Abstract
Depending on the production method, traditional paper mills often utilize the black liquor by burning it for energy. Hemicelluloses extracted from the raw material prior to pulping could be utilized to produce biochemical fuels. The aim of this study was to pre-extract hemicelluloses from wheat straw by treating the biomass with hot water and alkali (NaOH or the combination NaOH+NaBH4) at varying temperatures and chemical concentrations, and also to integrate resulting solid material to produce pulp and to produce bioethanol and biodegradable films from extracted liquor consisting mostly of xylan. Optimum hot water (135 °C) and alkali pre-extractions (16.7% NaOH at 50 °C) removed 16.5% and 33.6% of the xylan from the straw structure, respectively. The liquid portion of the hot water (135 °C) and alkali (16.7% NaOH at 50 °C) pre-extracted oven-dry (OD) straw yielded up to 7.79% and 6.81% (g/100 g soluble material) ethanol. Good-quality biodegradable films were produced when some gluten and nanocellulose was added to the extracted xylan. Although the hot water pre-extracted pulp yield was slightly lower, its physical and mechanical pulp properties were comparable to those of the corresponding conventional soda pulp.
Download PDF
Full Article
Integrated Production of Biofilm, Bioethanol, and Papermaking Pulp from Wheat Straw
Ayhan Tozluoğlu, Ömer Özyürek, Yalçın Çöpür,* and Hasan Özdemir
Depending on the production method, traditional paper mills often utilize the black liquor by burning it for energy. Hemicelluloses extracted from the raw material prior to pulping could be utilized to produce biochemical fuels. The aim of this study was to pre-extract hemicelluloses from wheat straw by treating the biomass with hot water and alkali (NaOH or the combination NaOH+NaBH4) at varying temperatures and chemical concentrations, and also to integrate resulting solid material to produce pulp and to produce bioethanol and biodegradable films from extracted liquor consisting mostly of xylan. Optimum hot water (135 °C) and alkali pre-extractions (16.7% NaOH at 50 C) removed 16.5% and 33.6% of the xylan from the straw structure, respectively. The liquid portion of the hot water (135 C) and alkali (16.7% NaOH at 50 C) pre-extracted oven-dry (OD) straw yielded up to 7.79% and 6.81% (g/100 g soluble material) ethanol. Good-quality biodegradable films were produced when some gluten and nanocellulose was added to the extracted xylan. Although the hot water pre-extracted pulp yield was slightly lower, its physical and mechanical pulp properties were comparable to those of the corresponding conventional soda pulp.
Keywords: Wheat straw; Pre-extraction; NaBH4; Ethanol; Pulping; Biodegradable film
Contact information: Düzce Üniversitesi Orman Fakültesi, Orman Endüstri Mühendisliği Bölümü, Konuralp Yerleşkesi, 81000, Düzce, Turkey; *Corresponding author: yalcincopur@duzce.edu.tr
INTRODUCTION
Deforestation is a major global problem that raises the demand for alternatives to wood for the production of pulp and paper, as well as for chemicals, fuel, and other purposes. On the other hand, fibrous wheat straw is abundant and is likely to increase in quantity as the needs of human cereal consumption are met. Wheat is the world’s most widely grown crop, and its annual production is estimated to be over 650 Tg (Atwell 2001) with wheat residues of 850 Tg produced annually (Talebnia et al. 2010). Recycling these organic raw materials would not only diminish environmental pollution, but would also utilize these wastes as a source of energy (Rajoka 2005).
The physical structure of straw makes it applicable for obtaining pulp fibers, and several researchers have investigated using straw for nanocomposite production (Helbert et al. 1996) and in the fermentation (Yasin et al. 2010) and medical (Kumar et al. 2011) industries, among others.
Turkey, with its rich agricultural potential and vast agricultural regions, is one of the world’s most important wheat producers and subsequently is a source of large quantities of underutilized and inexpensive wheat straw. Approximately 40 to 53 million tons of straw is produced in Turkey per year (Ergudenler and Isigigur 1994). Similar to other biomass, straw contains cell walls made of highly lignified structural carbohydrates and small amounts of extraneous materials. Chemical analysis shows that straw is not only rich in carbohydrates, but also rich in bioactive compounds and vitamins (Slavin 2003). The cell walls consist primarily of cellulose and hemicellulose. In straw, cellulose and xylose are the predominant carbohydrate components (Liu et al. 2005). A few pectic compounds and mannans are also present in the structure. Lignin is covalently bound to the side chains of xylan and is an obstacle to carbohydrate digestion because of the phenolic compounds in the structure (Antongiovanni and Sargentini 1991). One major mineral component of straw is silica, which also tends to impede polysaccharide degradation.
The new concept of an integrated biorefinery proposed by Van Heiningen (2006) pre-extracts a part of the hemicellulose prior to pulp production. This application is expected to improve the competitiveness of pulp producers without disrupting the pulp and paper production. In conventional chemical pulp production, the black liquor, which includes a fraction of hemicelluloses, is burnt to recover pulping chemicals and to generate steam and electricity. On the other hand, hemicelluloses pre-extracted with hot water (Yoon et al. 2008) and mild alkali (Al-Dajani and Tschriner 2008) prior to pulping could be converted to soluble sugars and utilized to produce ethanol and other valuable chemicals (Xing et al. 2011). In addition, literature (Copur and Tozluoglu 2008; Copur et al. 2012) has disclosed that adding small amount of NaBH4 in alkali pulping improves pulping selectivity by preventing peeling reactions and hemicelluloses degradation. Using NaBH4 is expected to preserve more glucan and xylan and to degrade lignin more selectively.
Several researchers have examined this new concept regarding woody materials; however, there is limited information about agricultural residues (Hossein et al. 2012). In the present study the objective was to determine whether an integrated biorefinery is technically feasible for pulp producers using wheat straw as a raw material and to assess the potential benefits of pre-extractions with hot water and alkali (NaOH and NaBH4).
EXPERIMENTAL
Materials
Wheat straw (Triticum aestivum), locally obtained in the Düzce province of Turkey, was cut to suitable sizes (3 to 8 cm) using a garden chopper. The chopped material was air-dried at room temperature. The moisture content was determined according to the TAPPI T212 om-06 test method, and the material was sealed in plastic bags and stored at -5 °C.
Methods
The wheat straw was first pre-extracted using hot water, NaOH, and a mixture of NaOH+NaBH4. The extracted liquids were hydrolyzed and fermented to produce ethanol from xylan and arabinan. The solid parts were further cooked to produce soda pulps.
Pre-extractions
For this study, the pre-extractions were conducted in a 3-L rotating digester. In the hot water extractions, the equivalent of 100 g of oven-dry (OD) straw and the required amount of water were added to the digester. The liquor to straw ratio was 10:1. The digester was heated to the maximum temperature in 30 min and then kept at the maximum treatment temperature for 4 h. The maximum treatment temperatures in the hot water pre-extractions were 90, 120, 135, and 150 °C.
In the alkali (NaOH) pre-extractions, the equivalent of 100 g of OD straw and the required amount of alkali solutions were added to the digester. The liquor-to-straw ratio was 4:1. The digester was heated to the maximum temperature in 30 min and then kept at the maximum treatment temperature for 4 h. The effects of NaOH concentration (16.7%, 26.7%, and 33.3% w/w) and treatment temperature (50, 70, and 90 °C) were investigated in the alkali pre-extractions. The optimum alkali treatment condition was determined regarding the ratios of carbohydrates to lignin in the solid materials and NaBH4 was added (only for the optimum alkali concentration). Additions of 0.1%, 0.5%, 2%, and 4% NaBH4 were made to the alkali (16.7% NaOH w/w), and pre-extractions for the OD wheat straw were conducted at 50 °C for 4 h. The other conditions were kept constant.
After pre-extraction, the liquor portions were separated from the solids by filtering through a cloth, and the liquid and solid materials were collected for further testing.
Analysis of pre-extracted liquids
The pre-extraction liquors were first centrifuged (5000 rpm) to separate the resultant solids from the liquid. To hydrolyze the oligomers in the liquid portion into monomeric sugars, 50 mL of liquid was incubated in an autoclave with 1000 mL of 4% sulfuric acid at 121 C for 1 h (Li et al. 2010). The liquids were neutralized with NaOH to pH 7 before analyzing the monomeric sugar contents in the liquids using HPLC (Agilent 1200 series, USA). The above procedure was conducted to determine the total sugar content of the liquids before fermentation.
Fermentation and ethanol production
The obtained liquids were fermented in a bioreactor (Sartorius Stedim Biotech Fermenter) to produce ethanol. The pH was maintained at 6.0 throughout the fermentation by the automatic addition of 0.5 M NaOH and 0.5 M HCl. The medium was supplemented with 1.70 g/L (NH4)2SO4, 0.96 g/L K2HPO4, 0.17 g/L MgSO4.7H2O, 0.23 g/L CaCl2.2H2O, and 0.02 g yeast extract. For fermentation, Pichia stipitis from overnight cultures was added and incubated in a bioreactor. Fermentation was conducted with 100 rpm for 48 h at 30 °C. Samples were taken out periodically (3, 6, 12, 24, and 48 h) and centrifuged at 10,000 × g for 10 min; then, the supernatants were filtered through 0.45-µm filters and stored at -20 °C until HPLC analysis. The fermentation experiments were performed in duplicate.
Biodegradable film production
The pre-extracted liquid was first filtered and then centrifuged (5000×g) for 5 min to completely remove small particles. Hemicellulose in liquid was precipitated with 2500 mL of refrigerated acetic acid-ethanol (1:10 v/v). The precipitate was desalted while washing with deionized water (200 mL) and ethanol (600 mL) three times. The hemicellulose left was dried at room temperature.
One gram of extracted xylan and mixtures of 0.6 g of xylan and 0.4 g of xylitol were stirred in deionized water (15 mL), and then the solutions were poured into polystyrene petri dishes (9 cm). The samples were dried in a conditioned room (50% relative humidity and 23 °C) for 3 days. In addition, 0.10 g of gluten and 0.025 g of nanocellulose (Özmen et al. 2013) were used in biodegradable film production to improve the mechanical properties.
The produced films were conditioned according to TAPPI standard method (T402 sp-08). The thickness of the samples was determined with a digital micrometer (40 EXL, Mahr GmbH, Esslingen, Germany) with a sensitivity of 0.1 μm. The tensile strength of the samples was tested (Zwick/Roell Z250 Strength Testing Device with 100 N/10 kN load cell). Samples for testing were prepared according to TAPPI standard method (T494 om-96). Elastic modulus and tensile strength are reported.
Pulping
As a control, conventional soda pulp was prepared from wheat straw to provide a basis for comparison with hot water (135 °C) pre-extracted pulps. On the other hand, the alkali and NaBH4 pre-extracted materials resulted in lower yields and mechanical pulps were produced from them according to method explained by Chang et al. 2012.
The liquor-to-straw ratio was 10:1. Regarding the optimum alkali pre-extraction condition, the soda pulps were cooked at 16.7% alkali charge. Furthermore, the samples were cooked to the target H factor of 460 at 160 °C for 65 min. The maximum temperature was achieved in 30 min. The H factor of the hot water pre-extraction was calculated and excluded from the total H factor. Pulps obtained from each cook were defibrillated in a laboratory disintegrator and thoroughly washed on a 200-mesh screen with warm (40 °C) tap water. To determine paper properties, the control and the hot water pre-extracted pulps were subjected to refining. A Waring blender (Chang et al. 2012) was used to evaluate the refining response, and pulps were refined for 0, 2, and 4 min. The freeness level of the pulps was determined according to ISO standard method (5267-1). Handsheets were produced in a Rapid Kothen machine in accordance with ISO standard method (5269-2). The paper physical properties were determined according to TAPPI standard method (T220 sp-01). The optical properties of the pulps were tested according to ISO standard methods (2469 and 2471).
Analytical tests
The straw for the study was sampled and prepared according to TAPPI standard method (T257 cm-02) for chemical tests. The ash content was determined by TAPPI standard method (T211 om-85). Alcohol-benzene, hot water, and 1% NaOH solubilities were examined according to TAPPI standard methods of T204 om-88, T207 om-88, and T212 om-88, respectively.
The yield determined by gravimetric measurements was calculated from the dry weight of the straw and the weight of the initial sample. The moisture content was obtained by drying the samples to a constant weight at 105 °C. The kappa number and the viscosity of the pulps was determined according to TAPPI standard method of T236 om-99 and SCAN standard method of cm 15:88, respectively.
The laboratory analytical procedures from the National Renewable Energy Laboratory (NREL) were used to determine the sugar (glucose, xylose, and arabinose) and lignin (acid-insoluble and -soluble) contents of the samples (Sluiter et al. 2004). The sugar and ethanol contents were determined using HPLC (Agilent 1200 series, USA) equipped with a Shodex SH1011 sugar column (mobile phase: 5 mM H2SO4, flow rate: 0.5 mL/min, column temperature: 60 °C) and a refractive index detector. The acid-insoluble lignin was obtained by weighing the solid samples. The acid-soluble lignin was analyzed at the adsorption of 320 nm against blank deionized water.
The percentage of solids recovered was calculated on an oven-dry basis as follows in Eq. 1,
Percentage of solids recovered = (W2 ÷ W1) × 100 (1)
where W1 is the dry weight of the sample before pretreatment (g) and W2 is the dry weight of the treated sample (g).
The reduction in lignin was calculated with respect to the initial dry weight of lignin in the untreated material (LU) and the dry weight of lignin in the remaining solids after treatment (LP). The percentage of lignin reduction was calculated with the following equation,
Percentage of lignin reduction = [(LU-LP) ÷ LU)] × 100 (2)
where LP is the dry weight of lignin in the pre-treated sample and LU is the dry weight of lignin in the untreated biomass.
In addition, the solubilization of glucan and xylan in the pre-treated samples was calculated in the same manner by substituting the appropriate percentage for glucan and xylan.
During fermentation, the ethanol yield was calculated as a percentage of the theoretical maximum yield (Kim and Lee 2005) using the following equation,
Percentage of theoretical ethanol yield = E ÷ (X × 0.511) × 100 (3)
where E and X represent ethanol (g) produced during fermentation and xylose (g) in the liquid, respectively. The constant 0.511 is the theoretical yield of ethanol produced from xylose and arabinose.
RESULTS AND DISCUSSION
Chemical Composition
Table 1 summarizes the chemical composition of the wheat straw and the literature values for hard/softwood (Fengel and Wegener 1984). The polysaccharides content (sum of glucose, xylose, and arabinose) was 60.1%, and lignin and ash accounted for 24% of the dry wheat straw. The wheat straw had higher extractives and ash contents but lower lignin and carbohydrate contents compared to hard/softwood. In addition, all solubility values of the wheat straw were noticeably higher than those of hard/softwood. In the straw structure, the main part of glucan was cellulose and xylan comprised most of the hemicelluloses.
Table 1. Chemical Composition of Wheat Straw and Hard/Softwood
The obtained data were within the range found in the literature (Mosier et al. 2005). Slight differences in values could be due to growth environment, soil conditions, or other factors (Utne and Hegborn 1992).
Hot Water Pre-Extraction
The loss percentages of weight, glucan, xylan, and lignin in the wheat straw pre-extracted with hot water at varying treatment temperatures are shown in Fig. 1. The results revealed that treatment temperature had a major effect and that an increase in treatment temperature removed more material from the structure. The weight loss in the straw resulting from the removal of hemicellulose, lignin, and other extraneous materials was greater for higher treatment temperatures, and when the material was pre-extracted at 150 °C, up to 38.5% of the material was removed from the structure. A material loss of almost 17% was reported by Hossein et al. (2012) when the straw was treated at 150 °C for 90 min.
Fig. 1. Glucan, xylan, lignin, and weight loss for hot water pre-extracted wheat straw at various treatment temperatures
For pre-extraction temperatures of 90 and 120 °C, greater lignin removal was observed compared to xylan removal. Similar amounts of xylan and lignin removal were obtained when the treatment temperature was raised to 135 °C. Above this treatment temperature (150 °C), a large amount of xylan (41.5%) was removed from the structure; however, the xylan removal ratio was higher than that of lignin. The removal of lignin with hemicelluloses could be attributed to the covalent bond chains of xylan and lignin in the straw structure. The removal of both components was almost linear. For a similar H factor (794), the hot water extraction of loblolly pine (Li et al. 2012) resulted in a weight loss of 15.0%, which is less than half as much as the wheat straw treated in this study (38.5%). This finding could be due to the lower degree of polymerization (DP) of the hemicelluloses of the straw (Antongiovanni and Sargentini 1991).
As can be seen in Fig. 1, the cellulose fraction was undamaged up to the treatment temperature of 135 °C. When the material was pre-extracted with hot water at 150 °C, 3.79% of the glucan was removed from the structure. The small amount of cellulose degradation observed in this study could be explained by the low cellulose crystallinity of straw (Liu et al. 2005).
Alkali (NaOH) Pre-Extraction
The major effect of alkali (NaOH) pre-extraction on hemicelluloses is the removal of lignin and acetyl as well as the various uronic acid groups. Figures 2a through 2d show the alkali pre-extraction results for removal of weight, glucan, xylan, and lignin from the wheat straw structure. The alkali used in this study was capable of reducing the contents of both hemicellulose and lignin from the straw structure under the applied treatment conditions. The alkali pre-extraction under various conditions resulted in weight losses ranging from 34.5% to 52.4% (Table 2). The results indicated that treatment temperature and alkali charge had a noticeable effect on the pre-extraction yield. An increase in both alkali charge and treatment temperature extracted more material from the straw structure. The rate of material extraction was lower for alkali concentrations of 26.7% to 33.3% compared to concentrations of 16.7% to 26.7% (Fig. 2a).
Fig. 2. (a) Weight loss, (b) glucan, (c) xylan loss, and (d) lignin loss in NaOH pre-extracted wheat straw at various treatment temperatures and chemical concentrations
The pre-extraction also dissolved some xylan in the straw structure. The results showed that both alkali charges and treatment temperatures greatly affected the xylan extraction (Fig. 2b) and increases in both resulted in higher xylan extraction from the structure. An increase in the alkali charge from 16.7% to 26.7% resulted in extraction of more xylan; however, the xylan extraction was lower for a higher (33.3%) alkali charge. The maximum xylan extraction was 59.2%, i.e., approximately 13.5% based on the original straw, when the wheat straw was treated at 90 °C and 33.3% alkali (NaOH) charge. In the literature, ground extracted wheat straw treated with 12% NaOH for 4 h at 80 °C resulted in the removal of 90% of the pentosans and 80% of the lignin (Cunningham et al. 1986). The lower xylan extractions in this study could be due to the straw size. In addition, nearly linear xylan extraction was observed when the treatment temperature was increased from 50 to 70 and 90 °C. One objective in this study was to optimize the pulping yield and paper properties after pre-extraction, and for that reason, some hemicelluloses had to be preserved in the material structure. The purpose was to collect pulp from pre-extracted material having almost equal pulp and paper properties of control soda pulp.
Table 2. Glucan, Xylan, Lignin, and Weight Loss of NaOH Pre-Extracted Wheat Straw at Varying Treatment Temperatures and Chemical Concentrations
As with xylan extraction, increases in treatment temperature and alkali charge removed more glucan from the straw structure. Compared with the original wheat straw, consisting of 34.9% glucose, glucan degradation ranged from 2.41% to 16.2% (based on OD straw), depending on the treatment temperature and alkali charge (Table 2). Even with alkali extractions performed at low treatment temperatures (lower than 100 °C), results in this study indicated that some delignification and degradation reactions occurred. The mild extraction temperatures could explain this lower glucan removal. Taherzadeh and Karimi (2008) observed similar results. Pulping potential and paper properties are all affected by cellulose degradation, and the lower glucan degradation in this study could improve paper properties produced from extracted solid materials.
The results displayed higher lignin removal from the straw structure for higher treatment temperatures and alkali charges (Fig. 2c). In this study, the rate of lignin removal was greater than that of xylan removal.
When the extraction rates of glucan, xylan, and lignin under varying treatment conditions were compared, it may be observed that the treatment temperature, as opposed to the alkali (NaOH) charge, was more effective. The improved solubility of the structural straw components with high alkali concentrations most likely occurred because the delignification reactions were more complete at higher alkali concentrations.
Optimized pre-extraction conditions could thus be set as a guide regarding hemicellulose extraction and pulp properties. The optimum treatment condition was determined for the rate of lignin and xylan removal from the structure; the optimum alkali pre-extraction charge was found to be 16.7% at 50 °C.
NaBH4-modified Alkali Extraction
The addition of a small amount of NaBH4 in alkali pulping improves pulping selectivity by preventing peeling reactions and hemicelluloses degradation (Copur et al. 2012). The NaBH4 utilized in this study was expected to preserve more glucan and xylan and to degrade lignin more selectively (Copur and Tozluoglu 2008). To gain a more efficient delignification, the alkali extraction was modified by adding 0.1%, 0.5%, 2%, and 4% (OD material) of NaBH4 at the optimum NaOH extraction conditions (16.7% NaOH at 50 C). The data in Fig. 3 show that modifying the alkali extraction with NaBH4 had only a slight effect on xylan degradation. The resulting glucan loss after modification was very small and thus was not reported in this study. On the other hand, the xylan loss was much higher than that of glucan. This finding could be explained by the chemical bonds of xylan and lignin in the straw structure (Yoon et al. 2008). The effect of modification on lignin delignification was remarkable; the addition of 0.1% NaBH4 removed 6.40% of the lignin from the straw structure. While the treatment with 2% NaBH4 led to a slight increase in xylan solubilization (1.60%), it resulted in more lignin delignification (10.2%) when compared to the weight loss of the straw without the addition of NaBH4. Statistical analysis indicated that adding NaBH4 to 16.7% NaOH had significant effect on weight (p<0.001), lignin (p<0.001) and xylose (p<0.01) losses.
Fig. 3. Xylose, lignin, and weight loss of NaBH4+NaOH pre-extracted wheat straw at various NaBH4chemical concentrations
Composition of Liquids and Ethanol Production
The liquid portion of some pre-extracted straw (hot water, alkali, and NaBH4– modified) was further examined for this study. The liquid portion had some oligomers, including glucan, xylan, arabinan, and water-soluble lignin. The yield of glucan in the liquid portion was very low, which indicated that the glucan was unhydrolyzed in pre-extraction. This could be explained by the mild treatment conditions utilized in this study.
Almost 94.1% of the xylan was recovered as monomeric sugar (xylose) when the straw was treated with hot water at 135 C. The higher treatment temperature (150 C) resulted in lower (81.3%) monomeric sugar recovery. The lowest xylose recovery (41.2%) was observed when the straw was treated with 16.7% alkali (Table 3). This variability in xylose recovery could be related to lignin solubilization, which impedes the recovery of xylose sugars (Mok and Antal 1992). Alkali treatment under mild conditions may prevent lignin condensation and result in high lignin solubility. In addition, mild treatment conditions inhibit conversion of degradation sugars to furfural, HMF, and organic acids (Chang and Holtzapple 2000), as was the case in this study. For this investigation, the xylose and arabinose sugars in the extraction liquids were converted to ethanol (Figs. 4a-4d). The theoretical ethanol yield for both 135 C (Fig. 4a) and 150 C (Fig. 4b) hot water pre-extracted straw was found to be 98%.
Table 3. Sugars in Pre-Extracted Solids and Liquids
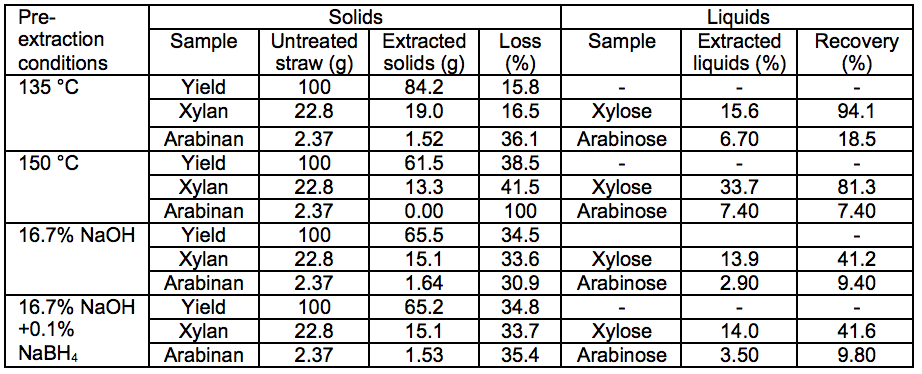
Fig. 4. The change in xylose, arabinose, and ethanol concentrations of (a) 135 C hot water pre-extracted, (b) 150 C hot water pre-extracted, (c) 16.7% NaOH pre-extracted, and (d) 16.7% NaOH + 0.1% NaBH4 pre-extracted wheat straw liquids fermented with Pichia stipitis
In the current study, ethanol yields of 7.79 (g/100 g soluble material) and 16.9% (g/100 g soluble material) were obtained, respectively. As seen in Fig. 4c, the liquid portion of the alkali-extracted straw (16.7% and 50 C) had a theoretical ethanol yield of 96.5%, and 6.81% (g/100 g soluble material) ethanol was obtained.
The lowest theoretical ethanol yield (59.2%) was observed when the straw was pre-extracted with 0.1% NaBH4 (Fig. 4d), and 4.22% (g/100 g soluble material) of ethanol was obtained for that sample. The lower xylose recovery and the alkali media in the samples could explain the lower ethanol yield in the alkali and NaBH4-modified pre-extracted samples.
Biodegradable Films
Films produced using only xylan developed cracks (Fig. 5), as was also observed in the literature (Gabrielli and Gatenholm 1998; Göksu 2005). To eliminate this problem, some xylitol (Seber 2010; Gonçalves 2011) was added, resulting in nice films (Fig. 5). The mechanical properties of the obtained films were low. However, they were improved by adding some gluten and nanocellulose (Table 4). Results indicated that even gluten addition improved the tensile strength. A dramatic increase was observed when small amounts (0.025 g) of nanocellulose were added to the solution (Table 4).
Table 4. Thickness and Mechanical Properties of Biodegradable Films
Fig. 5. Films obtained only using extracted xylan (left) and extracted xylan and xylitol (right)
Pulp and Paper Properties
Mill scale pulp of wheat straw is usually made using the soda method. The main problem after cooking is the high silica content in recovery (Schal et al. 2009). Mustajoki et al. (2010) pointed out that hot water pre-treated soda pulps resulted in better pulp bleachability. In the present study, to minimize the disadvantages of pulp production from wheat straw, the results obtained for the pre-extracted and the control soda pulps were evaluated. The properties of the control and the hot water (135 C) pre-extracted soda pulps, as well as the properties of the 16.7 % (OD straw) alkali pre-extracted and 0.1 % (OD straw) NaBH4-modified mechanical pulps, are given in Table 5.
Similar to the findings of Yoon et al. (2010), the hot water pre-extracted pulp gave a lower pulp yield (39.3% OD straw) as compared to the control soda pulp (47.6% OD straw). On the other hand, Hossein et al. (2012) found a higher pulp yield for pre-extracted straw soda-AQ pulps. As seen in Table 5, the control pulp had a much higher rejection rate (6.40% OD straw). The hot water pre-extracted soda pulp had slightly less xylose, but more lignin in the structure; this could be caused by the removal of some hemicelluloses with the lignin (LCC complexes) (Yoon et al. 2008). Similar to results reported by Zhang and Yang (2011), the hot water pre-extracted (135 C) soda pulp had lower viscosity than the control pulp; this could be due to the straw structure being more open to cooking chemicals that cause more cellulose degradation (Yoon et al. 2008). The hot water pre-extracted straw resulted in lower kappa pulp. Hot water pre-extraction could improve lignin reactivity and holes because hemicellulose removal from the structure may increase the ability of the cooking chemicals to reach the inner structures. The kappa and yield for the soda pulps obtained in this study were higher than the results in the literature (Fengel and Wegener 1984); this may be a consequence of the mild cooking conditions applied.
The alkali (16.7% OD straw) pre-extracted and NaBH4 (0.1% OD straw) modified mechanical pulps had similar yields. The results showed that NaBH4 modification preserved cellulose (55.7% OD pulp) and decreased lignin (13.0% OD pulp) content.
The material after alkali (16.7% OD straw) pre-extraction and NaBH4 (0.1% OD straw) modification resulted in lower glucose and xylose contents compared to the hot water (135 C) pre-extraction. On the other hand, there was a much higher lignin content in the structure. The reason for this may be that the pre-extraction temperature and the lower temperature (50 C) in the alkali and NaBH4 modifications dissolved less lignin from the straw structure. In contrast, the alkali utilized in pre-extraction as well as the NaBH4 modification dissolved more cellulose and xylose.
The soda pulps (control and hot water pre-extracted) were refined to 0, 2, and 4 min and the mechanical pulps (16.7% alkali pre-extracted and 0.1% NaBH4-modified) were fiberized for 10 min prior to hand-sheet preparation. The freeness, mechanical (tensile, tear and burst), and physical (brightness and opacity) properties of the pulps were measured, and the results are given in Table 6.
Table 5. Data on Pulps
The refining response of the hot water pre-treated pulp was lower than that of the control soda pulp. This could be due to the lower hemicellulose content of the hot water pre-extracted straw, causing lower swellability of the fibers (Yoon et al. 2008; Zang and Yang 2011).
Refining resulted in better mechanical properties (tensile and burst) for the soda pulps, which may be due to the fibrillation of the fibers during refining. On the other hand, the tear index value slightly improved at first, but then decreased with refining intensity. This decrease might be attributed to the damage to individual fibers during refining. The control soda pulp exhibited higher tensile and burst properties compared to the hot water pre-extracted soda pulp. These higher tensile and burst strength values could be explained by better fiber flexibility because of improved inter-fiber bonding of the higher hemicellulose content of the control soda pulp (Fengel and Wegener 1984). When the chemical and mechanical pulps were compared, the mechanical pulps had lower tensile and burst index values. This finding could be explained by the higher lignin content of the mechanical pulps, which had lower fiber flexibility and inter-fiber bonding (Çöpür 2007; Fengel and Wegener 1984).
Table 6. Paper Properties
* Pre-extracted soda chemical pulps
**Pre-extracted mechanical pulps
The tear value usually depends on individual fiber strength, but infrequently can depend on the degree of inter-fiber bonding. Refining decreases the tear strength of pulps after reaching an initial maximum. The control soda pulp with higher xylose content was expected to exhibit lower tear strength; however, similar tear index values for the control and the hot water pre-extracted soda pulps were observed in this study.
When the physical properties of the paper were considered, the control soda pulp was brighter than the hot water pre-extracted soda pulp. Although refining appeared to have had little effect on pulp brightness and opacity, both properties were diminished slightly.
The control soda pulp gave better brightness than the hot water pre-extracted chemical pulp and 16.7% NaOH pre-extracted mechanical pulp. This finding could be due to the higher lignin content of mechanical pulp and the effect of heat in hot water pre-extracted pulp.
Mass Distribution of Pre-Extracted Compounds
The mass balance was performed on the basis of 1000 kg OD wheat straw, which was extracted with hot water under the conditions of 135 °C for 4 h (Fig. 6). The results indicated that approximately 26 kg of xylan could be extracted from 1000 kg of OD wheat straw. Conversely, 2.48 kg of glucan was lost and 25 kg of lignin was delignified during the pre-extraction. The dissolved sugars were fermented to obtain bio-ethanol; 12.3 kg of ethanol was produced in this study.
The extracted solid wheat straw was cooked with the soda method, and 373 kg of straw pulp was produced. The pulp consisted of 253 kg of glucan, 93 kg of xylan, and 21 kg of lignin. In contrast, 412 kg of soda pulp was obtained when the control cook was accomplished for the equal H factor of the pre-extracted soda cook. The control soda pulp had 276 kg of glucan, 107 kg of xylan, and 18 kg of lignin in the structure. Compared to the control pulp, the hot water pre-extracted pulp had almost 10% lower xylan in the structure. Furthermore, the hot water pre-extraction resulted in 373 kg of soda pulp in addition to the 12.3 kg of ethanol.
Fig. 6. Mass balance for hot water pre-extracted wheat straw
CONCLUSIONS
- The results indicated that cellulose fraction was undamaged up to the treatment temperature of 135 °C. Consequently, the hot water pre-extraction at 135 C for 4 h provided the optimum pre-extraction conditions. Under other conditions carried out in this study, more than 30% of the xylan in the structure was dissolved.
- The hot water pre-extracted pulp gave a slightly lower pulp yield, but exhibited comparable mechanical and physical properties. On the other hand, ethanol (12.3 kg/1000 kg of straw) was produced from the hot water pre-extracted hydrolyzate.
- Biodegradable films produced using only xylan with hot water pre-extraction resulted in crack formation. Adding gluten and nanocellulose gave nice film formation and dramatically improved the mechanical properties of the biodegradable films.
ACKNOWLEDGMENTS
The authors are grateful for the support of the The Scientific and Technological Reseach Council of Turkey (TUBITAK-project no: 111O502).
REFERENCES CITED
Al-Dajani, W. W., and Tschriner, U. W. (2008). “Pre-extraction of hemicellulose and subsequent kraft pulping. Part I: Alkaline extraction,” TAPPI J. 7(6), 3-8. DOI: 10.1515/hf.2010.064
Antongiovanni, M., and Sargentini, C. (1991). “Variability in chemical composition of straws. Options Mediterraneenness: Serie A,” Seminaires Mediterraneens 49-53.
Atwell, W. A. (2001). Wheat Flour, Eagan Press, St. Paul, MN.
Chang, V. S., and Holtzapple, M. T. (2000). “Fundamental factors affecting biomass enzymatic reactivity,” Enzyme Eng. and Biotechnol. 84-86, 5-37. DOI: 10.1385/ABAB:84-86:1-9:5
Chang, X. F., Olson, J. A., and Beatson, R. P. (2012). “A comparison between the effects of ozone and alkaline peroxide treatments on TMP properties and subsequent low consistency refining,” BioResources 7(1), 99-111. DOI: 10.15376/biores.7.1.0099-0111
Çöpür, Y. (2007). “Refining of polysulfide pulps,” J. Appl. Sci. 7(2), 280-284.
Copur, Y., and Tozluoglu, A. (2008). “A comparison of kraft, PS, kraft-AQ and kraft-NaBH4 pulps of brutia pine,” Bioresour. Technol. 99(5), 909-913. DOI: 10.1016/j.biortech.2007.04.015
Copur, Y., Tozluoglu, A., and Ozyurek, O. (2012). “Sodium borohydrate (NaBH4) pretreatment for efficient enzymatic saccharification of wheat straw,” Bioresour. Technol. 107, 258-266. DOI: 10.1016/j.biortech.2011.12.076
Cunningham, R. L., Carr, M. E., and Bagby, M. O. (1986). “Hemi-cellulose isolation from annual plants,” Biotechnol. Bioeng Symp. 17, 159-168.
DOI: 10.1385/ABAB:124:1-3:1119.
Ergudenler, A., and Isigigur, A. (1994). “Agricultural residues as a potential resource for environmentally sustainable electric power generation in Turkey,” Renewable Energy 5(2), 786-790. DOI: 10.1016/0960-1481(94)90088-4
Fengel, D., and Wegener, G. (1984). “Wood Chemistry Ultrastructure, Reactions, Vol 1, Walter de Gruyter, Berlin.
Gabrielli, I., and Gatenholm, P. (1998). “Preparation and properties of hydrogels based on hemicelluloses,” J. Appl. Polym. Sci. 69(8), 1661-1667. DOI: 10.1002/(SICI)1097-4628(19980822)69:8
Göksu, E. I. (2005). Hemicellulose Based Biodegradable Film Production, M.S. thesis, METU, Ankara, Turkey.
Gonçalves, A. M. d. C. (2011). Preparation and Evaluation of Material Properties of Biofilms from Spruce Xylan, Integrated Master in Chemical Engineering, Chalmers University of Technology, Göteborg, Sweden.
Helbert, W., Cavaille, J. Y., and Dufresne, A. (1996). “Thermoplastic nanocomposites filled with wheat straw cellulose whiskers. 1. Processing and mechanical behavior,” Polym. Compos. 17(4), 604-611.
Hossein, R., Hossein, K., Fadavi, F., and Feizmand, M. (2012). “Effect of hot-water and mild alkaline extraction on soda AQ pulping of wheat straw,” Lignocellulose 1(1), 71-80.
ISO 2469. (2014). “Paper, board and pulps — Measurement of diffuse radiance factor (diffuse reflectance factor),” International Organization for Standardization, Geneva, Switzerland.
ISO 2471. (2008). “Paper and board — Determination of opacity (paper backing) — Diffuse reflectance method,” International Organization for Standardization, Geneva, Switzerland.
ISO 5267-1. (1999). “Pulps–Determination of drainability–Part 2: Schopper-Riegler method,” International Organization for Standardization, Geneva, Switzerland.
ISO 5269-2. (2004). “Pulps — Preparation of laboratory sheets for physical testing — Part 2: Rapid-Köthen method,” International Organization for Standardization, Geneva, Switzerland.
Kim, T. H., and Lee, Y. Y. (2005). “Pretreatment of corn stover by soaking in aqueous ammonia,” Appl. Biochem. Biotechnol. 121-124, 1119-1131.
Kumar, P., Yadava, R. K., Gollen, B., Kumar, S., Verma, R. K., and Yadav, S. (2011). “Nutritional contents and medicinal properties of wheat. A review,” Life Sci. Med. Res. Volume 2011:LSMR-22.
Li, H., Saeed, A., Jahan, M. S., Ni, Y., and Van Heiningen, A. R. P. (2010). “Hemicellulose removal from hardwood chips in the pre-hydrolysis step of the kraft-based dissolving pulp production,” J. Wood Chem. Technol. 30(1), 48-60.
Li, K., Lee, Y. Y., Yoon, S.-H., Smith, A. J., and Krishnagopalan, G. A. (2012). “Ethanol production from the mixture of hemicellulose prehydrolysate and paper sludge,” BioResources 7(3), 3607-3626. DOI: 10.15376/biores.7.3.3607-3626
Liu, R. G., Yu, H., and Huang, Y. (2005). “Structure and morphology of cellulose in wheat straw,” Cellulose 12, 25-34. DOI: 10.1007/s10570-004-0955-8.
Mok, W. S., and Antal Jr., M. J. (1992). “Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water,” Ind. Eng. Chem. Res. 31(4), 1157-1161. DOI: 10.1021/ie00004a026
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pretreatment of lignocellulosic biomass,” Bioresour. Technol.96(6), 673-686. DOI:10.1016/j.biortech.2004.06.025
Mustajoki, S., Leopniemi, A., and Dahl, O. (2010). “Alkaline peroxide bleaching of hot water treated wheat straw,” BioResources 5(2), 808-826.
Özmen, N., Çetin, N.S., and Narlıoğlu, N. (2013). “Production of cellulose nanocrystallites from poplar wood (Populus nigra x Populus deltoides),” SDU Faculty of Forestry Journal, 14, 58-63.
Rajoka, M. I. (2005). “The enzymatic hydrolysis and fermentation of pretreated wheat straw and bagasse to ethanol,” Afr. Tech. Dev. Forum 2(2), 29-35.
SCAN cm-15:88. (1988) “Pulps – Viscosity in cupri-ethylenediamine solution,” Scandinavian Pulp, Paper and Board Testing Committee, Sweden.
Schal, N., Krüger, E., Blum, R., and Rubenacker, M. (2009). “Soda-AQ pulping of wheat straw and its blending effect on old corrugated cardboard pulp properties,” Tappsa J. 30-35.
Seber, A. G. (2010). Preparation of Antimicrobial Films From Agricultural Biomass, M.S. thesis, Middle East Technical University (METU), Turkey.
Slavin, J. (2003). “Why whole grains are protective: Biological mechanisms,” Proc. Nutr. Soc. 62(1), 129-134. DOI:10.1079/PNS2002221 Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2004). Determination of Structural Carbohydrates and Lignin in Biomass, Biomass Analysis Technology Team Laboratory Analytical Procedures, National Renewable Energy Laboratory, Golden, CO.
Taherzadeh, M. J., and Karimi, K. (2008). “Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review,” Int. J. Mol. Sci. 9(9), 1621-1651.
Talebnia, F., Karakashev, D., and Angelidaki, I. (2010). “Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation,” Bioresour. Technol. 101(13), 4744-4753. DOI:10.1016/j.biortech.2009.11.080
TAPPI T204 om-88. (1988). “Wood extractives in ethanol-benzene mixture,” TAPPI Press, Atlanta, GA.
TAPPI T207 om-88. (1988). “Water solubility of wood and pulp,” TAPPI Press, Atlanta, GA
TAPPI T211 om-85. (2002). “Ash in wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T212 om-02. (2002). “One percent sodium hydroxide solubility of wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T220 sp-01. (2001). “Physical testing of pulp handsheets,” TAPPI Press, Atlanta, GA.
TAPPI T236 om-99. (1999). “Kappa number of pulp,” TAPPI Press, Atlanta, GA.
TAPPI T257 cm-02. (2002). “Sampling and preparing wood for chemical analysis,” TAPPI Press, Atlanta, GA
TAPPI T402 sp-08. (2008). “Standard conditioning and testing atmospheres for paper board, pulp handsheets and related products,” TAPPI Press, Atlanta, GA.
TAPPI T494 om-96. (1996). “Tensile properties of paper and paperboard,” TAPPI Press, Atlanta, GA.
Utne, B., and Hegbom, L. (1992). “Microscopy studies of wheat straw and rice straw as raw materials for the pulp and paper industry,” Second International Non-wood Fiber Pulping and Papermaking Conference Proceedings, China Technical Association of the Paper Industry, Beijing, p. 583.
Van Heiningen, A. (2006). “Converting a kraft pulp mill into an integrated forest biorefinery,” Pulp Pap.-Can. 107(6), 38-43.
Xing, R., Qi, W., and Huber, G. W. (2011). “Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries,” Energy Environ. Sci. 4, 2193-2205. DOI:10.1039/C1EE01022K
Yasin, M. A., Bhutto, W., Bazmi, A. A., and Karim, S. (2010). “Efficient utilization of rice-wheat straw to produce value added composite products,” Int. J. Chem. Env. Eng. 1(2), 136-143.
Yoon, S. H., Tunc, M. S., and Van Heiningen, A. (2010). “Near-neutral pre-extraction of hemicelluloses and subsequent kraft pulping of southern mixed hardwoods,” TAPPI J. 10(1), 7-15.
Yoon, S., Macevan, K., and Van Heiningen, A. (2008). “Hot-water pre-extraction from loblolly pine (Pinus taeda) in an integrated forest products biorefinery,” TAPPI J. 7(6), 27-31.
Zhang, S., and Yang, H. (2011). “Effect of hot water pre-extraction on alkaline pulping properties of wheat straw,” Adv. Mater. Res. 236-238, 1174-1177. DOI: 10.4028/www.scientific.net/AMR.236-238.1174
Article submitted: June 17, 2015; Peer review completed: September 23, 2105; Revised version received and accepted: September 28, 2015; Published: October 5, 2015.
DOI: 10.15376/biores.10.4.7834-7853
