Abstract
Diverse renewable resources, especially those obtained from residual agricultural wastes, are being exploited to reduce the impact of environmental damage. This study presents a method to produce purified cellulose extracted from locally planted pineapple leaves (Ananas comosus). The cellulose was extracted by maceration pretreatment. The heating times were varied. This method is a simpler and more effective approach to delignify the pineapple leaf fibers compared with conventional chemical pulping and bleaching processes. The chemical composition of the cellulose was investigated according to TAPPI standards and by structural analyses, namely Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD). The results indicated that the hemicellulose and lignin were partially removed from the cellulose. Chemical analysis confirmed that the cellulose content increased from 25.8% (pineapple leaf fibers) to 70.9% (macerated cellulose). The optimum heating time was 3 h. However, XRD showed that the extracted cellulose had a higher crystallinity index than the initial pineapple leaf fibers. These results indicated that pretreatment via maceration has good potential applications in the production of macerated cellulose.
Download PDF
Full Article
Isolation and Characterization of Macerated Cellulose from Pineapple Leaf
Naziratulasikin Abu Kassim,a Ainun Zuriyati Mohamed,a,* Edi Syams Zainudin,b,* Sarani Zakaria,c Siti Khaulah Zakiah Azman,a and Hazwani Husna Abdullah a
Diverse renewable resources, especially those obtained from residual agricultural wastes, are being exploited to reduce the impact of environmental damage. This study presents a method to produce purified cellulose extracted from locally planted pineapple leaves (Ananas comosus). The cellulose was extracted by maceration pretreatment. The heating times were varied. This method is a simpler and more effective approach to delignify the pineapple leaf fibers compared with conventional chemical pulping and bleaching processes. The chemical composition of the cellulose was investigated according to TAPPI standards and by structural analyses, namely Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD). The results indicated that the hemicellulose and lignin were partially removed from the cellulose. Chemical analysis confirmed that the cellulose content increased from 25.8% (pineapple leaf fibers) to 70.9% (macerated cellulose). The optimum heating time was 3 h. However, XRD showed that the extracted cellulose had a higher crystallinity index than the initial pineapple leaf fibers. These results indicated that pretreatment via maceration has good potential applications in the production of macerated cellulose.
Keywords: Pineapple leaf; Macerated cellulose; Pretreatment
Contact information: a: Pulp and Paper & Pollution Control Program, Laboratory of Biopolymer and Derivatives, Institute of Tropical Forestry and Forest Products, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; b: Faculty of Engineering, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; c: Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia; *Corresponding authors: ainunzuriyati@upm.edu.my, edisyams@upm.edu.my
INTRODUCTION
Cellulose is the most abundant polymer available worldwide and is a renewable source of raw materials (Klemm et al. 2005). Cellulose can be obtained from many lignocellulosic agricultural byproducts that are substantial and inexpensive. These byproducts have suitable chemical compositions, anatomical structures, and properties for use in composite, textile, and pulp and paper manufacturing. They can also be used to produce fuel, chemicals, enzymes, and food.
Byproducts from the cultivation of corn, wheat, rice, sorghum, barley, sugarcane, pineapple, banana, oil palm, and coconut are the major sources of agro-based biofibers (Reddy and Yang 2005). Agricultural crop residues such as leaf, stem, cob, husk, coir, bagasse, frond, fruit bunch, and other parts have been widely studied in terms of their cellulose characteristics and potential applications in advanced products.
Pineapple is planted in Malaysia for domestic distribution and export purposes. In 2006, the total pineapple cultivation area was about 8,731 hectares, comprised of 6,380 hectares from the farm sector and 2,351 hectares from the mills. The Malaysian Pineapple Industry Board reported an annual positive increase in the area of planted pineapple (MOA, 2011). Malaysia is one of the world’s largest producers of pineapple. During harvesting, pineapple plant leaves are discarded. Approximately 80 to 85% of pineapple leaves were wasted from 2008 to 2010 (Yusof et al. 2015). However, pineapple leaf is an abundantly available potential source of cellulose.
Micro-scale and nano-scale cellulose can be produced from pineapple leaves and then used as reinforcement agents in plastic (Kengkhetkit and Amornsakchai 2012) or in pulp and paper making (Arimu et al. 2015), composites (Mangal et al. 2003), textiles, and biomedical applications (Cherian et al. 2011). According to Kengkhetkit and Amornsakchai (2012), pineapple leaf fiber that is isolated by different methods has potential applications similar to other natural fibers in plastic reinforcement or in sound and thermal insulation. Composites of soy-based plastics and pineapple leaf fibers have good characteristics in terms of mechanical properties (Wanjun et al. 2005). The dynamic mechanical properties are enhanced by the addition of pineapple leaf fibers and continue to increase with increased pineapple leaf fiber loading. Furthermore, tensile strength and the modulus of composites increase with the pineapple leaf fiber content.
Numerous methods can be applied to isolate highly pure cellulose, including delignification and alkali extraction, steam explosion, alkaline peroxide extraction, organic solvent extraction, hydrochloric acid, a mixture of acetic acid and nitric acid (as catalyst), oxidative pretreatments (addition of an oxidizing compound such as hydrogen peroxide), and ammonia and carbon dioxide pretreatment (addition of external acid to improve the effect of thermal steam or liquid hot water) (Hendriks and Zeeman 2009; Liu and Sun 2010).
Maceration is a well-known term that is used in many fields especially in food processing technology. The process involves soaking stage using a mixture or solution, leading to softening of materials or fibers. The purpose is to ease the separation into smaller elements. Maceration of pineapple leaf fiber using acetic acid and hydrogen peroxide with the aid of high temperature helps to solubilize hemicellulose and lignin, disrupts the cellulose structure, and enhances accessibility of the cellulose for further chemical treatment (Kim et al. 2017). Hydrogen peroxide is broadly used in the bleaching process; it reacts with lignin effectively when 70-90 °C temperature conditions are applied (Zeinaly et al. 2009). Pulping with an organic acid such as acetic acid is also an effective method to delignify and fractionate fibers. Acetic acid is the most effective at delignification and removal of non-cellulose polysaccharides and also does not have any undesirable effects on cellulose properties such as intrinsic viscosity (Liu and Sun 2010). Peracetic acid (PAA) is also a conventional non-chlorine bleaching agent; it is a powerful oxidizing agent and is quite selective towards the lignin structure. Practically, it is prepared by the reaction of acetic acid and hydrogen peroxide. It oxidizes the aromatics in lignin, generating dicarboxylic acid and their lactones (Zhao et al. 2007).
Pretreatment is an important stage that is based on the successive combination of chemical and mechanical treatments prior to producing high purified cellulose, especially from pineapple leaf fibers as a lignocellulosic source (Deepa et al. 2011). The common pretreatment methods take longer periods of time due to the sequences involved. Pretreatment can include preparation of raw materials, alkali treatment stage, bleaching stage, and steam explosion to the fibers.
In this study, pineapple leaf cellulose was extracted after pretreatment by maceration with a range of heating times. The chemical and structural properties of the cellulose were examined. The aim of this research was to develop a simpler isolation process in gaining macerated cellulose or purified cellulose and to study its properties relative to potential applications in products.
EXPERIMENTAL
Materials
The pineapple leaves of MD2 cultivar were harvested from the Malaysian Pineapple Industry Board (MPIB) plantation area in Banting, Selangor, Peninsular Malaysia. Hydrogen peroxide and acetic acid were purchased from Sigma-Aldrich (Missouri, USA). Ethanol and toluene for extraction, sodium chlorite, and acetic acid were applied for holocellulose determination, while sodium hydroxide and sulphuric acid were used for α-cellulose and lignin determination accordingly. These chemicals were purchased from J. T. Baker (Pennsylvania, USA) and SYSTERM (Shah Alam, Malaysia).
Preparation of Macerated Pineapple Leaf Cellulose
Pineapple leaf fibers were prepared using green (G) and dried (D) leaves. These materials were compared to find the best condition for producing pineapple cellulose. Both types of leaves were cut into 1 cm × 3 cm uniform pieces. The green leaves were immersed in water prior to the boiling stage and were boiled in water until the leaves remained at the bottom of the beaker. The water boiling stage is very important to release air from the leaves, which softens them. Dried leaves were prepared by drying the leaves at 60 °C for 48 h. During the next stage—pretreatment via maceration—approximately 60 g of oven-dried pineapple leaf fibers were immersed in 200 mL of a mixture of hydrogen peroxide (H2O2, 37%) and acetic acid (CH3COOH, glacial) and incubated at 80 °C to 85 °C for 2 h, 3 h, and 4 h. The pineapple leaf cellulose was washed and air-dried for 48 h. After pre-treatment, pineapple leaf cellulose was ground, sieved, and characterized by its chemical composition and structural properties. In general, there are two types of fibers prepared in this study namely, green and dried fibers. Fibers with and without treatment contain a T or UT, respectively, in the sample name. The numbers 2, 3, and 4 indicate the maceration time in hours. Therefore, the 8 samples prepared in the study are listed as GUT, GT2, GT3, GT4, DUT, DT2, DT3, and DT4.
Characterization of Pineapple Leaf Cellulose
Chemical analysis
Fibers were ground and sieved to pass through BS 40 mesh (425 µm) but be retained at BS 60 mesh (250 µm). The chemical compositions of pineapple leaves with and without treatments were examined according to TAPPI standard methods for extractives (TAPPI T 264 cm-97 1997), holocellulose (TAPPI T 19 m-54 1954), α-cellulose (TAPPI T 203 cm-99 1999), and lignin (TAPPI T 222 om-98 2008). The moisture content (TAPPI T 210 om-58 1991). All tests were done in three replicates. The chemical analysis was performed for both ground pineapple leaf (before the maceration process) and cellulose after maceration.
Scanning electron microscopy (SEM)
The surface observation of the untreated and treated cellulose obtained at different duration of maceration time was analyzed using a JEOL JSM-6400 scanning electron microscope. Each sample was dried and sputtered with gold prior to SEM analysis.
Fourier transform infrared (FTIR) spectroscopy
All samples were dried, ground, and made into pellets using potassium bromide. The spectra were recorded from 4,000 cm-1 to 650 cm-1 at 4 cm-1 resolution on an FTIR spectrophotometer (Spectrum-100, Perkin Elmer, Massachusetts, USA).
X-ray diffraction
The crystallinity of all samples was tested by placing the milled powder in the sample holder prior to testing. The samples were analyzed using an X-ray diffractometer (D8 Advance, Bruker AXS Germany, Karlsruhe, Germany) at room temperature with a monochromatic CuK radiation source ( = 0.15406 nm) in the step scan mode with a 2 angle ranging from 10° to 50° with a step of 0.025 and scanning time of 0.1 s/step. To characterize the crystallinity of the diferent samples, the crystallinity index, CrI, was determined based on the reflected intensity data according to the method of Segal et al. (1959),
CrI (%) = I002 – Iam / I002 x 100 (1)
where I002 is the maximum intensity of the (002) lattice diffraction peak and Iam is the intensity scattered by the amorphous part of the sample. The diffraction peak for plane (002) is located at diffraction angle 2= 22°, and the intensity scattered by the amorphous part was measured as the lowest intensity at a diffraction angle 2 = 18°.
RESULTS AND DISCUSSION
Effect of Maceration Time on Chemical Composition of Pineapple Leaf Fibers
The chemical composition of pineapple leaves at different maceration heating times is shown in Fig. 1. The pineapple leaf without treatment consisted of 18.2% extractives, 34.6% hemicelluloses, 25.8% cellulose, and 20.3% lignin. After treating the leaves with CH3COOH and H2O2 mixture based on different heating time, the cellulose content increased, while lignin, hemicelluloses, and extractives decreased as expected. As reported by Daud et al. (2013) and Cherian et al. (2011), the lowest and highest cellulose content of pineapple leaf without treatment ranges between 66% and 81%. However, in this study, the α-cellulose content fiber without treatment was only 25.8%. This is expected because the leaf used in this study had not undergone any elimination of the unwanted tissues at the surface of the leaf. Pineapple leaves are comprised of vascular systems that are small and bound together by pectin (Rahman 2011). The pineapple leaf without any elimination of the upper surface is waxy due to the presence of the pulpy tissues in the leaf, which can be well separated by extraction processes such as scrapping, retting, or decorticating (Rahman 2011; Wan Nadirah et al. 2012). As shown in Fig. 1, the α-cellulose composition declined for T4. This may have been due to cellulose degradation after a certain time of maceration. The maceration treatment affected the content of hemicelluloses, which was reduced to 8.5%, 11.0%, and 15.0% for the different heating times of samples T2, T3, and T4, respectively. This was caused by cleavage of the ester-linked substances of hemicelluloses (Sheltami et al. 2012).
Table 1 presents the lignin, hemicellulose, and cellulose contents of various natural fibers, including pineapple leaf fiber data from this study. The cellulose content of pineapple leaves is higher than red grape skin fiber and lower than other fiber sources. Laftah and Abdul Rahaman (2015) conducted a study on chemical pulping of pineapple leaf by applying a mixture of less concentrated acetone. By introducing certain pressures during pulping process, pulp yielded differently, which includes the differences of lignin content. This can be detected via the appearance of a brownish color on the surface of the produced paper. In this study, the lignin content was reduced from 20.3% to 5.9% of UT and treated cellulose for T4 treatment.
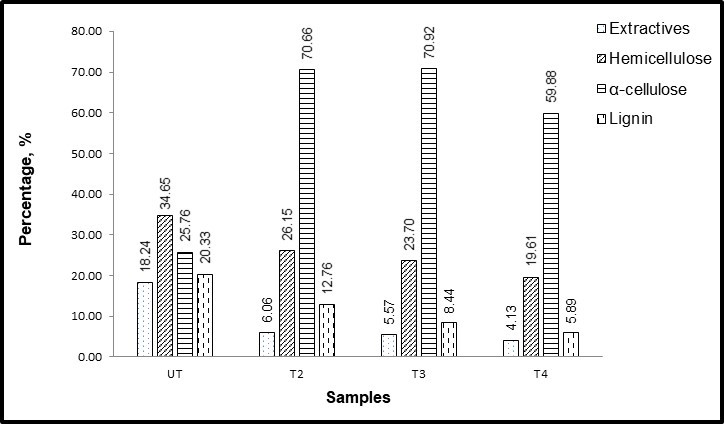
Fig. 1. Chemical composition of pineapple leaf fiber and pineapple leaf cellulose with and without pretreatment
Table 1. Chemical Compositions of Other Lignocellulosic Sources
Effect of Maceration Time on Morphology of Pineapple Leaf Fibers
The effect of pretreatment on the morphological of the untreated and treated pineapple leaf was obtained from SEM observation, as shown in Fig. 2. The cellulose diameters were measured as 6.13 µm, 3.23 µm, 3.66 µm, and 2.79 µm for UT, T2, T3, and T4, respectively. Based on the results, it was shown that the cellulose reduction in terms of fiber width occurred after completing the maceration process. The micrographs also showed that pretreatment or maceration time were sufficient to cause individualization of the fibers. This also means that the cementing materials, namely lignin and hemicellulose, were removed, resulting in the individual fibrils form (Fig. 2 b-d).
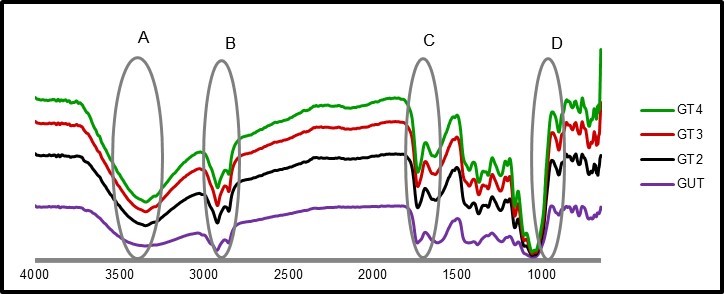
Fig. 2. SEM micrographs of dried pineapple leaf that have been undergone with and without treatment (A: UT, B: T2, C: T3, D: T4) at 100 and 1,000 magnifications.
Effect of Maceration Time on Chemical Changes of Pineapple Leaf Fibers
Fourier transform infrared spectroscopy is used to observe chemical changes in materials. As shown in Fig. 2 and Fig. 3, the peaks indicated slight differences between the green and dried leaf fibers.
Region A
Region A of the spectrum, for all samples, represents a range from 3500 to 3200 cm-1. The broad bands in this range represented free O-H stretching vibration of the OH groups in cellulose molecules. The presence of O-H indicates the presence of moisture content where hydroxyl is found in cellulose, hemicellulose, and lignin. Both samples of 3 h and 4 h treatments with acetic acid and hydrogen peroxide in both green and dried leaves exhibited narrower peaks in this region compared to the fibers without treatment. This result suggested that more lignin was dissolved during the treatment. Lignin is less hygroscopic than hemicellulose and amorphous cellulose.

Fig. 2. FTIR spectroscopy for untreated and treated samples of green leaf
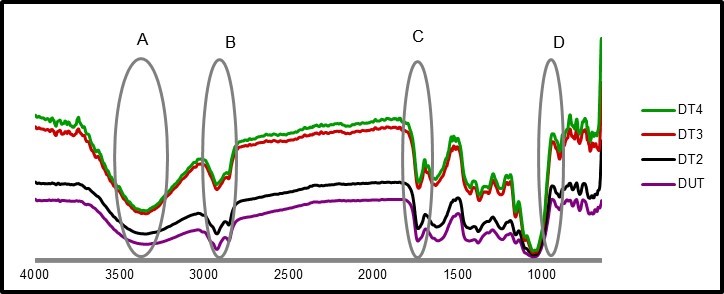
Fig. 3. FTIR spectroscopy for untreated and treated samples of dried leaf
Region B
Absorbances in the spectral range of 2880 to 2925 cm-1 were attributed to aliphatic saturated C-H stretching associated with methylene groups in cellulose.
Region C
Lignin is one of the important components in fiber; it binds cellulose together as a matrix. Lignin contains carbonyl, phenol hydroxyl, aromatic rings, and methoxyl functional groups. The spectral range at 1724-1736 cm-1 was assigned to the C=O stretching of the acetyl. The absorption at the spectrum area of 1730 cm-1 for the untreated sample could be attributed to uronic ester groups of residual hemicelluloses or to the ester linkage of carboxylic group of the ferulic and ρ-coumaric acids of lignin. It disappeared for chemical heated treatment due to the removal of the lignin and hemicellulose.
Region D
Lignin has characteristic bands in the range of 1500 to 1600 cm-1 due to aromatic ring vibrations. Untreated fiber absorbed at 1510 cm-1, and this absorbance was absent from the spectra of treated fibers due to the elimination of lignin (Lamaming et al. 2015).
Effect of Maceration Time on Crystallinity of Pineapple Leaf Fibers
XRD studies of the untreated and treated pineapple leaf fibers for green and dried leaves were implemented to investigate the influence of maceration treatment time on the crystalline behavior of each fiber.
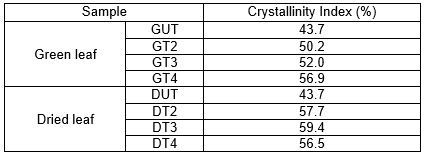
Table 2. Crystallinity Index of Pineapple Leaf Fibers at Different Treatment

In all samples, the crystalline index was higher for treated than for untreated leaves, which were attributed to partial removal of the hemicelluloses and lignin during the treatment. The compatible crystallinity indices are listed in Table 2. In all samples, diffraction peaks in the range of 16° to 22° indicated that the native cellulose I crystal structure was preserved (Liu et al. 2016). The 2θ value was located at 22.25° for green leaves and 22.26° for dried leaves, which was related to the crystalline structure of cellulose I. However, the amorphous region was characterized by the 2θ values of 17.66° and 17.79° for green and dried leaves, respectively. The increment of crystallinity indices for all treated samples may be due to more removal of non-cellulosic polysaccharides and dissolution of the amorphous regions (Cherian et al. 2010) except for DT4. This situation was directly expected because excessive time for chemicals attack at higher temperature (80°C) leads to devastation of carbohydrate fractions (Zhang et al. 2010). The distraction of crystalline oriented arrangement destroys the cellulose internal structure. There were slight increments in the crystallinity values. Pretreatment affects the crystallinities and polymorphs of the cellulose. Therefore, it also affected the yield and influenced the morphology of the nanoparticles (Yang and Zhong 2013). The crystallinity index of untreated pineapple leaf fibers in this study was higher than that reported for leaf sheath (Uma Maheswari et al. 2012) and coconut husks (Rosa et al. 2010).
CONCLUSIONS
This study suggested a simple method for the extraction of cellulose from pineapple leaves pretreated via maceration, with a range of heating times. The fundamental properties of cellulose such as morphology, crystallinity, dimension, and surface chemistry varied, depending on the raw material and extraction process. Chemical analysis, FTIR, and XRD showed that the cellulose produced in this study was comparable to cellulose that is treated by conventional processes such as bleaching or acid hydrolysis.
The chemical analysis confirmed higher percentage of cellulosic component and lower non-cellulosic components in the treated cellulose for 2, 3, and 4 h compared to untreated leaf. These results indicated that the pretreatment is successful in producing cellulose, which can be used in other applications. The cellulose content of pineapple leaf fibers increased from about 26% (in untreated specimens) to the range 60 to 71%, depending on the composition of the native fiber.
Hemicellulose and lignin content were reduced in the treated samples. The XRD results demonstrated increased crystallinity in treated samples.
FTIR analysis demonstrated that the maceration treatment successfully removed the hemicellulose and lignin content in the extracted or macerated cellulose.
XRD results exhibited an increment of crystallinity index of all treated cellulose (extracted or macerated cellulose).
In sum, by conducting this technique, the extracted or macerated cellulose showed wide potential use of cellulose such as in the reinforcement of biocomposites or specialty papers.
ACKNOWLEDGEMENTS
The authors thank MPIB for the pineapple leaves used in the experiments and the INTROP Pulp and Paper Lab members for technical guidance. The authors also would like to thank Ministry of Higher Education for Malaysia Higher Institution Centres of Excellence (HiCOE) for research financial support.
REFERENCES CITED
Arimu, M. O., Rafiu, M. A., and Adedeji, K. K. (2015). “Pulp and paper production from Nigerian pineapple leaves and corn straw as substitute to wood source,” International Research Journal of Engineering and Technology 2 (4), 1180-1188.
Cherian, B. M., Leão, A. L., de Souza, S. F., Costa, L. M. M., de Olyveira, G. M., Kottaisamy, M., Nagarajan, E. R., and Thomas, S. (2011). “Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications,” Carbohydrate Polymers 86(4), 1790-1798. DOI: 10.1016/j.carbpol.2011.07.009.
Cherian, B. M., Leão, A. L., de Souza, S. F., Thomas, S., Pothan, L. A., and Kottaisamy, M. (2010). “Isolation of nanocellulose from pineapple leaf fibres by steam explosion,” Carbohydrate Polymers 81(3), 720-725. DOI: 10.1016/j.carbpol.2010.03.046.
Daud, Z., Zainuri, M., Hatta, M., Sari, A., Kassim, M., Awang, H., and Aripin, M. A. (2013). “Analysis the chemical composition and fiber morphology structure of corn stalk,” Australian Journal of Basic and Applied Sciences 7(9), 401-405.
Deepa, B., Abraham, E., Cherian, B. M., Bismarck, A., Blaker, J. J., Pothan, L. A., Leao, A. L., de Souza, S. F., and Kottaisamy, M. (2011). “Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion,” Bioresource Technology 102(2), 1988-97. DOI: 10.1016/j.biortech.2010.09.030.
Hendriks, A. T. W. M. and Zeeman, G. (2009). “Pretreatments to enhance the digestibility of lignocellulosic biomass,” Bioresource Technology 100, 10-18. DOI: 10.1016/j.biortech.2008.05.027.
Hsieh, Y. (2013). “Cellulose nanocrystals and self-assembled nanostructures from cotton, rice straw and grape skin : A source perspective,” Journal of Materials Science 48, 7837-7846. DOI: 10.1007/s10853-013-7512-5.
Kengkhetkit, N. and Amornsakchai, T. (2012). “Utilisation of pineapple leaf waste for plastic reinforcement: 1. A novel extraction method for short pineapple leaf fiber,” Industrial Crops and Products 40, 55-61. DOI: 10.1016/j.indcrop.2012.02.037.
Kim, D., Orrego, D., Ximenes, E. A., and Ladisch, M. R. (2017). “Cellulose conversion of corn pericarp without pretreatment, ” Bioresource Technology 245, 511-517. DOI: 10.1016/j.biortech.2017.08.156.
Klemm, D., Heublein, B., Fink, H. P., and Bohn, A. (2005). “Cellulose: Fascinating biopolymer and sustainable raw material,” Angewandte Chemie International Edition 44(22), 3358-3393. DOI: 10.1002/anie.200460587.
Laftah, W. A. and Abdul Rahaman, W. A. W. (2015). “Chemical pulping of waste pineapple leaves fiber for kraft paper production,” Journal of Materials Research and Technology 4(3), 254-261. DOI: 10.1016/j.jmrt.2014.12.006.
Lamaming, J., Hashim, R., Sulaiman, O., Leh, C. P., Sugimoto, T., and Nordin, N. A. (2015). “Cellulose nanocrystals isolated from oil palm trunk,” Carbohydrate Polymers 127, 202-208. DOI: 10.1016/j.carbpol.2015.03.043.
Liu, C.-F., and Sun, R.-C. (2010). “Cellulose,” in: Cereal straw as a Resource for Sustainable Biomaterials and Biofuels (1st Ed.), R.-C. Sun (ed.), Elsevier, Amsterdam, Netherlands, pp. 131-168. DOI: 10.1016/B978-0-444-53234-3.00005-5.
Liu, C., Li, B., Du, H., Lv, D., Zhang, Y., and Yu, G. (2016). “Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods,” Carbohydrate Polymers 151, 716-724. DOI: 10.1016/j.carbpol.2016.06.025.
Luzi, F., Fortunati, E., Puglia, D., Lavorgna, M., Santulli, C., Kenny, J. M., and Torre, L. (2014). “Optimized extraction of cellulose nanocrystals from pristine and carded hemp fibres,” Industrial Crops and Products 56, 175-186. DOI: 10.1016/j.indcrop.2014.03.006.
Mangal, R., Saxena, N. S., Sreekala, M. S., Thomas, S., and Singh, K. (2003). “Thermal properties of pineapple leaf fiber reinforced composites,” Materials Science and Engineering: A 339(1-2), 281-285. DOI: 10.1016/S0921-5093(02)00166-1.
MOA (Ministry of Agriculture and Agro-Based Industry Malaysia). 2011. Agrofood Statistics. Retrieved October 21, 2017, from http://www.moa.gov.my/c/document_library/get_file?uuid=c953e8c1-b8fb-4a02-95b6-6365693e32c1&groupId=43204.
Rahman, A. M. (2011). “Study on modified pineapple leaf fibre,” Journal of Textile and Apparel, Technology and Management 7(2), 1-16.
Reddy, N. and Yang, Y. (2005). “Biofibres from agricultural byproducts for industrial applications,” Trends in Biotechnology 23(1), 22-7. DOI: 10.1016/j.tibtech.2004.11.002.
Rosa, M. F., Medeiros, E. S., Malmonge, J. A., Gregorski, K. S., Wood, D. F., Mattoso, L. H. C., Glenn, G., Orts, W. J., and Imam, S. H. (2010). “Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior,” Carbohydrate Polymers 81(1), 83-92. DOI: 10.1016/j.carbpol.2010.01.059.
Segal, L., Creely, J. J., Martin, A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer,” Textile Res. J. 29(10), 786-794. DOI: 10.1177/004051755902901003.
Siqueira, G., Bras, J., and Dufresne, A. (2010). “Luffa cylindrica as a lignocellulosic source of fiber, microfibrillated cellulose, and cellulose nanocrystals,” BioResources 5(2), 727-740.
Sheltami, R. M., Abdullah, I., Ahmad, I., Dufresne, A., and Kargarzadeh, H. (2012). “Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius),” Carbohydrate Polymers 88(2), 772-779. DOI: 10.1016/j.carbpol.2012.01.062.
Sonia, A. and Priya Dasan, K. (2013). “Chemical, morphology and thermal evaluation of cellulose microfibers obtained from Hibiscus sabdariffa,” Carbohydrate Polymers 92(1), 668-674. DOI: 10.1016/j.carbpol.2012.09.015.
TAPPI Method T 264 cm-97. (1997). “Preparation of wood for chemical analysis,” Technical Association of the Pulp and Paper Industry, Atlanta, GA, USA.
TAPPI Method T 19 m-54. (1954). “Holocellulose in wood,” Technical Association of the Pulp and Paper Industry, Atlanta, GA, USA.
TAPPI Method T 203 cm-99. (1999). “Alpha-, beta-, and gamma- cellulose in pulp,” Technical Association of the Pulp and Paper Industry, Atlanta, GA, USA.
TAPPI Method T 222 om-98. (1998). “Acid-insoluble lignin in wood and pulp,” Technical Association of the Pulp and Paper Industry, Atlanta, GA, USA.
TAPPI Method T 210 om-58. (1991). “Weighing, sampling and testing pulp for moisture,” Technical Association of the Pulp and Paper Industry, Atlanta, GA, USA.
Uma Maheswari, C., Obi Reddy, K., Muzenda, E., Guduri, B. R., and Varada Rajulu, A. (2012). “Extraction and characterization of cellulose microfibrils from agricultural residue – Cocos nucifera L.,” Biomass and Bioenergy 46, 555-563. DOI: 10.1016/j.biombioe.2012.06.039.
Wan Nadirah, W. O., Jawaid, M., Al Masri, A. A., Abdul Khalil, H. P. S., Suhaily, S. S., and Mohamed, A. R. (2012). “Cell wall morphology, chemical and thermal analysis of cultivated pineapple leaf fibres for industrial applications,” Journal of Polymers and the Environment 20(2), 404-411. DOI: 10.1007/s10924-011-0380-7.
Wanjun, L., Manjusri, M., Per, A., Lawrence, T. D., and Amar, K. M. (2005). “’Green’ composites from soy based plastic and pineapple leaf fiber: Fabrication and properties evaluation,” Polymer 46(8), 2710-2721. DOI: 10.1016/j.polymer.2005.01.027.
Yang, D. and Zhong, X. P. L. (2013). “Effects of pretreatments on crystalline properties and morphology of cellulose nanocrystals,” Cellulose 20, 2427-2437. DOI: 10.1007/s10570-013-9997-0.
Yusof, Y., Yahya, S. A., and Adam, A. (2015). “Novel technology for sustainable pineapple leaf fibers productions,” Procedia CIRP 26, 756-760. DOI: 10.1016/j.procir.2014.07.160.
Zeinaly, F., Deghani, M., and Mirmehdi, M. (2009). “Bleaching of kenaf bast soda pulp with alkali peroxide,” Iranian Journal of Wood & Forest Science and technology 16(1), 105-113.
Zhao, X., Wang, L., and Liu, D. (2007). “Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis,” Journal of Chemical Technology & Biotechnology 82(12), 1115-1121. DOI: 10.1002/jctb.1775.
Zhao, X., van der Heide, E., Zhang, T., and Liu, D. (2010). “Delignification of sugarcane bagasse with alkali and peracetic acid and characterization of the pulp,” BioResources 5(3), 1565-1580. DOI: 10.15376/biores.5.3.1565-1580
Article submitted: October 5, 2017; Peer review completed: December 4, 2017; Revised version received and accepted: August 20, 2018; Published: December 19, 2018.
DOI: 10.15376/biores.14.1.1198-1209
