Abstract
Ball-milled rice straw was dissolved in a lithium chloride/dimethyl sulfoxide (LiCl/DMSO) solvent system, regenerated, and subjected to enzymatic hydrolysis to obtain regenerated cellulolytic enzyme lignin (RCEL). The structure of the isolated lignin was characterized by elemental analysis, gel permeation chromatography (GPC), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), and proton nuclear magnetic resonance (1H NMR). Alkaline nitrobenzene oxidation (NBO) was conducted to analyze the structural characteristics of the in-situ lignin. The results showed that the rice straw RCEL was composed of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) phenylpropane units, with relatively high amounts of H units. The yield of RCEL is about 5% units higher than that of cellulolytic enzyme straw lignin (CEL) on the basis of total lignin in the original rice straw. When compared to the CEL obtained by the traditional method, there were no observed differences versus RCEL in terms of the elemental compositions, NBO product yields, and S/G ratio. The weight-average molecular weight of RCEL was 6835, which was lower than that of CEL, indicating that some rice straw lignin linkages were cleaved during LiCl/DMSO dissolution.
Download PDF
Full Article
Isolation of Cellulolytic Enzyme Lignin from Rice Straw Enhanced by LiCl/DMSO Dissolution and Regeneration
Wenjuan Wu,a,b,* Zhiguo Wang,a Yongcan Jin,a Yuji Matsumoto,b and Huamin Zhai a
Ball-milled rice straw was dissolved in a lithium chloride/dimethyl sulfoxide (LiCl/DMSO) solvent system, regenerated, and subjected to enzymatic hydrolysis to obtain regenerated cellulolytic enzyme lignin (RCEL). The structure of the isolated lignin was characterized by elemental analysis, gel permeation chromatography (GPC), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), and proton nuclear magnetic resonance (1H NMR). Alkaline nitrobenzene oxidation (NBO) was conducted to analyze the structural characteristics of the in-situ lignin. The results showed that the rice straw RCEL was composed of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) phenylpropane units, with relatively high amounts of H units. The yield of RCEL is about 5% units higher than that of cellulolytic enzyme straw lignin (CEL) on the basis of total lignin in the original rice straw. When compared to the CEL obtained by the traditional method, there were no observed differences versus RCEL in terms of the elemental compositions, NBO product yields, and S/G ratio. The weight-average molecular weight of RCEL was 6835, which was lower than that of CEL, indicating that some rice straw lignin linkages were cleaved during LiCl/DMSO dissolution.
Keywords: Rice straw; LiCl/DMSO; Cellulolytic enzyme lignin (CEL); Regeneration
Contact information: a: Jiangsu Provincial Key Lab of Pulp and Paper Science and Technology, Nanjing Forestry University, Nanjing 210037, China; b: Department of Biomaterial Sciences, Graduate School of Agricultural & Life Sciences, The University of Tokyo, Tokyo 113-8657, Japan;
* Corresponding author: wenjuanwu@njfu.edu.cn
INTRODUCTION
Lignin, a major wood component along with cellulose and hemicelluloses, is comprised of three different p-hydroxycinnamyl alcohol precursors: coniferyl, sinapyl and p-coumaryl alcohols (Boerjan et al. 2003; Higuchi 1997; Ralph et al. 2004). The structure and biosynthesis pathways have not yet been completely elucidated (Boerjan et al. 2003). A primary problem in characterizing the structure of native lignin is that it cannot be chemically isolated without significantly altering its in situ structure (Ikeda et al. 2002). Milled wood lignin (MWL) has been widely used to elucidate the chemical structure of lignin (Capanema et al. 2005; Holtman et al. 2006). However, whether the information obtained from MWL is representative of the protolignin is still unclear because the yields of MWL are low (Hu et al. 2006). Cellulolytic enzyme lignin (CEL) has been found to be structurally similar to milled wood lignin, but it can be obtained in a higher yield and is therefore more representative of the native wood lignin (Chang et al. 1975). Recently, a new and facile method, using a combination of enzymatic and mild acidic hydrolysis, was established by Wu and Argyropoulos (2003), and the lignin isolated from this method was termed enzymatic mild acidolysis lignin (EMAL). It has been reported that EMAL is more representative of the overall lignin present in milled wood, and this isolation process offers higher yields and purities of lignin than those obtained by MWL and CEL (Guerra et al. 2006; Wu and Argyropoulos 2003).
Generally, cellulose is highly crystalline and a large proportion of it is inaccessible to enzymes (Chandra et al. 2007). The enzymatic hydrolysis efficiency can be significantly improved by first dissolving the fiber in an ionic liquid solvent followed by subsequent regeneration (Papa et al. 2012; Sun et al. 2013). Wang et al. (2009) reported a novel dissolution system for lignocellulosic biomass; wood samples milled for 2 h using a planetary ball mill were completely dissolved in an ionic LiCl/DMSO solvent system. The resulting lignin structure did not change significantly during the ball milling. In this paper, a modified method is presented for the isolation of lignin in high yield from rice straw. In contrast with the conventional preparation of straw CEL, the regenerated straw CEL included the total dissolution of ball-milled straw in a LiCl/DMSO solvent system, followed by its regeneration and enzymatic hydrolysis. We have carried out comparative studies of the chemical structure of both CEL and RCEL from rice straw by elemental analysis, gel permeation chromatography (GPC), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), and proton nuclear magnetic resonance (1H NMR). As one of the most important wet chemical methods employed for elucidating the structure of lignin, alkaline nitrobenzene oxidation (NBO) was also used in this work to characterize the lignin structure of rice straw.
EXPERIMENTAL
Materials
Rice straw (Oryza sativa L.) collected at a farm near Tokyo, Japan was used as the raw material. The straw sample was ground and the 40 to 80 mesh fraction collected. The straw meal was extracted with a mixture of benzene:ethanol (2:1, v/v) for 8 h to remove most organic solvent-soluble extractives. The primary chemical components of the extractive-free rice straw are listed in Table 1.
Table 1. Chemical Compositions of Extractive-free Rice Straw Powder, w/w% on a Bone Dry Basis
Methods
Ball milling
The vacuum-dried, extractive-free rice straw meal (2 g per bowl) was milled in a planetary ball-mill (Fritsch GMBH; Idar-Oberstein, Germany) for 1 h. A zirconium dioxide bowl (45 mL) with 18 zirconium dioxide balls (1 cm in diameter) was used, and the mill rotated at 600 rpm. The milling was conducted in a cold room (-20 °C), and a 5-min cooling break was provided after every 15 min of milling to prevent overheating.
DMSO/LiCl dissolution and regeneration
An ionic solvent system of dimethyl sulfoxide (DMSO) containing 8% lithium chloride (LiCl/DMSO, w/w) was used for the dissolution of the straw sample. A rice straw sample after 1 h of ball milling was suspended into the LiCl/DMSO solvent system at a concentration of 7.5% (w/w); this suspension was first stirred at room temperature for 48 h and then stirred at 60 °C for 24 h. The dissolved rice straw material was regenerated by placing the reaction mixture in deionized water and washing the resulting regenerated cellulose in a dialysis tube until no chloride (Cl–) was detected in the dialysate. The regenerated material was freeze-dried for subsequent enzymatic hydrolysis.
Isolation and purification of lignin
The isolation procedure for MWL, CEL, and RCEL is summarized in Fig. 1.
Fig. 1. Isolation procedure for MWs ytic enzyme lignin (cL, CEL, and RCEL
Rice straw milled wood lignin (MWL) was isolated according to the standard method described by Björkman (1956). The ball milled straw was extracted with 96% 1,4-dioxane in water (twice, 24 h). After centrifuging, the supernatant was collected and evaporated. The residue was dried under vacuum at room temperature. The dried residue was dissolved in 90% acetic acid (10 mL for each gram of straw residue) and centrifuged, and the supernatant was slowly introduced into a 10 x volume of deionized water. The precipitated solid was centrifuged, washed with water three times until the odor of acetic acid had disappeared, and dried under a vacuum. The dried lignin was dissolved in a 2:1 mixture of 1,2-dichloroethane/ethanol. The solution was centrifuged, and the clear solution was introduced into a large volume of diethyl ether. This suspension was centrifuged, and the residue was washed with diethyl ether twice to obtain pure lignin.
Cellulolytic enzyme straw lignin (CEL) was isolated according to the method of Chang et al. (1975). Ten grams of ball-milled rice straw, either untreated or regenerated from LiCl/DMSO dissolution, was suspended in 200 mL of an acetate buffer (pH 4.8). Commercial cellulase (Celluclast 1.5L) and β-glucosidase (Novozyme 188) from Novo Nordisk A/S (Demark) were added to the suspension, which was then incubated at 50 °C for 72 h. The enzyme activity loading was based on the cellulose activity of 60 filter paper units (FPU) per gram of substrate. The ratio of cellulase to β-glucosidase was 1 FPU:1 CBU (cellobiose unit). The manufacturer specified activity of Celluclast 1.5L and Novozyme 188 were directly used to calculate loading. The enzymatic hydrolysis residue was separated from the hydrolysate by centrifugation and freeze-dried. The freeze-dried residue was extracted with 1,4-dioxane/water (96:4, v/v) to prepare a crude CEL or RCEL, which was then purified by the procedure described above for isolating straw MWL. Typical yields of MWL, CEL, and RCEL (w/w, based on the amount of original Klason lignin of rice straw) were 5.4%, 15.3%, and 20.1%, respectively.
Acetylation of lignin
The lignin samples were acetylated for determination of GPC and 1H NMR according to the method of Lundquist (1992).
Chemical composition analysis
The Klason method was used for the determination of Klason lignin (KL) and acid-soluble lignin (ASL) content in all straw samples (Dence 1992). Ash in the Klason residue was measured to correct the lignin content. Acid-soluble lignin was determined from the UV/VIS absorbance at 205 nm using a Shimadzu UV-240 UV-VIS spectrophotometer (Shimadzu Corporation; Kyoto, Japan). The hydrolysate from the Klason method was used for the sugar analysis according to the method described by Borchardt and Piper (1970).
Alkaline nitrobenzene oxidation analysis
The lignin aromatic structures were analyzed by alkaline nitrobenzene oxidation. Alkaline nitrobenzene oxidation of all samples was carried out according to procedures described by Chen (1992).
Elemental analysis
Elemental analysis was performed to determine the carbon, hydrogen, and nitrogen contents in the isolated lignin samples using a Vario EL III Elemental Analyzer (Elementar; Hanau, Germany). The oxygen content was calculated by the difference.
Thermogravimetric (TG) analysis
The thermal decomposition characteristics of the lignin samples were determined using a HTG-3 instrument (Henven; Beijing, China). The TG analysis was performed at a constant heating rate of 20 °C/min. up to 900 °C. An inert atmosphere was maintained by a 25-mL/min N2 flow.
Fourier transform-infrared (FT-IR) spectroscopy analysis
The FT-IR spectra of the lignin samples were recorded from a KBr disk containing 1% of the finely ground samples with a FTIR-650 spectrophotometer (Gangdong; Tianjin, China) over the wave number range of 400 to 4,000 cm-1.
Gel permeation chromatography (GPC) analysis
The weight-average (Mw) and number-average (Mn) molecular weights of these acetylated lignin preparations were determined using a Waters 1515 gel permeation chromatographer (GPC) equipped with a Waters 2414 refractive index detector (Milford, MA), using Styragel® HR1, HR3, and HR4 columns (7.8 mm × 300 mm). Calibration was performed using polystyrene standard samples (molecular weight range 100 to 500,000). Solutions of the lignin samples were prepared at 5 to 10 mg/mL in tetrahydrofuran (THF). The THF at 30 °C was used as the eluent at a flow rate of 1.0 mL/min.
X-ray diffraction (XRD) analysis
The straw samples were converted into pellets using a disk apparatus for IR measurement and subjected to X-ray diffraction analysis at diffraction angles 2θ from 0° to 30° using the reflection method. A Rigaku RINT 2000 (Rigaku Corporation; Tokyo, Japan) instrument was used with Ni-filtered Cu-Kα radiation (λ = 0.15418 nm) at 40 kV and 40 mA.
1H NMR spectroscopy analysis
The 1H NMR spectra were obtained using a JEOL Alpha 500 spectrometer (Tokyo, Japan). Deuterated chloroform (CDCl3) and tetramethylsilane (TMS) were used as the solvent and internal standard, respectively.
RESULTS AND DISCUSSION
Yield and Elemental Composition
Generally, the cellulose contained in lignocellulose is highly crystalline, and this characteristic protects it from chemical and biological degradation. Lignin is a highly cross-linked polymer based on aromatic phenylpropane units that binds cellulose and hemicelluloses, thus imparting rigidity and microbial resistance to the cell wall. One feasible method for lignin isolation includes the disruption of the cellulose’s crystallinity without degrading the lignin by its dissolution and regeneration in ionic liquids. In this study, two lignin samples were prepared from rice straw subjected to 1 h of ball milling. One lignin sample isolated from the straw that was dissolved in DMSO/LiCl (with 8% LiCl), diluted and washed with water to regenerate, and subsequently subjected to enzymatic hydrolysis. The other lignin sample was isolated from a similar sample, which was not dissolved and regenerated. The yield and purity of both isolated lignins were evaluated, and the results are presented in Table 2. The combination of ball milling and treatment with DMSO/LiCl led to a higher yield of lignin. The yield of isolated lignin from the rice straw just subjected to 1 h of ball milling (MWL) was only 5.4% due to the limited period of ball-milling time. The yield of CEL was 15.3%; this indicated that the removal of polysaccharides by enzymatic hydrolysis could effectively improve the dissolution of lignin in 96% 1,4-dioxane. Compared with CEL, rice straw RCEL exhibited a higher yield of 20.1%. This observation showed that the yield of isolated lignin could be further increased from straw by DMSO/LiCl dissolution and water regeneration. As the yield of rice straw MWL was the lowest among the three lignin isolation procedures, only the structural characteristics of CEL and RCEL were comparatively investigated in the next step to determine whether there are any differences between these two lignin samples.
As shown in Table 3, the elemental composition of RCEL was almost the same as that of CEL. These results confirmed that the isolation of lignin from the mild ball-milling of the straw after its dissolution in DMSO/LiCl and its regeneration in water is a more efficient isolation procedure.
Table 2. Isolation Yield and Sugar Content of Lignin Samples, w/w% (mean ± SD)
Table 3. Elemental Analysis of CEL and RCEL Isolated from Rice Straw, w/w%
Table 4. Average Molecular Weight and Polydispersity Index (Mw/Mn) of CEL and RCEL
Molecular Weight and Molecular Weight Distribution
The weight-average (Mw) and number-average (Mn) molecular weight of the lignin samples isolated from rice straw and their polydispersity (Mw/Mn) are given in Table 4. The weight-average molecular weight of the RCEL was 6835, which was clearly lower than that of CEL (8464). This difference might be caused by different contents of carbohydrate impurities. One hypothesis is that the carbohydrate chains linked to the isolated lignin can increase the hydrodynamic volume of lignin and therefore increase the lignin’s apparent molar mass during GPC analysis (Jääskeläinen et al. 2003). This is in line with the results of the carbohydrate analysis shown in Table 2. However, the total sugar in the RCEL was only about 0.2% higher than that in the CEL. A more reasonable explanation for this phenomenon might be the cleavage of some lignin linkages during the DMSO/LiCl dissolution. Both lignin samples presented a relatively narrow molecular weight distribution, as shown by the polydispersity index, Mw/Mn, which was about 1.7.
FT-IR Spectroscopy
The FT-IR spectra of the lignin samples are illustrated in Fig. 2. There were no significant differences for structural features between the CEL and the RCEL. The bands at 1598, 1506, and 1420 cm-1correspond to aromatic skeletal vibrations and are characteristic absorption patterns for isolated lignin. A wide absorption band at 3424 cm-1 originated from the O-H stretching vibration in aromatic and aliphatic OH groups, whereas the bands at 2932 and 2854 cm-1 are assigned to the C-H asymmetric and symmetrical vibrations in methyl and methylene groups, respectively. The absorption at 1656 cm-1, shown in Fig. 2, was attributed to the carbonyl stretching in conjugated p-substituted aryl ketones. The C-H deformation, combined with aromatic ring vibration, at 1454 cm-1, were present in both lignins. Syringyl and condensed guaiacyl absorptions were clearly visible at 1334 cm-1, whereas guaiacyl ring breathing with C=O stretching appeared at 1268 cm-1. This confirmed that the aromatic structure of lignin did not dramatically change during its regeneration from the DMSO/LiCl solvent system.
X-ray Diffraction Pattern
The effect of ionic dissolution and regeneration of ball-milled rice straw on the product crystallinity was investigated by X-ray diffraction. Figure 3 shows the crystallinity of the original rice straw, the ball-milled rice straw, and the rice straw after ball milling, dissolution, and regeneration of ball-milled rice straw. The I200 diffraction peak represents both crystalline and amorphous material, while the IAMdiffraction peak represents only the amorphous material (Segal et al. 1959). The degree of crystallization of the sample was defined as the ratio of the I200 – IAM difference to I200. The degree of crystallization calculated from these results was 50% for rice straw, 23% for ball-milled rice straw, and 33% for regenerated rice straw. Some carbohydrates, removed during dissolution and regeneration from the regenerated sample, may have originated from the amorphous region; hence, the value of regenerated straw was higher than that of the ball-milled sample. Generally, lignocellulosic materials with lower crystallinity are more affected by enzymatic saccharification (Chandra et al. 2007). It has been demonstrated that rice straw pretreatment with the DMSO/LiCl system improved the enzymatic hydrolysis of polysaccharides in ball-milled wood, thus increasing the yields of isolated lignin by aqueous 1,4-dioxane extraction.
| Fig. 2. FT-IR spectra of CEL and RCEL isolated from rice straw | Fig. 3. X-ray diffraction pattern of original rice straw (S), ball-milled rice straw (MS), and regenerated rice straw with ball milling (RMS) |
Table 5. Nitrobenzene Oxidation (NBO) Analysis and S/G Molar Ratio of Purified Lignin Samples (mean ± SD)
Effects of DMSO/LiCl Dissolution on the Monomeric Composition of Lignins
Alkaline nitrobenzene oxidation is commonly used for the structural analysis of lignin because it gives information about the constitutive monomeric composition of the lignin samples. Uncondensed p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units are primarily oxidized to their corresponding p-hydroxybenzaldehydes, vanillins, and syringaldehydes, respectively. Table 5 shows the proportion of the various phenylpropanoid units comprising the isolated rice straw lignin based on the nitrobenzene oxidation analysis. The H, G, and S values presented in Table 5 are the sum of p-hydroxybenzaldehyde and p-hydroxybenzoic acid, the sum of vanillin and vanillic acid, and the sum of syringyl and syringic acid, respectively. All NBO products were detected in each sample, which confirmed that the lignin in rice straw is a typical grass-type (i.e., HGS-type) lignin with significant amounts of p-hydroxyphenyl units. No obvious differences in NBO products, their yields, and H:G:S unit ratios was found for the CEL versus the RCEL.
Thermal Stability
The thermal stability of both lignins was investigated by thermogravimetric (TG) analysis. The results obtained are shown in Fig. 4. The TG curves indicate the weight loss of the lignin samples in relation to the temperature. The thermal degradation covers a wide temperature range for the CEL, between 270 and 900 °C, and a broader range for the RCEL. Also, the CEL started decomposing and reached a maximum thermal degradation degree at lower temperatures than did the RCEL. Thus, the RCEL was more thermally stable than the CEL. Sun et al. (2000) proposed that the thermal stability of lignin increases as its molecular weight increases. According to these authors, the greater degree of branching and condensation of the lignin macromolecule increases the thermal energy required for bond cleavage. The observed results from the TG analysis in this study are in line with the GPC analysis presented earlier on the molecular weight distribution of each lignin sample.
Fig. 4. Thermogravimetric analyses of CEL and RCEL
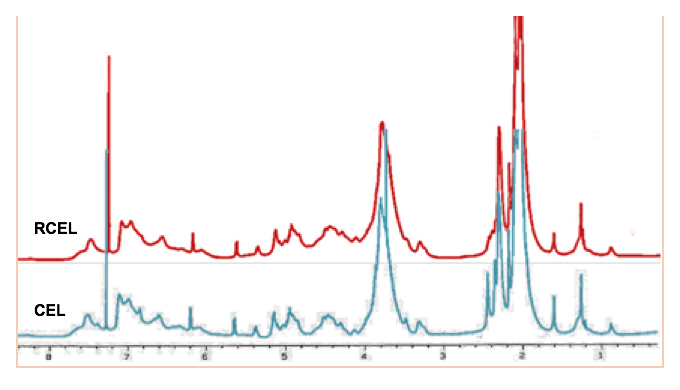
Fig. 5. 1H NMR spectra of CEL and RCEL
1H NMR Spectra
The structural features of the CEL and the RCEL were explored with 1H NMR spectroscopy as presented in Fig. 5. The signals between 1.60 and 2.22 ppm correspond to aliphatic acetate protons, while the peaks from 2.22 to 2.50 ppm can be attributed to aromatic acetate protons (Lundquist 1982). The syringyl, guaiacyl, and p-hydroxyphenyl units were characterized by signals at 6.25 to 6.80, 6.80 to 7.20, and 7.30 to 7.60 ppm, respectively. The strong peaks at 3.48 to 4.00 ppm originated from methoxyl protons.
CONCLUSIONS
- The combined procedure of ball milling, dissolution in DMSO/LiCl solvent, and regeneration in water, followed by enzymatic hydrolysis, made it possible to isolate a larger proportion of lignin from the rice straw than the procedure that does not include dissolution and regeneration.
- A detailed comparison of the lignin structure revealed no significant differences between these two lignin products. As this procedure recovers a large proportion of plant protolignin, it appears likely that the chemical structure of recovered lignin is more representative of the in situ lignin of the living plant.
ACKNOWLEDGMENTS
The authors are grateful for the financial support of the National Basic Research Program of China (973 Program, Grant No. 2010CB732205), the National Science Foundation of China (Grant Nos. 31070524, 31370571 and 31200444), and the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
REFERENCES CITED
Björkman, A. (1956). “Studies on finely divided wood. Part I. Extraction of lignin with neutral solvents,” Sven. Papperstidn. 59(2), 477-485.
Boerjan, W., Ralph, J., and Baucher, M. (2003). “Lignin biosynthesis,” Annu. Rev. Plant Biol. 54, 519-546.
Borchardt, L. G., and Piper, C. V. (1970). “A gas chromatographic method for carbohydrates as alditol-acetates,” TAPPI J. 53(2), 257-260.
Capanema, E., Balakshin, M., and Kadla, J. F. (2005). “Quantitative characterization of a hardwood milled wood lignin by nuclear magnetic resonance spectroscopy,” J. Agric. Food Chem. 53(25), 9639-9649.
Chandra, R. P., Bura, R., Mabee, W. E., Berlin, A., Pan, B., and Saddler, J. N. (2007). “Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics?” Adv. Biochem. Eng. Biotechnol. 108, 67-93.
Chang, H. M., Cowling, E. B., Brown, W., Adler, E., and Miksche, G. (1975). “Comparative studies on cellulolytic enzyme lignin and milled lignin of sweetgum and spruce,” Holzforschung 29(5), 153-159.
Chen, C. L. (1992). “Nitrobenzene and cupric oxide oxidations,” in: Methods in Lignin Chemistry, Lin, S. Y., and Dence, C.W. (eds.), Springer-Verlag, Berlin, pp. 301-321.
Dence, C. W. (1992). “The determination of lignin,” in: Methods in Lignin Chemistry, Lin, S. Y., and Dence, C. W. (eds.), Springer-Verlag, Berlin, pp. 33-61.
Guerra, A., Filpponen, I., Lucia, L., Saquing, C., Baumberger, S., and Argyropoulos, D. S. (2006). “Toward a better understanding of the lignin isolation process from wood,” J. Agric. Food Chem. 54(16), 5939-5947.
Higuchi, T. (1997). Biochemistry and Molecular Biology of Wood, Springer-Verlag, London.
Holtman, K., Chang, H. M., Jameel, H., and Kadla, J. (2006). “Quantitative 13C-NMR characterization of milled wood lignins isolated by different milling techniques,” J. Wood Chem. Technol. 26(1), 21-34.
Hu, Z. J., Yeh, T. F., Chang, H. M., Matsumoto, Y., and Kadla, J. F. (2006). “Elucidation of the structure of cellulolytic enzyme lignin,” Holzforschung 60(4), 389-397.
Ikeda, T., Holtman, K., Kadla, J. F., Chang, H. M., and Jameel, H. (2002). “Studies on the effect of ball milling on lignin structure using a modified DFRC method,” J. Agric. Food Chem. 50(1), 129-135.
Jääskeläinen, A. S., Sun, Y., Argyropoulos, D. S., Tamminen, T., and Hortling, B. (2003). “The effect of isolation method on the chemical structure of residual lignin,” Wood Sci. Technol. 37(2), 91-102.
Lundquist, K. (1992). “Proton (1H) NMR spectroscopy,” in: Methods in Lignin Chemistry, Lin, S. Y., and Dence, C. W. (eds.), Springer-Verlag, Berlin, pp. 242-249.
Papa, G., Varanasi, P., Sun, L., Cheng, G., and Stavila, V. (2012). “Exploring the effect of different plant lignin content and composition on ionic liquid pretreatment efficiency and enzymatic saccharification of Eucalyptus globulus L. mutants,” Bioresour. Technol. 117(2012), 352-359.
Ralph, J., Lundquist, K., Brunow, G., Lu, F. C., Kim, H., Schatz, P. F., Marita, J. M., Hatfield, R. D., Ralph, S. A., Christensen, J. H. and Boerjan, W. (2004). “Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids,” Phytochem. Rev. 3(1-2), 29-60.
Segal, L., Creely, J. J., Martin Jr., A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,” Text. Res. J.29(10), 786-794.
Sun, R. C., Tomkinson, J., and Lloyd, J. G. (2000). “Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre,” Polym. Degrad. Stab. 68(1), 111-119.
Sun, S., Li, M., Yuan, T., Xu, F., and Sun, R. C. (2013). “Effect of ionic liquid/organic solvent pretreatment on the enzymatic hydrolysis of corncob for bioethanol production. Part 1: Structural characterization of the lignins,” Ind. Crop. Prod. 43, 570-577.
Wang, Z., Yokoyama, T., Chang, H. M., and Matsumoto, Y. (2009). “Dissolution of beech and spruce milled woods in LiCl/DMSO,” J. Agric. Food Chem. 57(14), 6167-6170.
Wu, S., and Argyropoulos, D. S. (2003). “An improved method for isolating lignin in high yield and purity,” J. Pulp Pap. Sci. 29(7), 235-240.
Article submitted: February 18, 2014; Peer review completed: May 14, 2104; Revised version received and accepted: May 27, 2014; Published: June 5, 2014.
