Abstract
Ozone is a non-chlorine bleaching agent that can reduce pollution in the pulp bleaching stage. In this work the ozone bleaching of eucalyptus kraft pulp was performed as part of a kinetics study to explain factors affecting the properties of bleached pulp. The bleaching efficiency was closely related to the rates of mass transfer and self-decomposition, as well as the intensity of ozonation. For ozone bleaching of 3% consistency pulp, a brightness of 68% ISO, viscosity of 579 mL/g, and kappa values of 7.9 were achieved under an optimal condition with pH 2 and organic reagent NP-10 supplied. In this condition, the ozone mass transfer and intensity of ozonation were promoted, while self-decomposition declined.
Download PDF
Full Article
Kinetics of Ozone Bleaching of Eucalyptus Kraft Pulp and Factors Affecting the Properties of the Bleached Pulp
Tian He,a,# Mingyou Liu,a,# and Xiaofei Tian b,c,*
Ozone is a non-chlorine bleaching agent that can reduce pollution in the pulp bleaching stage. In this work the ozone bleaching of eucalyptus kraft pulp was performed as part of a kinetics study to explain factors affecting the properties of bleached pulp. The bleaching efficiency was closely related to the rates of mass transfer and self-decomposition, as well as the intensity of ozonation. For ozone bleaching of 3% consistency pulp, a brightness of 68% ISO, viscosity of 579 mL/g, and kappa values of 7.9 were achieved under an optimal condition with pH 2 and organic reagent NP-10 supplied. In this condition, the ozone mass transfer and intensity of ozonation were promoted, while self-decomposition declined.
Keywords: Ozone bleaching; Low consistency pulp; Kinetic studies
Contact information: a: State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou, CN 510641, China; b: School of Biology and Biological Engineering, South China University of Technology, Guangzhou, CN 510006, China; c: Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control, College of Light Industry and Food Engineering, Guangxi University, Nanning, CN 530004, China; # Co-first authors; *Corresponding author: xtien@scut.edu.cn
INTRODUCTION
Ozone has been widely applied in intensive delignification and bleaching pretreatment (Shatalov and Pereira 2006; Coca et al. 2016). To avoid inducing environmental pollution (Sheats et al.2010), ozone is considered to be an emerging substitution for conventional chlorine-based reagents for pulp bleaching (Rounsaville and Rice 1996;Arooj et al. 2014). With the rapid development of an effective ozone generator or ozone reaction kettle during the last two decades, an industrialized application of ozone could be realized (Freire et al. 2006; Arooj et al. 2014). The efficiency of ozone delignification relies on the ozone dosage supplied during the reaction (Bajpai 2012). However, due to the simultaneous occurrence of degradation of cellulose, the maximum dosage of ozone in the pulp should be limited to no more than 1.2% (v/w) (Shatalovand Pereira 2008; Coca et al. 2016).
Ozone bleaching is a complex mass transfer process including stages of solid chemical absorption and gas-liquid mass transfer, accompanied by self-decomposition and oxidation reactions (Seisto et al. 2000; Perincek et al. 2007). For ozone bleaching of low consistency pulp, the reactivity and selectivity of the ozonation reaction mainly depend on the efficiency of ozone transfer and diffusion within the reaction system (Cogo et al. 1999; Roncero et al. 2003a).
Due to low pulp consistency, fibers are more dispersed in the reaction system. Based on the two-film theory (Fig. 1) (Fang et al. 2008), a dilute-solution-chemical-absorption-diffusion model (Eq. 1) was introduced to simplify the mass transfer process (Cogo et al. 1999):
(1)
Terms used in each equation in the article are defined in a list that appears after the Conclusions section.
Fig. 1. Model of the two-film theory
The two-film theory is based on three hypotheses: (1) the existence of a stagnant interface between gas and liquid phases (thin film layers of stagnation are found close to each side of the interface) and diffusion of absorbing molecules that are transferred through this two-layered film from the gas into the liquid phase; (2) the equilibrium of the composition in gas and liquid phases at the interface; (3) the uniformity of the composition contained in the gas or liquid phases beyond the interface in fully turbulent fluids.
At a constant temperature, the equilibrium for partial-pressure of the ozone above a dilute ozone solution could be described by the Henry Theorem (Eqs. 2 and 3).
(2)
(3)
The amount of ozone transferred in the pulp can be calculated by Eqs. 4 and 5.
(4)
(5)
As ozone is nearly insoluble in water, it was supposed that the resistance of mass transfer could be formed on the film. In this case, KL≈ kL, and the value of H would be around zero. It would be necessary to reduce the film resistance to improve the transfer rate. In ozone bleaching for low consistency pulp, only a limited amount of ozone was provided with a high decomposition rate. Therefore, Eq. 5 could be converted to Eq. 6.
(6)
In contrast, solid chemical absorption plays an important role in effecting the reactivity and selectivity of ozone bleaching of high consistency pulp, as the oxidation reaction of lignin mainly occurred in the cell wall.
To better describe the process of pulp bleaching with high-concentration ozone, a solid-chemical-absorption-and-diffusion model was developed to simplify the mass transfer process (Fig. 2).
Fig. 2. Graphical model of the high-concentration ozone bleaching process (Fang et al. 2008)
a. Cellulose concentration change, b. ozone concentration change, c. lignin concentration change
The diffusion equation of ozone in the cell wall (Fick’s second law) can be found in Eqs. 7 through 9.
(7)
(8)
(9)
The equation of mass transfer is shown as Eqs. 10 and 11, which are simplified as Eq. 12.
(10)
(11)
(12)
It could be of significance to study the kinetics of ozone bleaching for improved efficiency and higher selectivity of delignification. By studying the kinetics of a bleaching system with different pulp consistencies, the research team can clearly evaluate the rates of mass-transfer and self-decomposition, as well as the intensity of ozonation during different bleaching processes. In the meantime, other factors such as pH and chemical additives, which also affect the properties of bleached pulp, may also need to be evaluated to observe optimal conditions for an application of the ozone bleaching technology.
EXPERIMENTAL
Materials
Eucalyptus kraft pulp (EKP) was obtained from a local paper mill (Nanning, Guangxi, China). The brightness, viscosity, and kappa value of the EKP sample were determined as 34.6% ISO, 880 mL/g, and 16, respectively.
Methods
Oxygen delignification (O)
60 g (dry weight, DW) of EKP was mixed with 700 mL H2O containing 10% (w/v) NaOH, 1% (w/v) MgSO4, and 30% (v/v) H2O2. The mixture was sealed in a 2-L reactor (Dawn Precision Instrument Co., Ltd, Yantai, China) and incubated at 100 °C for 100 min with stirring at 180 rpm. The pressure was 0.6 MPa at 100 °C. After the oxygen delignification, the pulp was washed thoroughly with distilled water until it reached pH 7.
Acid pretreatment (A)
After oxygen delignification, 30 g (DW) of EKP was sealed in a plastic bag containing 700 mL of H2O. The pH was adjusted to 2.0 by adding 2 M H2SO4. The mixture was incubated at 25 °C for 30 min.
Ozone bleaching of low-consistency EKP
Ozone was produced by a laboratory generator (KCF-SF100B, Koner, Jiangsu, China) using compressed wet air. A flow of 120 L/Nm3 ozone was pumped into a 2.5-L bleaching reactor (Fig. 3) through Teflon® tubes at the flow rate of 800 mL/min.
Fig. 3. Reactors for O3 bleaching (a) for low-consistency; and (b) for high- and medium-consistency
The reaction was performed at 25 °C with a stirring speed of 500 to 1500 rpm. After the reaction, the residual ozone was collected by passing through two 200-mL bottles containing 35 mL of 200 g/L KI solution. The concentration of the residual ozone in the gas was determined by titration of the generated iodine with 1 M Na2S2O3 standard solution (Torres et al. 2010). The mass of ozone reacted was calculated by Eq. 13.
Mass of reacted ozone (g) = Mass of the ozone supplied (g) – Mass of ozone collected (g) (13)
Ozone bleaching of medium- and high consistency EKP
After the acid treatment, the EKP was squeezed to a certain solid consistency. The pulp content applied for medium- and high-consistency bleaching were 15% and 35%, respectively. A total of 60 g (DW) of the pulp was loaded in and uniformly dispersed in the rotary reactor (Xiao et al. 2013) with a rolling speed of 60 r/min. The ozone flow was continuously pumped in the reactor at 800 mL/min and the bleaching process was performed at 25 °C. The consumption of the ozone during the reaction was determined and calculated as described above (Roncero et al. 2003b,c).
Analytical assays
The brightness of the bleached EKP was determined using a digital brightness meter (CTP-ISO, Technidyne Corporation, Inc., New Albany, IN, USA). Preparation of the sample was according to TAPPI T-452 om-08 (2008). The viscosity and kappa values were determined by the copper ethylenediamine method and KMnO4 method (Shi and He 2012), respectively. The ozonation performance (KLa, C*L, σ) and the efficiency (Eq. 14)were determined according to the method reported by Cogo et al. (1999). After ozone bleaching, the pulp was washed by distilled water three times. The decline of viscosity and kappa number of the pulp were calculated as Eqs. 15 and 16:
Efficiency = Degree of delignification /(ozone consumed/quantity of pulp) (14)
Decline of viscosity (%) = (Initial viscosity before bleaching – Viscosity after bleaching) / (initial viscosity) (15)
Decline of kappa (%) = (Kappa before bleaching- Kappa after bleaching) / (initial Kappa before bleaching) (16)
RESULTS AND DISCUSSION
Ozone Bleaching of the Low-, Medium- and High- Consistency EKP
During the ozone bleaching process, the self-decomposition of ozone could affect the bleaching efficiency (Roncero et al. 2003a). The self-dissociation rate from ozone can be obtained from Eq. 17:
![]() (17)
(17)
According to the models of ozone mass transfer (Eq. 13) and self-decomposition (Eq. 17) (Fariha et al. 2015), the rate of ozone bleaching would be independent from initial consistency of the pulp (Piccoli et al. 2014). To determine the effect of the pulp consistency on bleaching efficiencies, medium- and high- consistency pulp was used in the ozone bleaching process and properties of bleached pulp were determined. The results are shown in Table 1. The brightness and kappa of the pulp had no remarkable change while the viscosity of the bleached EKP declined gradually with an increase of the consistency.
Table 1. Effect of Pulp Consistency on the Properties of Bleached EKP
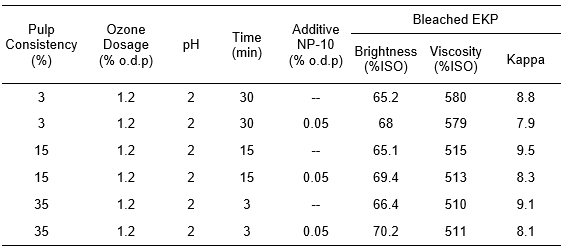
As shown in Table 1, the pulp brightness from low-consistency bleaching method was only slightly lower than that from the medium- or high- consistency bleaching process. However, it achieved the highest selectivity by low-consistency bleaching with the highest pulp viscosity but the lowest kappa value.
The NP-10 is an active polyoxyethylene-ether nonionic surfactant which promoted the accessibility of ozone to the fiber surface. Adding the NP-10 had no negative effect on the viscosities of the bleached pulps. It led to a remarkable increase of the brightness but a decrease of the kappa value. The application of the NP-10 led to a high delignification without dissolving the carbohydrate polymers.
According to Eq. 11, because the solubility of ozone in the water was extremely low, H was almost 0. The resistance from liquid films affected the mass-transfer rate of ozone. Therefore, the ozone bleaching of high-consistency EKP could be simplified to a solid-chemical-absorption process. According to the absorption equation (Eq. 12), the mass-transfer rate of ozone was closely related to the diffusion rate of ozone. Because the diffusion rate was independent from the initial pulp consistency, the initial pulp consistency had an insignificant effect on mass-transfer rate.
Although the same ozone mass-transfer rate was used, the ozone bleaching of low-consistency EKP required a longer reaction time than the high-consistency pulp for obtaining a similar brightness level (Cogo et al. 1999). This could be explained by the low reaction intensity between ozone and fiber as a prolonged reaction time of 30 to 60 min could achieve higher brightness through the low-consistency bleaching process.
During the low-consistency bleaching process, an intensive shearing force caused an acceleration of contacting frequency between the ozone and fibers through the formation of a stable fluidization of a three-phase system containing ozone, fiber, and water. The low-consistency pulp was favorable for efficient penetration for ozone molecules onto the single surface fiber due to reduced adhesion of the fibers. As the dissolubility of ozone in the water phase was extremely low, the accumulation of the ozone in the aqueous mixture could cause rapid decomposition. For the efficient reaction of ozone with fiber in bleaching of low-consistency pulp, the self-decomposition of the ozone could be controlled at a low level.
Effect of Pulp Loading on the Properties of Bleached EKP through the Low- Consistency Ozone Bleaching Process
Through the low consistency bleaching method, the pulp loading had a strong influence on the properties of bleached EKP (Figs. 4 and 5). Along with the increase of pulp loading, the decline of viscosity and kappa of the bleached EKP increased gradually but turned to a decreasing trend after reaching peak values. The highest brightness of the bleached EKP was achieved with the pulp consistency of 3%. Similarly, the brightness showed a rising tendency and then decreased. The highest delignification occurred with the highest decline of kappa value achieved when the pulp loading was 3%.
Fig. 4. Effect of pulp consistency on the decline of viscosity and kappa of the bleached pulp
Fig. 5. Effect of pulp consistency on brightness of the belched EKP
The phenomenon could be explained by the accelerated access of ozone with increased contact to fibers, as the pulp suspension could be well dispersed and maintained at a stable fluidization state at a relative low consistency. When the loading amount was below 3%, the rate for ozone to access fibers declined. However, an overload of the pulp (above 3%) led to an unstable pulp fluidization in the reacting system and thus caused a limited time for ozone to penetrate the fibers. At the same time, the pulp loading had no obvious effect on the cellulose degradation, as the decline of viscosity was only slightly changed.
Effect of pH on Bleaching Properties of Pulp
As demonstrated in Eq. 16, the concentration of [OH–] in the reaction system contributed to a direct effect on the self-decomposition. The pH is an important factor for ozone bleaching efficiency. In this work, the pH levels of 1.5, 2, 2.5, 3, and 3.5 were applied with a 3% pulp consistency, and their effects on the properties of bleached pulps were determined (Figs. 6 and 7).
Fig. 6. Effect of pH on viscosity and kappa of the bleached EKP
Fig. 7. Effect of pH on brightness of bleached EKP
The increased pH resulted in increased viscosity and kappa of the bleached EKP, while the brightness decreased gradually. The brightness of the bleached pulp from pH 2 had a 10% to 15% increase from pH 3 to 3.5. The reason could be explained by the equation of ozone self-decomposition in the aqueous system: higher pH accelerates the self-decomposition of ozone to [OH]–. The consumption of the ozone by self-decomposition would lead to a decline of the reaction rate between the lignin composition and ozone (Blanca et al. 2003). Although the kappa value was getting lower when pH < 2, the viscosity declined as well, indicating a low selectivity of the ozone bleaching process. Combined with the experimental results, the optimum pH for ozone bleaching of low-consistency EKP was pH 2.
Effect of Organic Additives on Pulp Properties
The organic additives had a significant effect on the properties of bleached pulp (Cogo et al. 1999). Some organic additives, such as tert-butyl alcohol and dimethylformamide, could improve the qualities of bleached pulp. In this study, the effects of selected organic additives on the ozone mass transfer during ozone bleaching of low-consistency EKP are shown in Table 2.
Table 2. Effect of Chemical Additives on the Performance of Ozonation
Reaction conditions: pulp consistency=3%, ozone dosage=1.2% o.d.p., pH=2, temperature=25 °C, reaction time=30 min, NP-10=0.05 mol/L.
The addition of organic additives, especially the NP-10, could obviously improve the performance of ozone bleaching by promoting the ozone mass-transfer rate. Compared with the bleaching process without adding organic additive, adding the NP-10 could decrease the surface tension of the film layers when the KLA and C*L increased. The experimental selectivity was in good agreement with the theoretical selectivity. The mass-transfer model based on the gas-liquid-film theory could sufficiently describe the ozone bleaching of the low-consistency EKP.
CONCLUSIONS
The ozone bleaching of low-consistency Eucalyptus kraft pulp (EKP) was carried out, with attention paid to the kinetics. The mass-transfer model of chemical- absorption-diffusion was suggested for describing the ozone bleaching of the low-consistency EKP. The mass transfer rate in bleaching process based on the two-film theory was also studied. The effects of pulp consistency, pH, and organic additives on the mass-transfer rate and properties of the bleached pulp were investigated by controlling the reaction conditions at same levels.
- The techniques of low-, medium-, and high- consistency bleaching were applied on ozone bleaching. Their effects on the properties of bleached EKP were compared. Although the low-consistency bleaching required a longer reaction time, it had no negative effect on the mass-transfer rate with less non-productive decomposition of ozone. In contrast to the medium- or high- consistency bleaching, the low-consistency bleaching produced the EKP with the highest pulp viscosity.
- A pulp consistency of 3% achieved the properties of bleached pulp with a viscosity, brightness, and kappa of 579 mL/g, 68% ISO, and 7.9, respectively. At the same time, the effects of pH and organic additives on the mass transfer during ozone bleaching were investigated. When lower pH (pH 2) was applied with organic additives NP-10 added, the self-decomposition rate declined for ozone, while the mass-transfer rate was increased. Under this condition, the properties of bleached EKP could be improved.
- This study emphasized the promise of low-pollution, low-cost, high-efficiency for ozone bleaching of low-consistency EKP by demonstrating the feasibility of ozone bleaching for building an environmentally friendly industrial system.
NOMENCLATURE
KG Overall mass transfer coefficient for gas film (m·s-1)
D Conventional diffusion coefficient (m2/s)
De Effective diffusion coefficient (m2/s)
C Ozone concentration (kg O3/m3 H2O)
C0 Saturated ozone concentration in water (kg O3/m3H2O)
X Distance to the fiber surface (m)
t Diffusion time (s)
δ Thickness of reaction layer (m)
PAG Ozone pressure in gas phase (pa)
A Fiber surface area (m2)
P Fiber density in solution (kg/m3)
m Partition coefficient
PAi Ozone pressure in gas liquid equilibrium interface (pa)
CAL Ozone concentration in liquid film (mol·L-1)
CAi Ozone concentration in gas liquid equilibrium interface (mol·L-1)
C*L Ozone concentration in liquid phase in equilibrium with gas phase (kg·m-3)
σ Surface tension (N·M-1)
N Mass of ozone transferred (kg)
V Solution volume (m3)
kG Gas film mass transfer coefficient (m·s-1)
kL Liquid film mass transfer coefficient (m·s-1)
KL Overall mass transfer coefficient for liquid film (m·s-1)
a Interfacial area per unit of volume (m2m-3)
H Solubility coefficient (kmol·(kN·m)-1)
G Gas flow rate (m3h-1 at normal conditions)
ACKONWLEDGEMENTS
The research was financially supported by the State Key Laboratory of Pulp and Paper Engineering and National Engineering Research Center of Papermaking and Pollution Control (No. 2015ZD04) and the opening project of Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control (KF201603).
REFERENCES CITED
Arooj, F., Ahmad, N., and Chaudhry, M. N. (2014). “A pilot scale application of ozone to bleach raw cotton fabric using various additives,” Ozone: Science & Engineering 37(3), 203-215. DOI: 10.1080/01919512.2014. 956861
Bajpai, P. (2012). “Chapter Four- Ozone Bleaching,” Environmentally Benign Approaches for Pulp Bleaching (Second Edition), 59-95. DOI: 10.1016/B978-0-444-59421-1.00004-1
Blanca Roncero, M., Queral, M.A., José, F., Colom Teresa Vidal (2003). “ Why Acid pH Increases the Selectivity of the Ozone Bleaching Processes,” Ozone: Science & Engineering 25(6), 523-534, DOI: 10.1080/01919510390481838.
Coca, M., Gonález-Benito, G., and García-Cubero. (2016). “Chapter 18: Chemical oxidation with ozone as an efficient pretreatment of lignocellulosic materials,” in: Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, S. I. Mussatto (ed.), Elsevier, Amsterdam, Netherlands, pp. 409-429. DOI: 10.1016/B978-0-12 -802323-5.00018-9
Cogo, E., Albet, J., Malmary, G., Coste, C., and Molinier, J. (1999). “Effect of reaction medium on ozone mass transfer and applications to pulp bleaching,” Chemical Engineering Journal 73(1), 23-28. DOI: 10.1016/S1385-894 7(99)00011-X
Fang, H.C., Liu, M. Y., and Chen, J. (2008). “Kinetics studies of ozone bleaching model for low consistency pulp,” Paper Science& Technology 4, 26-28.
Fariha Arooj, Nasir Ahmad, Muhammad Nawaz Chaudhry. (2015). “A Pilot Scale Application of Ozone to Bleach Raw Cotton Fabric Using Various Additives,” Ozone: Science & Engineering 37:(3), 203-215, DOI: 10.1080/01919512.2014.956861.
Freire, C. S. R., Silvestre, A. J. D., Pascoal Neto, C., and Evtuguin, D. V. (2006). “Effect of oxygen, ozone and hydrogen peroxide bleaching stages on the contents and composition of extractives of Eucalyptus globulus kraft pulps,” Bioresource Technology 97(3), 420-428. DOI: 10.1 06/j.biortech.2005.03.006
Perincek, S. D., Duran, K., Korlu, A. E., and Bahtiyari, I. M. (2007). “An investigation in the use of ozone gas in the bleaching of cotton fabrics,” Ozone: Science & Engineering 29(5), 325-333. DOI: 10.1080/01919510701509578
Piccoli, H. H., Ulson de Souza, A. A., and Guelli Ulson de Souza, S. M. A. (2014). “Bleaching of knitted cotton fabric applying ozone,” Ozone: Science & Engineering 37(2), 549-566. DOI: 10.1080/01919512.2014.939742
Roncero, M. B., Queral, M. A., Colom, J. F., and Vidal, T. (2003a). “Why acid pH increases the selectivity of the ozone bleaching processes,” Ozone: Science & Engineering 25(6), 523-534. DOI: 10.1080/01919510390481838
Roncero, M. B., Torres, A. L., Colom, J. F., and Vidal, T. (2003b). “TCF bleaching of wheat straw pulp using ozone and xylanase. Part A: Paper quality assessment,” Bioresource Technology 87(3), 305-314. DOI: 10.1016/S0960-8524(02)00224-9
Roncero, M. B., Torres, A. L., Colom, J. F., and Vidal, T. (2003c). “TCF bleaching of wheat straw pulp using ozone and xylanase: Part B: Kinetic studies,” Bioresource Technology 87(3), 315-323.DOI: org/10.1016/S0960-8524(02)00225-0
Rounsaville, J., and Rice, R. G. (1996). “Evolution of ozone for the bleaching of paper pulps,” Ozone: Science & Engineering 18(6), 549-566. DOI: 10.1080/01919512. 1997.10382863
Seisto, A., Poppius-Levlin, K., and Fuhrmann, A. (2000). “Effect of ozone bleaching on the fibre properties of pine and birch kraft pulp,” Cellulosic Pulps, Fibres and Materials 2000, 137-147. DOI: 10.1533/9781845698546.137
Shatalov, A. A., and Pereira, H. (2006). “Polysaccharide degradation during ozone-based TCF bleaching of non-wood organosolv pulps,” Carbohydrate Polymers 67(3), 275-281. DOI: 10.1016/j. carbpol.2006.05.028
Shatalov, A. A., and Pereira, H. (2008). “Arundo donax L. reed: New perspectives for pulping and bleaching. 5. Ozone-based TCF bleaching of organosolv pulps,” Bioresource Technology 99(3), 472-478. DOI: 10.1016/j.biortech.2007.01.014
Sheats, Alan. (2010). “Ozone bleaching-Environmental production and cost benefits,” Paper Asia 26(3),24-28, DOI: ISSN: 02184540.
Shi, S. L., and He, F. W. (2012). Pulp and Paper Analysis and Inspection, China Light Industry Press, Beijing, China.TAPPI T452 om-08 (2008). “Brightness of pulp, paper and paperboard (directional reflectance at 457 nm),” TAPPI Press, Atlanta, GA.
Torres, A. L., Roncero, M. B., Colom, J. F., Martínez, J. A., and Vidal, T. (2010). “Application of an experimental design to modeling of ozone bleaching stage in TCF processes,” Ozone: Science & Engineering 26(5), 443-451. DOI: 10.1080/01919510490507685
Xiao, X.Y., Liu, M,Y., Li, Y,M. (2013). “A reversal mixing experimental device for ozone bleaching of high consistency paper paddle,” C.N. Patent No.2334236
Article submitted: June 24, 2017; Peer review completed: August 19, 2017; Revised version received and accepted: October 27, 2017; Published: November 22, 2017.
DOI: 10.15376/biores.13.1.425-436
