Abstract
Tannin and sucrose (TS) can be used in a biomaterial-based wood adhesive that requires a higher hot pressing temperature and longer time than traditional resins. To solve these problems, hydrochloric acid and citric acid were utilized as a catalyst during the curing process to decrease energy costs (forming TSH and TSC adhesives, respectively). Thermal analysis revealed that the addition of hydrochloric acid and citric acid resulted in decreases to the thermal thresholds associated with degradation and curing. The resultant insoluble matter ratio verified that the polymerization reaction happened at a lower temperature than the adhesive without acidic conditions. The FT-IR and solid state 13C NMR spectra showed that the addition of acid compounds acted catalytically and increased the generation of 5-HMF in uncured adhesives. The dimethylene-ether bridges and methylene bridge were formed during the heat treatment. The water resistance of the particleboards manufactured by TSC and TSH adhesives were notably enhanced when the hot-pressing temperature was 180 °C. However, an increased hot pressing temperature did not improve the mechanical properties of the particleboard bonded by TSH, which was due to cellulose and hemicellulose decomposition under strong acidity conditions.
Download PDF
Full Article
Influence of Acid on the Curing Process of Tannin-sucrose Adhesives
Shijing Sun a and Zhongyuan Zhao b,*
Tannin and sucrose (TS) can be used in a biomaterial-based wood adhesive that requires a higher hot pressing temperature and longer time than traditional resins. To solve these problems, hydrochloric acid and citric acid were utilized as a catalyst during the curing process to decrease energy costs (forming TSH and TSC adhesives, respectively). Thermal analysis revealed that the addition of hydrochloric acid and citric acid resulted in decreases to the thermal thresholds associated with degradation and curing. The resultant insoluble matter ratio verified that the polymerization reaction happened at a lower temperature than the adhesive without acidic conditions. The FT-IR and solid state 13C NMR spectra showed that the addition of acid compounds acted catalytically and increased the generation of 5-HMF in uncured adhesives. The dimethylene-ether bridges and methylene bridge were formed during the heat treatment. The water resistance of the particleboards manufactured by TSC and TSH adhesives were notably enhanced when the hot-pressing temperature was 180 °C. However, an increased hot pressing temperature did not improve the mechanical properties of the particleboard bonded by TSH, which was due to cellulose and hemicellulose decomposition under strong acidity conditions.
Keywords: Natural adhesive; Tannin; Sucrose; Citric acid; Hydrochloric acid
Contact information: a: College of Material Science and Engineering, Nanjing Forestry University, Nanjing 210037, China; b: College of of Furnishings and Industrial Design, Nanjing Forestry University, Nanjing 210037, China; *Corresponding author: zhaozy930@126.com
INTRODUCTION
Wood-based materials have been utilized as structural and non-structural materials for interior and exterior environments (Sellers 2001; Guan et al. 2016) such as plywood, oriented strand board (OSB), particleboard, and fiberboard. Examples of structural materials include glulam, laminated veneer lumber (LVL), oriented strand lumber (OSL), and parallel strand lumber (PSL) (Huang et al. 2017). As a composite material, wood-based panels are typically composed of wood elements and synthetic resins such as formaldehyde-based, isocyanate-based, vinyl acetate resin, etc., which are mostly derived from fossil resources (Li et al. 2015; Cheng et al. 2016). Because of the depletion of petroleum resources and concerns about formaldehyde emission, there is no doubt that the utilization of synthetic resin on wood-based materials will be restricted (Umemura et al. 2012; Kusumah et al. 2016). Therefore, the research into natural adhesives has been an important direction for the wood-based material industry, and scholars have developed protein-based, polysaccharide-based, tannin-based adhesives, etc. to manufacture eco-friendly materials (Pizzi et al. 2016; Li et al. 2018; Liu et al. 2018; Papacchini et al. 2018).
Tannin and sucrose (TS) adhesives have been used to manufacture particleboard. When the boards are bonded under optimal conditions, the properties satisfy the requirements of JIS A 5908 type 18 standard (2003) (Zhao and Umemura 2015; Zhao and Umemura 2017). However, the hot-pressing temperature and time for this adhesive were 220 °C and 10 min, respectively. Therefore, it is necessary to research a method to reduce the curing temperature or time. Condensed tannins, which is commonly utilized to produce leather (Khanbabaee and Van 2011; Maier et al. 2017), contain a high amount of polyphenols. The monomer of wattle tannin contains resorcinol A-rings and catechol or pyrogallol B-rings, and the free C6 or C8 sites on the A-ring, which could react with an active substance to form the adhesive due to their strong nucleophilicity (Kim and Kim 2003). Sucrose is a common disaccharide, and 5-hydroxymhthylfurfural (5-HMF) is generated by heating treatment, which has active chemical properties (Jeong et al. 2013; Han et al. 2014). Considering chemical characteristics and curing mechanism of TS adhesives, 5-HMF was generated at 200 °C and reacted with tannin to form a linkage. Hence, the high curing temperature between tannin and sucrose was due to the generation temperature of 5-HMF.
The 5-HMF formation from sucrose depends on many factors such as time, water activity, temperature, and amount and type of catalyst. Numerous studies have already indicated that acid is a common catalyst for a 5-HMF synthesis (Tan-Soetedjo et al. 2017). In acidic conditions, sucrose is easily hydrolyzable to its monomers, D-glucose and D-fructose, which can both be converted to 5-HMF in good yields. In this research, hydrochloric acid and citric acid were selected as catalysts and added to TS adhesives. The influence of organic acids and inorganic acids on the curing process and bonding properties were investigated.
EXPERIMENTAL
Materials
Wattle tannin (tannic acid ME) was purchased from the Fuji Chemical Industry Co. (Wakayama, Japan). Sucrose (guaranteed reagent) and citric acid (extra pure reagent) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan), and hydrochloric acid (concentration was 37%) was purchased from Wako Pure Chemical Industries Ltd. (Kyoto, Japan). Tannin, sucrose, and citric acid were used without further purification, but were dried in a vacuum oven at 60 °C for 15 h.
Methods
pH Adjustment of Adhesive Solution by Adding Acid
The acid content of both the hydrochloric acid and citric acid were determined through adjustments of pH values. Based on previous research, the optimal ratio of tannin to sucrose is 25:75 (Zhao and Umemura 2017). Therefore, 25 g of tannin and 75 g of sucrose were mixed in a beaker, and 150 g of distilled water was added into the mixture to blend a TS adhesive solution at a concentration of 40 wt%. Two beakers of this solution were prepared for further experiments. Citric acid and hydrochloric acid were also mixed with distilled water at a 40 wt% concentration. Subsequently, 0.1 g of the citric acid and hydrochloric acid solution was added in stepwise to the two beaker TS solutions, respectively. The viscosity and pH of the final solution at 20 °C were measured by a rotational viscometer (Viscolead One, Fungilab S.A., Barcelona, Spain) and a pH meter (D-51, Horiba Scientific, Kyoto, Japan), respectively. This process was repeated several times until the pH of the 40 wt% concentration tannin-sucrose-citric acid and tannin-sucrose-hydrochloric acid solutions reached 1.5. Basic information regarding the adhesives is presented in Table 1.
Table 1. Viscosity and pH of Adhesives
Note: a Tannin and sucrose adhesive; b Tannin-sucrose-citric acid adhesive; c Tannin-sucrose-hydrochloric acid adhesive
Thermal Analysis
Each type of adhesive shown in Table 1 was prepared for a 100 g solution. The liquid samples were poured into glass vials and then frozen using liquid nitrogen. Afterwards, the samples were dried by a freeze drying machine (Freezone 1L FZ-1 Instruments, Tokyo, Japan). The dried adhesives were pulverized to a < 250-μm mesh size to obtain the uncured adhesive powder. A thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were carried out using a TGA 2050 (TA Instruments, Tokyo, Japan) and DSC 2910 (TA Instruments, Tokyo, Japan), respectively. The samples were scanned from room temperature to 400 °C at a rate of 10 °C/min under nitrogen purging with a flow rate of 100 mL/min and 40 mL/min, respectively.
Measurement of insoluble matter ratio
Uncured adhesive powder was obtained in the same manner as the thermal analysis. Each of the uncured adhesive powders were divided into four parts and heated at 160 °C, 180 °C, 200 °C, and 220 °C for 10 min to obtain the cured adhesives. Then, 2 g samples of each cured adhesive were boiled in distilled water for 4 h and weighed to measure the wet insoluble matter. The boiling treatment was carried out in triplicate. All samples obtained from the heating treatments and boiling treatments were vacuum-dried at 60 °C for 15 h, and the weight of dried insoluble matter (M) was obtained. The insoluble matter ratio was calculated by Eq. 1,
(1)
Fourier transform infrared spectra
Fourier transform infrared spectra were measured to investigate the chemical changes between the uncured and cured adhesives. The uncured adhesive powder was obtained in the same method as the thermal analysis. In the cured adhesives, each uncured adhesive powder was heated at 200 °C for 10 min, and the insoluble matter was obtained. The infrared spectra were scanned by a Fourier transform infrared spectrophotometer (FT/IR-4200, JASCO Corporation, MD, USA) using the KBr disk method, and recorded with an average of 32 scans at a resolution of 4 cm-1.
13C CP/MAS NMR
A solid state 13C NMR spectrum was obtained from the insoluble matter of cured adhesives to investigate the chemical structure of the polymer. The 13C CP/MAS NMR spectra at an ambient temperature were measured on a Varian 400 NMR system spectrometer (Varian, Palo Alto, CA) with a Varian 5 mm CP-MAS and a multipulse probe. The powder samples were placed in Si3N4 rotors of 5 mm in diameter with an O-ring cap and spun at the magic angle at the frequency of 8 to 9 kHz according to the characteristics of the samples. The 13C NMR spectra were taken at 100.56 MHz for the 13C nuclei with a 40 msec acquisition period, 30.5 kHz spectral width, and a radiofrequency of 86 kHz. The pulse sequence was continuous 1H decoupling with a small phase incremental alteration (SPINAL) decoupling pulse sequence. The cross-polarization had a 2.0 msec contact time. The 13C CP-MAS NMR spectra were obtained using the regular CP-MAS sequence at a 500 μsec contact time with a 4.0 μsec long π/2 pulse and a 5.0 s of recycle delay.
Manufacture of particleboard
Based on the adhesive information showed in Table 1, the TS, TSC, and TSH adhesives were prepared at 40 wt% concentration. These adhesives were sprayed onto wood particles in a blender with 20 wt% resin dosage content based on the oven-dried weight of the particles. The sprayed particles were dried at 80 °C for 12 h until the moisture content was between 4 and 7%. The dried particles were mat-formed using a forming box of 300 mm ×300 mm. The mat was then hot-pressed at 180 °C and 200 °C for 10 min at a distance bar of 9 mm to control the thickness. The size of the manufactured board was 300 mm × 300 mm × 9 mm, and the target density was 0.8 g/cm3.
Evaluation of particleboard properties
The particleboards were conditioned for 1 week at 20 °C and 60% relative humidity (RH), and then evaluated according to the Japanese Industrial Standard for particleboard (JIS A 5908 2003). The static 3-point bending test was carried out on a 200 mm × 30 mm × 9 mm specimen from each board, and the effective span and loading speed were 150 mm and 10 mm/min, respectively. The modulus of rupture (MOR) was calculated from the bending test. The internal bond strength (IB) test was performed on a 50 mm × 50 mm specimen with a loading speed of 2 mm/min, and thickness swelling (TS) after water immersion for 24 h at 20 °C was measured in specimens of the same size. Each experiment was performed in five repetitions and the average values and standard deviations were calculated. The statistical significance was considered for p values < 0.5.
RESULTS AND DISCUSSION
Thermal Analysis
Figure 1 shows the thermogravimetric (TG) and derivative TG (DTG) curves of the TS, TSC, and TSH adhesives. From the TG curves, the adhesives mixed with acid compounds exhibited lower degradation temperatures than the TS adhesives. From the DTG curves, the TS adhesives showed a sharp peak at approximately 205 °C, which was considered to be the sucrose thermolysis and the products reacting with tannin (Zhao and Umemura 2015). There was a substantial weight loss peak of the TSC and TSH adhesives at 159 °C and 129 °C, respectively which was due to the sucrose pyrolysis or caramelization and tannin degradation (Dalluge et al. 2014). However, although the TSC and TSH adhesives were adjusted at the same pH values, the catalysis of hydrochloric acid seemed more effective than the citric acid. This was possibly attributed to the strong acidity of the hydrochloric acid.
Fig. 1. TG and DTG curves of adhesives
Fig. 2. DSC curves of adhesives
The DSC curves of each adhesive sample are shown in Fig. 2. The TS adhesives showed two endothermic peaks at 180 °C and 215 °C which were attributed to the sucrose melting and the polymerization reaction, respectively. A broad endothermic peak was observed at 155 °C from the TSC adhesives. Based on the TG analysis, this could be attributed to the sucrose pyrolysis or reactions among the three substances. In addition, there was an endothermic shoulder located at approximately 212 °C, which was attributed to the citric acid decomposition (Barbooti and Al-Sammerrai 1986). A sharp endotherm peak was detected from the TSH adhesive at approximately 129 °C, indicating that a violent endothermic reaction happened. Based on the results of the TG analysis, this reaction also led to weight loss of the TSH adhesives. Therefore, the endothermic reaction was possibly attributed to the sucrose caramelization or polymerization between the tannin and sucrose products (Ajandouz and Puigserver 1999). The thermal analysis indicated that both the hydrochloric acid and citirc acid reduced the reaction temperature between the tannin and sucrose. When the pH of the adhesive solutions was at same level, the hydrochloric acid exhibited stronger catalysis than the citric acid.
Fig. 3. Insoluble matter of adhesives
Insoluble Matter Ratio
Figure 3 shows the results of the insoluble matter ratios of the adhesives. The three series of adhesives were heated at 160 °C, 180 °C, 200 °C, and 220 °C for 10 min to obtain the cured adhesive. Afterwards, the cured adhesives were boiled for 4 h to calculate the insoluble matter ratio. A variance analysis (ANOVA) revealed no significant (p > 0.05) difference between the TSH and TSC adhesives heated at 220 °C. Across all samples, an increase of heat temperature resulted in a growth trend of the insoluble matter ratio. When the heating temperature was 160 °C and 180 °C for the TS adhesives, the insoluble matter ratio was found nearly 0% which indicated that the curing reaction didn’t happen under the 180 °C condition. In contrast, the insoluble matter ratios of the TSC and TSH adhesives at 160 °C and 180 °C were 23%, 42%, 49%, and 62%, respectively. Including the results from the thermal analysis, the addition of acid compounds led the curing process to occur at a lower temperature. When the heating temperature was at 200 °C, the insoluble matter of the TSH adhesives was 81%, which was higher than the TS adhesives heated at 220 °C. These observations reflected that acid compounds could reduce the curing reaction temperature between tannin and sucrose. Similarly, the results of the thermal analysis showed that hydrochloric acid exhibited better effects than citric acid at same pH.
FT-IR Spectroscopic Analysis
The FT-IR spectra of the uncured adhesives are shown in Fig. 4. Three absorption peaks of the uncured adhesives increased with an addition of acid compounds compared to the TS adhesives. The peak located at 1625 cm-1 was derived from aromatic C=C stretching, which was possibly attributed to the tannin hydrolysis. The peaks at 1509 cm-1 and 780 cm-1 were due to the characteristic of C=C stretching vibration and unsubstituted CH=CH of 5-HMF, respectively (Alakhras and Holze 2007; Zhang et al. 2012). Moreover, the peak at approximately 920 cm-1 was decreased as acid was added, which was attributed to the pyranose ring of the sucrose (Jing et al. 2016), which indicated the sucrose hydrolysis. Two specific peaks of the TSC adhesives were observed at 1734 cm-1 and 1209 cm-1 due to the C=O stretching derived from the carboxyl group, C-O stretching, and –OH bending motions from citric acid, respectively (Yang and Wang 1996; Lackovic et al. 2003; Schwanninger et al. 2004). The FT-IR spectra of the uncured adhesives indicated that the addition of each hydrochloric acid and citric acid led to the hydrolysis of sucrose to glucose and fructose, and eventual dehydration to 5-HMF.
Fig. 4. FT-IR spectra of uncured adhesives
Figure 5 shows the FT-IR spectra of insoluble matter of the cured adhesives heated at 200 °C for 10 min. The peaks located at 1705 cm-1, 1200 cm-1, and 780 cm-1 increased with the addition of acid compounds. The absorption bond located at 1705 cm-1 attributed to the C=O stretching derived from the carbonyl group (Vaz and Ribeiro-Claro 2003). This group was more noticeable in the TSC adhesives than the other adhesives, which were possibly due to the formation of carbopol by the carboxylic group from the citric acid. The peak at 1200 cm-1was associated with the –CO stretching of the benzene nucleus and/or dimethylene ether bridges (-CH2OCH2-) (Kim and Kim 2003). The peak at 780 cm-1 was due to the CH=CH of 5-HMF (Alakhras and Holze 2007). The results of FT-IR between uncured and cured adhesives show that the addition of both citric acid and hydrochloric acid led to the sucrose hydrolysis, and was succeeded by the glucose and fructose converting to 5-HMF. During the heating treatment, dimethylene ether bridges and carbonyl group formed in three kinds of the cured adhesives.
Fig. 5. FT-IR spectra of insoluble matter of cured adhesives
The 13C CP/MAS NMR Analysis
The solid-state 13C NMR spectra of the insoluble matter were obtained from TS, TSC, and TSH heated at 200 °C for 10 min. The peaks located at 110 ppm, 141 ppm, and 156 ppm were derived from C2 and C3 of furanic rings, which certified 5-HMF participated in the polymerization of the three kinds adhesives (Gandini and Belgacem 1997). The single peak observed at 110 ppm was also attributed to the C8 position of the tannin for methylene ether bridging (Pizzi and Scharfetter 1978). The carbon had the carbonyl result reduced and directly bonded with two resorcinolic rings was absorbed at 36 ppm. In addition, a small peak at 175 ppm was derived from the carbonyl group, which is possibly due to the unreacted aldehyde group of 5-HMF or the carbopol derived from sucrose caramelization (Grenier-Loustalot et al. 1996). A major peak at 70 ppm was intense in all three cured adhesives, which was due to the dimethylene-ether bridge (-CH2-O-CH2-) structures (Rego et al. 2004). The absorption at 30 ppm from the insoluble matter of the TS and TSC adhesives was due to methylene bridges (-CH2-) and chemical structures formed by self-reaction of 5-HMF (Tondi 2017). The results of the 13C NMR spectrum shown in Fig. 6 indicated that 5-HMF participated in the polymerization reaction, in which it reacted with C8 of resorcinol A-rings and formed methylene bridges. Also, a dimethylene-ether bridge was observed as a major chemical chain of the polymer. The addition of acid compounds acted as a catalytic, which improved the generation of 5-HMF in uncured adhesives.
Fig. 6. Sold state 13C NMR spectrum of adhesives
Fig. 7. Possible curing mechanism of TSC and TSH adhesives
In addition, tannin was degraded with the addition of acid compounds, and this possibly promoted the reaction rate between the tannin and 5-HMF. More carbopol was produced in the cured TSC adhesives. The possible curing process of the TSH and TSC adhesives is shown in Fig. 7.
Evaluation of Bonding Properties
Figure 8 shows the mechanical properties and water resistance of the particleboard bound by the TS, TSC, and TSH adhesives with 20 wt% resin content at 180 °C for 10 min.
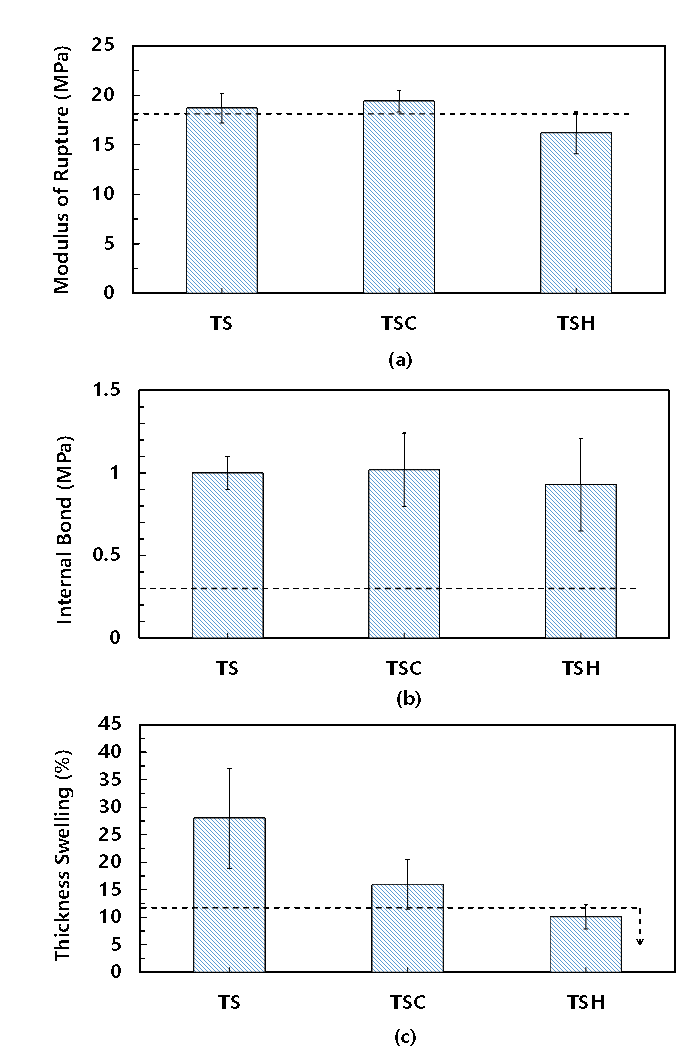
Fig. 8. Properties of the particleboards bonded at 180 °C for 10 min with TS, TSC and TSH adhesives. (a) Modulus of rupture, (b) internal bond, and (c) thickness swelling. The values of dotted line were chosen according to JIS A 5908 type 18.
The particleboard formulated with TSH adhesives exhibited a better performance than with the TS or TSC adhesives, and achieved JIS A5908 type 15 standard (2003). The thickness swelling of the board with TSC and TSH adhesives was 33% and 10.1%, while the particleboard bound by TS adhesives was destroyed, which indicated that the addition of acid improved the water resistance of the particleboard at lower hot-pressing temperatures. The properties of the particleboard bound with 20 wt% resin content at 200 °C for 10 min are shown in Fig. 9.
Fig. 9. Properties of the particleboards bonded at 200 °C for 10 min with TS, TSC and TSH adhesives. (a) Modulus of rupture, (b) internal bond, and (c) thickness swelling.The values of dotted line were chosen according to JIS A 5908 type 18.
In the bending and internal bond properties, the variance analysis (ANOVA) revealed no significant (p>0.05) difference between the particleboard manufactured by the TS and TSC adhesives, and the properties satisfied the requirement of JIS A5908 type 18 standard (2003). The TSH adhesives showed a relatively low mechanical property, although the insoluble matter ratio at 200 °C was higher than the other adhesives. In the thickness swelling properties, however, the TSH adhesives exhibited excellent water resistance at 9.1%, compared to boards with TS and TSC adhesives, which were at 28% and 16%. In contrast to the results of the two groups’ experiments, an increase of hot pressing temperature did not improve the mechanical properties of the particleboard bonded by the TSH adhesives, although the particleboard still exhibited excellent water resistance. This phenomenon was attributed to the cellulose and hemicellulose decomposition with the addition of hydrochloric acid and heating conditions (Saeman 1945), which led to an intense reduction of wood particles. In addition, both of the mechanical and water resistance properties of the TSC adhesives improved when the hot pressing temperature increased, this was possible due to the polycondensation reaction took place between the carboxyl group and chemical compounds which derived from citric acid and wood particles, respectively (Umemura et al. 2012).
CONCLUSIONS
- Hydrochloric acid and citric acid were used as catalysts to investigate the effects on the curing process and bonding properties of the particleboard. The thermal analysis revealed that an addition of hydrochloric acid and citric acid resulted in the weight loss and endothermic reaction temperature from 205 °C to 129 °C and 159 °C, respectively.
- The results of insoluble matter ratio verified that the polymerization reaction occurred at a lower temperature than the adhesives without acidic conditions. When the heating temperature was 160 °C and 180 °C, the insoluble matter ratio of the TS adhesives was 0%, while the TSC and TSH adhesives were 23%, 42%, 49%, and 62%, respectively.
- The FT-IR and solid state 13C NMR spectra revealed that the addition of acid compounds acted as a catalyst, which improved the generation of 5-HMF in uncured adhesives. In addition, dimethylene-ether bridges and methylene bridges were formed during the heating treatment.
- The bonding properties of the adhesives were evaluated by manufacturing the particleboards. The results showed that the water resistance of the particleboards manufactured by the TSC and TSH adhesives was visibly enhanced when the hot-pressing temperature was 180 °C. However, an increase in the hot pressing temperature did not improve the mechanical properties of the particleboard bonded by TSH, which was due to decomposition of cellulose and hemicellulose at strong acidity conditions.
ACKNOWLEDGMENTS
The authors are grateful for the support of Scientific Research Foundation of Nanjing Forestry University (GXL2018013) and the Priority Academic Program Development of JiangsuHigher Education Institutions (PAPD).
REFERENCES CITED
Ajandouz, E., and Puigserver, A. (1999). “Nonenzymatic browning reaction of essential amino acids: Effect of pH on caramelization and Maillard reaction kinetics,” J. Agr. Food. Chem.47(5), 1786-1793. DOI: 10.1021/jf980928z
Alakhras, F., and Holze, R. (2007). “In situ UV-vis-and FT-IR-spectroscopy of electrochemically synthesized furan–thiophene copolymers,” Synthetic Met. 157(2-3), 109-119. DOI: 10.1016/j.synthmet.2006.12.011
Barbooti, M., and Al-Sammerrai, D. (1986). “Thermal decomposition of citric acid,” Thermochim. Acta. 98, 119-126. DOI: 10.1016/0040-6031(86)87081-2
Cheng, H., Ford, C., Dowd, M., and He, Z. (2016). “Soy and cottonseed protein blends as wood adhesives,” Ind. Crop. Prod. 85, 324-330. DOI: 10.1016/j.indcrop.2015.12.024
Dalluge, D., Daugaard, T., Johnston, P., Kuzhiyil, N., Wright, M., and Brown, R. (2014). “Continuous production of sugars from pyrolysis of acid-infused lignocellulosic biomass,” Green Chem. 16, 4144-4155. DOI: 10.1039/c4gc00602j
Gandini, A., and Belgacem, M. (1997). “Furans in polymer chemistry,” Prog. Polym. Sci. 22(9), 1203-1379. DOI: 10.1016/S0079-6700(97)00004-X
Grenier-Loustalot, M., Larroque, S., Grenier, P., and Bedel, D. (1996). “Phenolic resins: 4. Self-condensation of methylolphenols in formaldehyde-free media,” Polymer 37(6), 955-964. DOI: 10.1016/0032-3861(96)87277-6
Guan, C., Zhang, H., Hunt, J., and Yan, H. (2016). “Determining shear modulus of thin wood composite materials using a cantilever beam vibration method,” Constr. Build. Mater. 121, 85-289. DOI: 10.1016/j.conbuildmat.2016.06.007
Han, J., Lei, T., and Wu, Q. (2014). “High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism,” Carbohyd. Polym. 102, 306-316. DOI: 10.1016/j.carbpol.2013.11.045
Huang, C., He, J., Narron, R., Wang, Y., and Yong, Q. (2017). “Characterization of kraft lignin fractions obtained by sequential ultrafiltration and their potential application as a biobased component in blends with polyethylene,” ACS Sustain. Chem. Eng. 5(12), 11770-11779. DOI: 10.1021/acssuschemeng.7b03415
Jeong, J., Antonyraj, C., Shin, S., Kim, S., Kim, B., Lee, K., and Cho, J. (2013). “Commercially attractive process for production of 5-hydroxymethyl-2-furfural from high fructose corn syrup,” J. Ind. Eng. Chem. 19(4), 1106-1111. DOI: 10.1016/j.jiec.2012.12.004
Jing, L., Zong, S., Li, J., Surhio, M., and Ye, M. (2016). “Purification, structural features and inhibition activity on α-glucosidase of a novel polysaccharide from Lachnum YM406,” Process Biochem. 51(10), 1706-1713. DOI: 10.1016/j.procbio.2016.08.007
JIS A 5908 (2003). “Particleboard,” Japanese Standards Association, Tokyo, Japan.
Khanbabaee, K., and Van, T. (2011). “Tannins: Classification and definition,” Nat. Prod. Rep. 18, 641-649. DOI: 10.1039/b101061l
Kim, S., and Kim, H. (2003). “Curing behavior and viscoelastic properties of pine and wattle tannin-based adhesives studied by dynamic mechanical thermal analysis and FT-IR-ATR spectroscopy,” J. Adhes. Sci. Technol. 17(10), 1369-1383. DOI: 10.1163/156856103769172797
Kusumah, S., Umemura, K., Yoshioka, K., Miyafuji, H., and Kanayama, K. (2016). “Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard I: Effects of pre-drying treatment and citric acid content on the board properties,” Ind. Crop. Prod. 84, 34-42. DOI: 10.1016/j.indcrop.2016.01.042
Lackovic, K., Johnson, B., Angove, M., and Wells, J. (2003). “Modeling the adsorption of citric acid onto Muloorina illite and related clay minerals,” J. Colloid. Interf. Sci. 267(1), 49-59. DOI: 10.1016/S0021-9797(03)00693-3
Li, J., Luo, J., Li, X., Yi, Z., Gao, Q., and Li, J. (2015). “Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine,” Ind. Crop. Prod. 74, 613-618. DOI: 10.1016/j.indcrop.2015.05.066
Li, R., Gutierrez, J., Chung, Y., Frank, C., Billington, S., and Sattely, E. (2018). “A lignin-expoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesive,” Green Chem. 20, 1459-1466. DOI: 10.1039/c7gc03026f
Liu, C., Mei, C., Xu, B., Chen, W., Yong, C., Wang, K., and Wu, Q. (2018). “Light stabilizers added to the shell of co-extruded wood/high-density polyethylene composites to improve mechanical and anti-UV ageing properties,” Roy. Soc.Open Sci. 5, 180074. DOI: 10.1098/rsos.180074
Maier, M., Oelbermann, A., Renner, M., and Weidner, E. (2017). “Screening of European medicinal herbs on their tannin content – New potential tanning agents for the leather industry,” Ind. Crop. Prod. 99, 19-26. DOI: 10.1016/j.indcrop.2017.01.033
Papacchini, A., Leggieri, M. R., Zucchini, L., Ortenzi, M. A., Ridi, F., Giomi, D., and Salvini, A. (2018). “Modified α,α’-trehalose and D-glucose: Green monomers for the synthesis of vinyl copolymers,” Roy. Soc. Open Sci. 5, 171313. DOI: 10.1098/rsos.171313
Pizzi, A. (2016). “Wood products and green chemistry,” Ann. Forest Sci. 73(1), 185-203. DOI: 10.1007/s13595-014-0448-3
Pizzi, A., and Scharfetter, H. (1978). “The chemistry and development of tannin-based adhesives for exterior plywood,” J. Appl. Polym. Sci. 22(6), 1745-1761. DOI: 10.1002/app.1978.070220623
Rego, R., Adriaensens, P., Carleer, R., and Gelan, J. (2004). “Fully quantitative carbon-13 NMR characterization of resol phenol-formaldehyde prepolymer resins,” Polymer 45(1), 33-38. DOI: 10.1016/j.polymer.2003.10.078
Saeman, J. (1945). “Kinetics of wood saccharification-hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature,” Ind. Eng. Chem. Res. 37(1), 43-52. DOI: 10.1021/ie50421a009
Schwanninger, M., Rodrigues, J., Pereira, H., and Hinterstoisser, B. (2004). “Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose,” Vib. Spectrosc. 36(1), 23-40. DOI: 10.1016/j.vibspec.2004.02.003
Sellers, T. J. (2001). “Wood adhesive innovations and applications in North America,” Forest. Prod. J. 51(6), 12–22.
Tan-Soetedjo, J., Bovenkamp, H., Abdilla, R., Rasrendra, C., Ginkel, J., and Heeres, H. (2017). “Experimental and kinetic modeling studies on the conversion of sucrose to levulinic acid and 5-HMF using sulfuric acid in water,” Ind. Eng. Chem. Res. 56 (45), 13228-13239. DOI: 10.1021/acs.iecr.7b01611
Tondi, G. (2017). “Tannin-based copolymer resins: Synthesis and characterization by solid state 13C NMR and FT-IR spectroscopy,” Polymers 9(6), 223-239. DOI: 10.3390/polym9060223
Umemura, K., Ueda, T., Munawar, S., and Kawai, S. (2012). “Application of citric acid as natural adhesive for wood,” J. Appl. Polym. Sci. 123(4), 1991-1996. DOI: 10.1002/app.34708
Vaz, P., and Ribeiro-Claro, P. (2003). “C–H…O hydrogen bonds in liquid cyclohexanone revealed by the vC=O splitting and the vC–H blue shift,” J. Raman. Spectrosc. 34(11), 863-867. DOI: 10.1002/jrs.1066
Yang, C., and Wang, X. (1996). “Formation of cyclic anhydride intermediates and esterification of cotton cellulose by multifunctional carboxylic acids: An infrared spectroscopy study,” Text. Res. J. 66(9), 595-603. DOI: 10.1177/004051759606600908
Zhang, M., Yang, H., Liu, Y., Sun, X., Zhang, D., and Xue, D. (2012). “Hydrophobic precipitation of carbonaceous spheres from fructose by a hydrothermal process,” Carbon 50(6), 2155-2161. DOI: 10.1016/j.carbon.2012.01.024
Zhao, Z., and Umemura, K. (2015). “Investigation of a new natural particleboard adhesive composed of tannin and sucrose. 2. Effect of pressing temperature and time on board properties, and characterization of adhesive,” BioResources 10(2), 2444-2460. DOI: 10.15376/biores.10.2.2444-2460
Zhao, Z., and Umemura, K. (2017). “Investigation of a new natural particleboard adhesive composed of tannin and sucrose,” J. Wood. Sci. 60(4), 269-277. DOI: 10.1007/s10086-014-1405-3
Article submitted: June 9, 2018; Peer review completed: July 13, 2018; Revised version received: July 31, 2018; Accepted: August 13, 2018; Published: August 24, 2018.
DOI: 10.15376/biores.13.4.7683-7697
