temperature for 10 min
As shown in Fig. 7, the paper samples achieved higher wet strength with a longer duration of dipping treatment, correlating to more weight gains, as shown in Table 1. By increasing the dipping time, more MA and chitosan were absorbed onto the paper samples, so that more covalent bonds were formed, thus resulting in the increased wet strength. However, in reality, one would have to consider the adsorption limit of the paper samples; also, a long dipping time might not be practical because it would slow down the production process.
Fig. 7. Effect of dipping treatment duration on the wet strength of treated paper samples. Paper samples were treated with 1% MA dissolved in acetone solution for prescribed duration, dried in air, then treated with 1% chitosan in 2% acetic acid solution for an equal time, air dried, and cured at 90 °C for 10 min.
CONCLUSIONS
- Maleic anhydride (MA) was used for the grafting of chitosan onto cellulosic fibers. This was attributed to the formation of esters between MA and fibers, as well as the formation of amides between chitosan and MA, both of which were validated based on FT-IR results.
- The chitosan grafting led to increases in both the temporary and permanent wet strength properties.
- Increasing the concentration of dipping treatment solutions, curing temperature, and dipping duration led to increases in the wet strength of the treated paper samples.
- Under the conditions of 1% MA and chitosan concentration, 12 h dipping duration, and 90 °C curing temperature, the temporary and permanent wet strengths of the treated paper were 31.6% and 29.7%, respectively. At a higher curing temperature under the same conditions, both the temporary and permanent wet strengths achieved further increased.
ACKNOWLEDGMENTS
This project was supported by the Science and Research Programs of Education Department of Jilin Provence of P. R. C. (No. 201684) and the Canada Research Chairs program of the Government of Canada (CRC 950213263). The authors are also very grateful to the support of China Scholarship Council (201707790004).
REFERENCES CITED
Aranaz, I., Mengíbar, M., Harris, R., Paños, I., Miralles, B., Acosta, N., Galed, G., and Heras, A. (2009). “Functional characterization of chitin and chitosan,” Curr. Chem. Biol. 3(2), 203-230. DOI: 10.2174/187231309788166415
Cheng, D., Yang, X., He, Z., and Ni, Y. (2016). “Potential of cellulose-based materials for lithium-ion batteries (LIB) separator membranes,” Journal of Bioresources and Bioproducts 1(1), 18-21.
Chen, Z., Li, C., Song, Z., and Qian, X. (2014). “Wet strength and antibacterial performance of cellulosic paper induced by maleic anhydride-acylated chitosan,” BioResources 9(3), 4503-4509. DOI: 10.15376/biores.9.3.4503-4509
Chen, Z., Zhang, H., Song, Z., and Qian, X. (2013). “Preparation and application of maleic anhydride-acylated chitosan for wet strength improvement of paper,” BioResources 8(3), 3901-3911. DOI: 10.15376/biores.8.3.3901-3911
Fan, J., Li, T., Ren, Y., Qian, X., Wang, Q., Shen, J., and Ni, Y. (2017). “Interaction between two oppositely charged starches in an aqueous medium containing suspended mineral particles as a basis for the generation of cellulose-compatible composites,” Industrial Crops and Products 97, 417-424. DOI: 10.1016/j.indcrop.2016.12.048
Hubbe, M. A., Rojas, O. J., and Lucia, L. A. (2015). “Green modification of surface characteristics of cellulosic materials at the molecular or nano scale: A review,” BioResources 10(3), 6095-6206. DOI: 10.15376/biores.10.3.Hubbe
Hamzeh, Y., Sabbaghi, S., Ashori, A., Abdulkhani A., and Soltani, F. (2013). “Improving wet and dry strength properties of recycled old corrugated carton (OCC) pulp using various polymers,” Carbohydr. Polym. 94(1), 577-583. DOI: 10.1016/j.carbpol.2013.01.078
Kontturi, E. and Vuorinen, T. (2009). “Indirect evidence of supramolecular changes within cellulose microfibrils of chemical pulp fibers upon drying,” Cellulose 16(1), 65-74. DOI: 10.1007/s10570-008-9235-3
Lindström, T., Wågberg, L., and Larsson, T. (2005). “On the nature of joint strength in paper – A review of dry and wet strength resins used in paper manufacturing,” 13th Fundamental Research Symposium, Cambridge, UK.
Liu, K., Lin, X., Chen, L., and Huang, L. (2016). “Preparation of guanidine-modified starch for antimicrobial paper,” Journal of Bioresources and Bioproducts 1(1), 3-6.
Liu, Y., and Kim, H. I. (2012). “Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites,” Carbohydr. Polym. 89(1), 111-116. DOI: 10.1016 /j.carbpol. 2012. 02. 058
Liu, S. (2010). “Woody biomass: Niche position as a source of sustainable renewable chemicals and energy and kinetics of hot-water extraction/hydrolysis,” Biotechnol. Adv. 28(5), 563-582. DOI: 10.1016/j.biotechadv.2010.05.006
Luo, L., van der Voet, E., and Huppes, G. (2010). “Biorefining of lignocellulosic feedstock – Technical, economic and environmental considerations,” Bioresource Technology 101(13), 5023-5032. DOI: 10.1016/j.biortech.2009.12.109
Mosier, N. S., Sarikaya, A., Ladisch, C. M., and Ladisch, M. R. (2001). “Characterization of dicarboxylic acids for cellulose hydrolysis,” Biotechnology Progress, 17(3), 474-480. DOI: 10.1021/bp010028u
Mourya, V. K., and Inamdar, N. N. (2008). “Chitosan-modifications and applications: Opportunities galore,” React. Funct. Polym. 68(6), 1013-1051. DOI: 10.1016/j.reactfunctpolym.2008.03.002
Nada, A. M. A., El-Sakhawy, M., Kamel, S., Eid, M. A. M., and Adel, A. M. (2006). “Mechanical and electrical properties of paper sheets treated with chitosan and its derivatives,” Carbohyd. Polym. 63(1), 113-121. DOI: 10.1016/ j. car bpol. 2005.08.028
Niekraszewicz, A., Struszczyk, H., Malinowska, A., and Szymański, A. (2001). “Chitosan application to modification of paper,” Fib. Text. East. Eur. 9(3), 58-63.
Qian, L., Guan, Y., He, B., and Xiao, H. (2008). “Synergy of wet strength and antimicrobial activity of cellulose paper induced by a novel polymer complex,” Mater. Lett. 62(21-22), 3610-3612. DOI: 10.1016/j.colsurfb.2009.11.014
Ranjbar-Mohammadi, M., Arami, M., Bahrami, H., Mazaheri, F., and Mahmoodi, N. M. (2010). “Grafting of chitosan as a biopolymer onto wool fabric using anhydride bridge and its antibacterial property,” Colloids Surf. B: Biointerfaces. 76 (2), 397-403. DOI: 10.1016/j.colsurfb.2009.11.014
Shen, J., Fatehi, P., and Ni, Y. (2014). “Biopolymers for surface engineering of paper-based products,” Cellulose 21(5), 3145-3160. DOI: 10.1007/s10570-014-0380-6
Shen, J., Kaur, I., Baktash, M. M., He, Z., and Ni, Y. (2013). “A combined process of activated carbon adsorption, ion exchange resin treatment and membrane concentration for recovery of dissolved organics in pre-hydrolysis liquor of the kraft-based dissolving pulp production process,” Bioresource Technology 127, 59-65. DOI: 10.1016/j.biortech.2012.10.031
Saeed, A., Fatehi, P., and Ni, Y. (2011). “Chitosan as a flocculant for pre-hydrolysis liquor of kraft-based dissolving pulp production process,” Carbohydr. Polym. 86(4), 1630-1636. DOI: 10.1016/j.carbpol.2011.06.075
Saito, T., and Isogai, A. (2006). “Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation,”Colloid Surf. A – Physicochem. Eng. Asp. 289(1-3), 219-225. DOI: 10.1016/j.colsurfa.2006.04.038
Sun, S., An, Q., Li, X., Qian, L., He, B., and Xiao, H. (2010). “Synergistic effects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper,” Bioresour. Technol.101(14), 5693-5700. DOI: 10.1016/j.biortech.2010.02.046
TAPPI T456 om-3 (2005). “Tensile breaking strength of water-saturated paper and paperboard (“wet tensile strength”),” TAPPI Press, Atlanta, GA.
Vallapa, N., Wiarachai, O., Thongchul, N., Pan, J., Tangpasuthadol, V., Kiatkamjornwong, S., and Hoven, V. (2011). “Enhancing antibacterial activity of chitosan surface by heterogeneous quaternization,” Carbohyd. Polym. 83(2), 868-875. DOI: 10.1016/j.carbpol.2010.08.075
Villaverde, J. J., Sandín-España, P., Sevilla-Morán, B., López-Goti, C., and Alonso-Prados, J. L. (2016). “Biopesticides from natural products: Current development, legislative framework, and future trends,” BioResources 11(2), 5618-5640. DOI: 10.15376/biores.11.2.Villaverde
Wang, J. P., Chen, Y. Z., Zhang, S. J., and Yu, H. Q. (2008). “A chitosan-based flocculant prepared with gamma-irradiation-induced grafting,” Bioresour. Technol. 99(9), 3397-3402. DOI: 10.1016/j.biortech.2007.08.014
Yuan, J., Wang, T., Huang, X., and Wei, W. (2016). “Effect of wet-end additives on the results of alkyl ketene dimer sizing after adding bacterial cellulose,” BioResources 11(4), 9280-9289. DOI: 10.15376/biores.11.4.9280-9289
Zakaria, S. (2004). “Development of wet-strength paper with dianhydride and diacid,” Mater. Chem. Phys. 88(2-3), 239-243. DOI: 10.1016/j.matchemphys.2003.09.035
Article submitted: January 26, 2018; Peer review completed: March 17, 2018; Revised version received: March 29, 2018; Accepted: March 31, 2018; Published: April 18, 2018.
DOI: 10.15376/biores.13.2.4018-4028
