Abstract
Eichhornia crassipes (water hyacinth) was pulped by means of a kraft pulping process with reagent loads of 10 and 20% on a dry matter basis to determine yield, rejects, kappa number, and ash. Fiber classification, brightness, opacity, and viscosity were measured in the brown pulp. Bleaching was performed by means of an O1O2D1(PO)D2HD3 sequence. Yield, kappa number, pH, ash, brightness, opacity, and viscosity were evaluated in the bleached pulp. Finally, a microanalysis of inorganic elements was carried out in both the bleached and unbleached pulp ash. The highest kraft pulp yield was 26.4%, with a 10% reagent load at 120 °C and 30 minutes cooking. It was determined that E. crassipes cellulosic pulp contains large amounts of fines. Results of the bleaching sequence indicate low brightness (58.0 %) and low viscosity (6.43 cP). The most abundant inorganic elements in the ash of both bleached and unbleached pulp were Ca, Mg, P, and Si. These results suggest that E. crassipes biomass might complement cellulosic fibers in pulping processes of low yield, such as the wood fibers used to produce handmade paper.
Download PDF
Full Article
Kraft Pulping and Bleaching of Eichhornia crassipes (Mart.) Solms (Water Hyacinth)
Luis Fernando Pintor-Ibarra,a José de Jesús Rivera-Prado,b Sarai Ramos-Vargas,c Teófilo Escoto-García,b Nancy Eloisa Rodríguez-Olalde,a and José Guadalupe Rutiaga-Quiñones a,*
Eichhornia crassipes (water hyacinth) was pulped by means of a kraft pulping process with reagent loads of 10 and 20% on a dry matter basis to determine yield, rejects, kappa number, and ash. Fiber classification, brightness, opacity, and viscosity were measured in the brown pulp. Bleaching was performed by means of an O1O2D1(PO)D2HD3 sequence. Yield, kappa number, pH, ash, brightness, opacity, and viscosity were evaluated in the bleached pulp. Finally, a microanalysis of inorganic elements was carried out in both the bleached and unbleached pulp ash. The highest kraft pulp yield was 26.4%, with a 10% reagent load at 120 °C and 30 minutes cooking. It was determined that E. crassipes cellulosic pulp contains large amounts of fines. Results of the bleaching sequence indicate low brightness (58.0 %) and low viscosity (6.43 cP). The most abundant inorganic elements in the ash of both bleached and unbleached pulp were Ca, Mg, P, and Si. These results suggest that E. crassipes biomass might complement cellulosic fibers in pulping processes of low yield, such as the wood fibers used to produce handmade paper.
Keywords: Cellulosic pulp; Kappa number; Bleaching process; Inorganic substances
Contact information: a: Facultad de Ingeniería en Tecnología de la Madera (FITECMA), Edificio “D”, Ciudad Universitaria, Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), Av. Fco. J. Múgica S/N, Col. Felicitas del Rio, Morelia, Michoacán, México, C. P. 58040; b: Departamento de Madera, Celulosa y Papel “Ing. Karl Augustin Grellmann”, Centro de Ciencias Exactas e Ingeniería, Universidad de Guadalajara, Zapopan, Jalisco, México, C.P. 45020; c: Facultad de Biología, Maestría en Ciencias en Ingeniería Ambiental, Edificio “R”, Ciudad Universitaria Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, México, C. P. 58040; *Corresponding author: rutiaga@umich.mx
GRAPHICAL ABSTRACT
INTRODUCTION
Pulp and paper production is almost exclusively based on wood. However, a growth in paper demand, together with a decline in the supply of fibers from forests, are forcing the pulp and paper industry to find alternative sources of fibers that are both technical and economically viable to complement forest resources (Jahan et al. 2008). The production of paper and paperboard in the world has increased as well. In 2009, the production was 371 million tons, while in 2015, 400 million tons were produced (FAO 2017). Likewise, in 2013 the Mexican paper industry required 5 million 144 thousand tons of fiber in order to produce 4 million 513 thousand tons of paper (INEGI 2013), increasing to approximately 6 million 822 thousand tons of fiber for the production of 5 million 956 thousand tons of paper by 2018 (Cámara del Papel 2018). Therefore, the global scarcity of fibrous resources has aroused great interest in the use of non-conventional fibrous raw materials (straw, sugar cane bagasse, bamboo, and miscellaneous raw materials) (Atchison 1996), (Ricinus communis, Cyperus papyrifera, Typha domingensis, Agave tequilana) (Escoto-García et al. 2013), which can be used to obtain cellulose for further paper production. Anupam et al. (2016) present a classification of non-woody fibers that could be used for the manufacture of paper: agricultural residues (sugarcane bagasse, cotton stalks, rice straw), natural growing plants (bamboo, reeds, sabai grass, kahi grass), non-wood crops grown primarily for its fiber content such as bast fiber (jute, hemp, kenaf), leaf fiber (sisal), and seed hair (cotton fiber, cotton linter).
E. crassipes is a floating aquatic plant, native to the Amazon basin in Brazil (Barrett 1980). It is an invasive plant that has spread within tropical and subtropical regions of the world (Villamagna and Murphy 2010), with extremely rapid proliferation (Malik 2007). Biomass of this plant amounts up to 1,800 to 2,700 tons of wet raw material or to 90 to 135 tons of absolute dry biomass per hectare (Shoyakubov and Aitmetova 1999). A mat of these medium-sized plants may contain approximately 2,000,000 plants per hectare with a weight between 270 and 400 tons (Malik 2007). Furthermore, it is estimated that over a 6-month period, 125 tons of wet weight are produced in an area of one hectare (Istirokhatun et al. 2015). Moreover, this invasive plant has been reported to cause serious ecological impacts, such as a loss of diversity and a hybridization with native species, alterations in ecosystem processes, and an increase in pests and diseases. Also, it can cause serious economic difficulties to navigation and irrigation systems (Rodríguez 2006; Villamagna and Murphy 2010; Mahamadi 2011; Stiers et al. 2011; Nguyen et al. 2015).
Due to its alarming reproductive and propagation capacity, E. crassipes is considered a threat to biodiversity (Istirokhatun et al. 2015; Tan et al. 2015). Even after the use of traditional mechanical methods for its elimination and phytoremediation of contaminated water, the problem of how to use this valuable lignocellulosic resource in a reasonable and efficient way remains (Feng et al. 2017).
Some previous scientific reports on this aquatic plant have dealt with topics of, for example, saccharification processes (Abdel-Fattah et al. 2012; Reales-Alfaro et al. 2013), obtaining bioethanol (Nigam 2002; Masami et al. 2008; Aswathy et al. 2010; Satyanagalakshmi et al. 2011; Bergier et al. 2012; Ganguly et al. 2012; Manivannan et al. 2012; Awasthi et al. 2013; Singh and Bishnoi 2013; Manivannan and Narendhirakannan 2014), acid-catalysed hydrolysis (Girisuta et al. 2008), biosorbent (Mahamadi 2011; Murithi et al. 2014; Vijetha et al. 2014), nutritional value (Mako et al. 2011; Saha and Ray 2011), pyrolysis process (Promdee et al. 2012), and antimicrobial activity of its extracts (Thamaraiselvi and Jayanthi 2012).
Regarding the application of E. crassipes for pulp and paper manufacture, the following reports stand out: Bagnall et al. (1974) proposed the use of fibers from this aquatic plant to make paper, as well as a nutrient absorber in treated wastewater, forage feed, and compost. Nolan and Kirmse (1974) obtained cellulosic pulp through four chemical processes, including the kraft process. Widyanto et al. (1983) propose the use of water hyacinth as an absorbent of pollutants in paper factories and to cultivate this plant to complement the raw material to make pulp and paper. Jeododibroto et al. (1983) studied the morphology of E. crassipes, obtained soda pulp, and carried out a chlorine bleaching process. Das et al. (2013) propose the use of this aquatic plant as an alternative raw material for the pulp and paper industry. Recently, the authors’ work team has carried out some studies on the chemical composition of water hyacinth (Fileto-Pérez et al. 2013; Fileto-Pérez et al. 2015; Lara-Serrano et al. 2016; Pintor-Ibarra et al. 2018). Thus, the aim of this research is to obtain cellulosic pulp by means of a kraft pulping process followed by a bleaching sequence, in order to use E. crassipes pulp as a complement to wood cellulosic fibers and as an alternative raw material for cellulosic fibers used in paper production. In the present study, in addition to other results, the ash analysis of the E. crassipes pulp is reported, and a bleaching sequence was applied, which was not previously reported.
EXPERIMENTAL
Materials
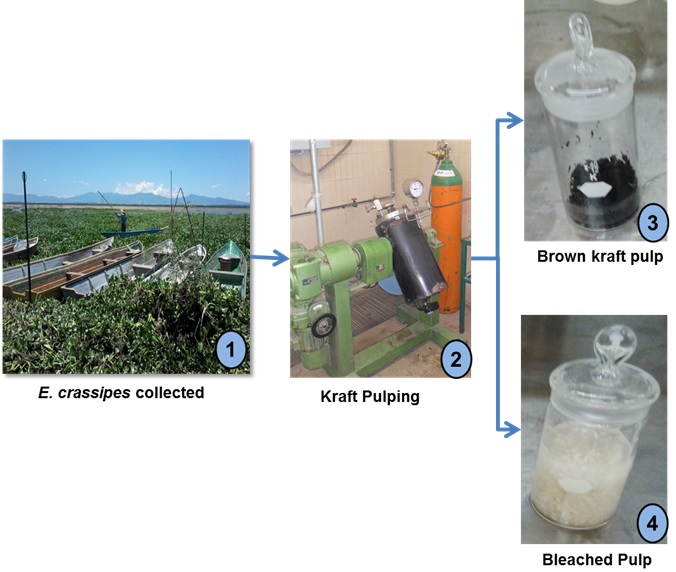
Samples of Eichhornia crassipes were collected at Cuitzeo Lake, located in the State of Michoacán, Mexico, between 19°53’15” and 20°04’34” North latitude and between 100°50’20” and 101°19’34” West longitude. E. crassipes samples were washed with abundant water at constant current to remove contaminants such as soil, seashells, and small stones, among others. The biomass was dried outdoors in the shade until it contained approximately 12% moisture (TAPPI T 412 om-06 2006a). Fibers were manually separated from the plant.
Kraft Pulping Process
Pulping conditions were as follows: white liquor sulfidity 26%, active alkali 100 g/L, and a liquor to wood ratio of 12:1 with a 10 and 20% reagent load, on a dry matter basis. The kraft cooking process was carried out in a 15 L heated reactor bath (Jayme type, Stober Deutsch & Newmann, Germany) using a 22 factorial design with a central point (Gutiérrez-Pulido and de la Vara-Salazar 2004). Factor A was temperature (120 and 150 °C), and factor B was cooking time at Tmax (10 and 30 min) (See Tables 2 and 3).
The obtained data were analyzed at a 95% confidence level and processed using the Statgraphics Version 4 Plus software to evaluate yield, rejects, kappa number (TAPPI T 236 cm-99 1999) and ash content (TAPPI T 413 om-93 1993). After each cooking stage, cellulosic pulp was washed with water at current flow and filtered on a 325-mesh sieve. The screened pulp was stored at room temperature for its later use. The residual black liquor obtained was filtered and stored for future research.
Only the brown kraft pulp with highest yield was screened in a diaphragm equipment (Type F1 117, Serie 1123, AB Lorentzen & Wettre, Stockholm, Sweden) with a 0.40 mm plate opening. The fiber classification was determined using Bauer-McNett equipment (AB Lorentzen & Wettre, Stockholm, Sweden) (TAPPI T 233 cm-06 2006). The following parameters were evaluated in standard sheets (60 g.m-2) of high-yield brown kraft pulp, which were formed in a conventional semi-automatic TAPPI equipment (TMI Testing Machines Inc, Amityville, NY, USA) (TAPPI T 205 sp-02 2002): brightness (TAPPI T 452 om-02 2002), opacity (TAPPI T 519 om-02 2002), and viscosity (SCAN-CM 15:88 1988).
Bleaching Process
Bleaching was performed only to high-yield brown kraft pulp by means of an O1O2D1(PO)D2HD3 sequence, which stands for oxygen, chlorine dioxide, peroxide oxygen, and sodium hypochlorite (Table 1). At the end of every bleaching stage, yield, kappa number (TAPPI T 236 cm-99 1999), pH (TAPPI T 625 cm-85 1984), and ash content (TAPPI T 413 om-93 1993) were determined. Brightness (TAPPI T 452 om-02 2002), opacity (TAPPI T 519 om-02 2002), and viscosity (SCAN-CM 15:88 1988) were determined in 60 g.m-2 standard sheets only at the end of the bleaching sequence.
Table 1. Bleaching Stages Conditions for Brown Kraft Pulp
Ash Microanalysis
Determination of inorganic elements in brown pulp ash and in ash after each stage of the bleaching process was performed using an X-ray spectrometer, connected to a Jeol JSM – 6400 scanning electron microscope (Tokyo, Japan), with operating conditions of 20 kV and 8.5s (Téllez-Sánchez et al. 2010).
RESULTS AND DISCUSSION
Kraft Pulping Process
The results obtained in each experimental run according to the design matrix are shown in Tables 2 and 3. The analysis of variance (ANOVA) for each response variable is presented in Table 4 (10 % reagent load), and Table 5 (20 % reagent load).
Yield
The ANOVA indicates that there was no statistically significant difference for yield, for both reagent loads used in the experimentation (Tables 4 and 5). Figure 1 shows the effect of temperature and time on yield during the kraft pulping process with 10 and 20% reagent loads on a dry basis.
Table 2. Results of the Design Matrix for 10% Reagent Loading
Table 3. Results of the Design Matrix for 20% Reagent Loading
Table 4. ANOVA for the Response Variables (10% reagent load)
Table 5. ANOVA for the Response Variables (20% reagent load)
The lowest yield was 14.5% and the highest was 26.4%, both at a 10% reagent load (Table 2). For the 20% reagent load, the lowest yield was 15.8% and the highest was 18.6% (Table 3). These results demonstrate that the higher the reagent load, the lower the yield obtained. Similarly, Widyanto et al. (1983) reported a decrease in yield as the reagent load increased with soda pulping for E. crassipes. In Fig. 1a it can be observed that to maximize pulp yield, the combination of T (120 °C) and t (30 min) should be used. In Fig. 2a. it is observed that the effect of increasing the time is positive (the yield increases) as long as the temperature is lowered (120 °C). So, for the case of using 10% reagent load, the equation of the fitted model to maximize yield is (optimum value = 25.3%):
(1)
where T is temperature (°C) and t is time (min).
For kraft pulping with 20% reagent loading, in Fig. 1b it is observed that to achieve maximum pulp yield, the combination of T (150 °C) and t (10 min) should be used. However, in Fig. 2b the effect of keeping the time low (10 min) on the highest yield is observed as long as the temperature is low (120 °C). It is known that the conclusion obtained only by the analysis of the main effects is not always correct (Gutiérrez-Pulido and de la Vara-Salazar 2004), as shown by these results. So, for the case of using 20% reagent load, the equation of the fitted model to maximize yield is (optimum value = 18.2 %):
(2)
where T is temperature (°C) and t is time (min).
The determination coefficients are low, both for pulping with 10% (R-squared is 47.0% and R-squared (adjusted) is 20.5%), and with 20% (R-squared is 5.6% and R-squared (adjusted) is 0.0%) reagent loading. In this case, low values that would indicate that the effect of these factors studied on pulp yield is small compared to the rest of the variability observed in the experiment, such once because the levels studied are narrow.
Fig. 1. Main effects on yield: a) 10% reagent load, b) 20% reagent load
Fig. 2. Interaction effects on yield: a) 10% reagent load, b) 20% reagent load
Figure 3 shows response surface plots for the response variable (pulp yield) and gives a visualization of what the fitted model (Eqs. 1 and 2) means over the experimentation region. It can be seen that the points where the surface takes higher values are precisely in the best treatment that had been found (10%: 120 °C and 30 min; 20%: 120 °C and 10 min).
The highest yield (26.4%) obtained with a reagent load of 10% at 120 °C and 30 min (Table 2) was within the 15 to 30% yield range obtained for this aquatic plant as reported by Bagnall et al. (1974). Kumar et al. (2015) obtained a higher yield value (33.9%) in stems and leaves of water hyacinth. Compared to other raw materials, the yield obtained in this work was lower than that reported (43.7%) for Cymbopogon winterianus (citronella grass) kraft pulp (Sharma et al. 2017), and than that obtained (51.7%) for Melia dubia kraft pulp (Deepika et al. 2018). Moreover, Lara-Serrano et al. (2016) studied the chemical components of E. crassipes and concluded that if biomass is used as raw material in the cellulosic pulp manufacturing process, the yield would be low due to its low holocellulose content. These low results contrast with the obtained yields for softwood and hardwood kraft pulping, which range from 44 to 51% (Libby 1980; Rutiaga-Quiñones et al. 1998; Juacida et al. 2002; Gabriel-Parra et al. 2018).
In addition, pulping conditions for wood and for water hyacinth are different. For wood, pulping conditions are a 20% reagent load, 90 to 120 min cooking time, and temperatures from 168 to 170 °C, contrasting with water hyacinth pulping conditions, which require lower operating conditions, resulting in lower pulping costs. This shows that the variability of organic chemical components in different fibrous species have different demands on reagents and pulping times (Juacida et al. 2002).
Fig. 3. Response surface for yield a) 10% reagent load, b) 20% reagent load
Rejects
ANOVA indicated that there were statistically significant differences for the response variable (rejects) (Tables 4 and 5). Figure 4a shows that the minimum of rejects was achieved with treatment T (150 °C) and t (30 min), while in the interaction plot (Fig. 4b) the effect of the shorter time was observed (rejects decreases) as long as the temperature was kept high (T = 150 °C). Then, the equation of the fitted model to minimize the content of rejections with optimal conditions (T = 150 °C and t = 10 min) was the following (optimum value = 0.63%),
(3)
where T is temperature (°C) and t is time (min).
It is known that taking conclusions from the analysis of the main effects of the studied factors is not always the correct way (Gutiérrez-Pulido and de la Vara-Salazar 2004), so it is better to rely on the analysis of iteration effects, as shown by these results.
For the case of using 20% reagent load, the analysis of both the main effects (Fig. 4b) and the interaction effects (Fig. 5b), it is concluded that to minimize the amount of rejects, the combination of T (150 °C) and t (10 min) should be used. In this case, the equation of the fitted model to minimize rejects (optimum value = 0.04%) is as follows:
(4)
where T is temperature (°C) and t is time (min).
The determination coefficients obtained were the following: R-squared = 88.7% and R-squared (adjusted) = 83.0% (for 10% reagent load), and R-squared = 87.2% and R-squared (adjusted) = 80.8% (for 20% reagent load). This means that the studied factors, together with their interactions, explained a high percentage of variability observed in the response variable (rejects).
With a 10% reagent load in the kraft pulping process, rejects values ranged from 0.37% to 6.11% (Table 2). On the other hand, with a 20% reagent load, results ranged from 0.17 to 2.21% (Table 3). Thus, the higher the reagent load percentage, the lower the rejects percentage and the higher the obtained yield. MacLeod (2007) points out that when the variables reagent load and temperature increase, the number of rejects in the pulping process decreases. Furthermore, the number of rejects generated in the pulping process of E. crassipes was similar to those reported for softwood and hardwood species, which range from 1.5 to 3.4% (Juacida et al. 2002).
Fig. 4. Main effects on yield: a) 10% reagent load, b) 20% reagent load
Fig. 5. Interaction effects on yield: a) 10% reagent load, b) 20% reagent load
The response surface plots for the response variable (rejects) visualize what the fitted model (equations 3 and 4) means in the experimental region (Fig. 6). It can be seen that the points where the surface takes lower values were precisely in the best treatment found (10%: 150 ° C and 10 min; 20%: 150 ° C and 10 min).
Fig. 6. Response surface for rejects, a) 10% reagent load, b) 20% reagent load
Kappa number
Statistically significant differences were found for the response variable (kappa number) only for the kraft pulp with 20% reagent load (Tables 4 and 5). Figure 7a shows the effects of the factors studied, and the minimum kappa number was obtained with 120 °C and 30 min. Likewise, the interaction plot (Fig. 8a) indicates that with a time of 30 min there was lower kappa number, as long as the process was carried out at 120 °C. For this case using 10% reagent load, the equation of the fitted model to minimize the kappa number (optimum value = 30.3) was as follows:
(5)
where T is temperature (°C) and t is time (min).
When using 20% reagent loading, it is seen in Fig. 7b that to achieve the low kappa number, the combination of 120 °C and 10 min must be used. Likewise, in the interaction plot (Fig. 8b) with 10 min of cooking kraft, a low kappa number was obtained, as long as the pulping process was carried out at 102 °C. Thus, the equation of the fitted model to minimize the kappa number was (optimum value = 21.2):
(6)
where T is temperature (°C) and t is time (min).
The determination coefficients obtained were the following: R-squared = 71.8% and R-squared (adjusted) = 57.8% (for 10% reagent load), and R-squared = 95.9% and R-squared (adjusted) = 93.8% (for 20% reagent load). This means that the studied factors, together with their interactions, explained a high percentage of variability observed in the response variable (kappa number).
The amount of residual lignin in water hyacinth pulp also differed when using a 10% and a 20% reagent load, ranging from 30.0 to 33.2 (Table 2) and from 20.6 to 31.6 (Table 3), respectively, coinciding with several reports (Casey 1990; MacLeod 2007; Wan Daud et al. 2009; Kumar et al. 2015). In general, values obtained for kappa number were high (21 to 33). Therefore, it can be predicted that for bleaching E. crassipes cellulosic pulp, high amounts of reagents will be required. During cooking, E. crassipes cellulosic pulp darkened, agreeing with what by Bagnall et al. (1974) reported. Additionally, kappa number values obtained here are comparable to those reported for kraft pulping (Kumar et al. 2015) and for soda pulping (Joedodibroto et al. 1983) for the same aquatic plant. They are also within the range of 18.9 to 31.0 reported for kraft pulping of softwood and hardwood species (Rutiaga-Quiñones et al. 1998; Juacida et al. 2002; Torres et al. 2005).
Fig. 7. Main effects on kappa number: a) 10% reagent load, b) 20% reagent load
Fig. 8. Interaction effects on kappa number: a) 10% reagent load, b) 20% reagent load
The response surface plots for the response variable (kappa number) visualize what the fitted model (Eqs. 5 and 6) means in the experimental region (Fig. 9). It can be seen that the points where the surface takes lower values were precisely in the best treatment found (10%: 120 °C and 30 min; 20%: 120 °C and 10 min).
Fig. 9. Response surface for kappa number, a) 10% reagent load, b) 20% reagent load
Ash content in high-yield brown kraft pulp
Table 4 presents the ANOVA for the ash content at 10% reagent load, and Table 5 shows it for 20% reagent load. In the main effects plot it is observed that the combination of 120 °C and 10 min (Fig. 10a) should be used to obtain low ash content in the kraft pulp. Likewise, the interaction plot shows that a low ash content was obtained with a cooking time of 10 min, as long as the cooking process was carried out at 120 °C. Under these conditions, for a 10% reagent load, the equation of the fitted model to minimize the ash content is the following (optimum value = 6.0%):
(7)
where T is temperature (°C) and t is time (min).
When the pulping process was carried out with a 20% reagent load, the main effects plot shows that to obtain low ash content, the combination of 120 °C and 30 min should be used. However, in the interaction plot it is observed that maintaining a high time (30 min) low ash content was achieved, but always when the cooking temperature was 150 °C. This same effect was observed in the case of the response variables yield and rejects. For this case, with the optimal conditions of 150 °C and 30 min, the equation of the model adjusted to minimize the ash content (optimum value = 6.5%) was as follows,
(8)
where T is temperature (°C) and t is time (min).
For this case, the coefficients of determination obtained were as follows: R-squared = 78.6% and R-squared (adjusted) = 67.8% (for 10% reagent load), and R-squared = 61.1% and R-squared (adjusted) = 41.7% (for 20% reagent load). This means that the studied factors temperature and time, together with their interactions, explained a high percentage of variability observed in the response variable (ash content).
Fig. 10. Main effects on ash content: a) 10% reagent load, b) 20% reagent load
Fig. 11. Interaction effects on kappa number: a) 10% reagent load, b) 20% reagent load
Previous reported values of inorganic substances content in the E. crassipes range from 19.1 to 22.9% (Fileto-Pérez et al. 2013; Pintor-Ibarra et al. 2018). This high ash content is related to its natural characteristic to absorb and concentrate toxic minerals and metals from the aquatic environment (Mahmood et al. 2005). The values obtained for cellulose pulp vary from 5.7 to 10.8% when pulping with a 10% reagent load (Table 2), while when carrying out the process with a 20% reagent load the results ranged from 5.1 to 16.6% (Table 3). These results were lower than those of the original raw material due to the fact that some inorganic substances are soluble in alkali (Doldan et al. 2011) and were eliminated during the pulping process. These results do not show a reduction in the percentage of ash in the cellulosic pulp by increasing the load of chemical reagents during the kraft pulping process, as it occurs in a soda pulping process (Widyanto et al. 1983).
The response surface plots for the response variable (ash) visualize what the fitted model (Eqs. 7 and 8) means in the experimental region (Fig. 12). It can be seen that the points where the surface takes lower values were precisely in the best treatment found (10%: 120 °C and 10 min; 20%: 150 °C and 30 min).
Fig. 12. Response surface for ash: a) 10% reagent load, b) 20% reagent load
Fiber classification
As shown in Table 6, E. crassipes fibers contained a high proportion of fines (47.8%), compared with Pinus douglasiana kraft pulp (Rutiaga-Quiñones et al. 1998), which has a long fibers retention of 87.1% in the 30 mesh. A high content of fines is also reported in cellulosic pulp of Cymbopogon winterianus (citronella grass) (Sharma et al. 2020). The high fines in E. crassipes is related to its structural fine elements, such as parenchyma and aerenchyma cells (Mahmood et al. 2005). Unfavorable results have been documented for drainage of E. crassipes pulp through sheet formers, due to its high content of fine cells (Bagnall et al. 1974). Although fine fibers may decrease drainage freedom, fines are needed because they increase contact zones of cellulosic surfaces and favor tensile strength of paper sheets (Swanson and Steber 1959; Casey 1990; Young 1991).
Brightness, opacity, and viscosity in high-yield brown kraft pulp
Brightness and opacity percentages (Table 6) were lower than those reported for Pinus douglasiana brown kraft pulp, with values of 32.9% and 99.5% for brightness and opacity, respectively (Rutiaga-Quiones et al. 1998), which is reflected in E. crassipes kraft pulp dark color. This coincides with previous literature reports that state that water hyacinth darkens during the cooking process (Bagnall et al. 1974). E. crassipes brown kraft pulp presented lower viscosity than kraft pulp from timber species. Viscosity value reported for Pinus douglasiana kraft pulp is 12.6 cP (Rutiaga-Quiñones et al. 1998) and for Pinus tecunumanii kraft pulp 34 cP (Torres et al. 2005). However, no previous data on literature about cellulosic pulp viscosity of E. crassipes were found. Table 6 shows the characteristics of the high-yield brown kraft pulp using a 10% reagent load.
Table 6. Characterization of High-yield Brown Kraft Pulp
Bleaching Process
Yield and kappa number
During the bleaching sequence the behavior for brown kraft pulp of E. crassipes was drastically different (Table 7). To begin with, pulp yields were low compared to Pinus douglasiana kraft pulp during a bleaching sequence applied (97.3 to 99.2%) (Rutiaga-Quiñones et al. 1998). No data were available on the yield of E. crassipes pulp during the bleaching process. The initial kappa number (30.0) (Tables 6 and 7) meant a 66.9% pulp delignification with double oxygen stage, which is higher than the delignification of pulps exposed to a single stage (41.7% to 63%) (Poukka et al. 1999; Suchy and Argyropoulos 2002). Delignification for E. crassipes pulp was greater than the delignification of Pinus tecunumanii kraft pulp (62%), with a double oxygen stage treatment (Torres et al. 2005).
Pulp delignification was determined based on the initial kappa number (Table 7). Then a kappa number was measured after applying chlorine dioxide. After the first application, a 46.6% delignification was achieved. This was followed by a 39.7% delignification on the second application until a 93.5% delignification was reached. The final kappa number was close to zero, which is related to a good delignification. Nonetheless, the brightness of the bleached pulp of E. crassipes (58.0%) was low compared to the kraft pulp of Pinus douglasiana (85.7%) using the bleaching sequence O1D1D2 (Rutiaga-Quiñones et al. 1998) and in comparison with P. tecunumanii (90%) using the sequence D0EoEopD1ED2 (Torres et al. 2005). The low percentage of bleaching in the cellulosic pulp of E. crassipes could have been caused by the high ash content and some extractives. It has been reported in the literature that high quantities of these substances produce a yellowish color in the cellulosic pulp and they also react with the bleaching agents, causing problems in the bleaching stages (Rapson 1963; MacDonald and Franklin 1969; Grace et al. 1996).
pH of pulp in the bleaching sequence
Concerning pH, values varied depending on the chemical reagent used at each stage of the bleaching process (Table 7).
Ash content of pulp in the bleaching sequence
Regarding ash percent, the initial value (9.9%) in the brown kraft pulp was lower compared to the value achieved after the first stage of bleaching (13.9%). Based on the results, the inorganic content persists, carbohydrates are degraded by bleaching reagents, as shown in the decrease in ash percentage of each bleaching stage (Table 7).
Brightness, opacity and viscosity in bleached kraft pulp
The final brightness achieved in the E. crassipes pulp was low with high opacity (Table 7), and its use may not be viable due to the number of stages applied in the bleaching sequence. However, the final brightness was high compared to that reported for Melia dubia kraft pulp (42.5%) (Anupam et al. 2018). On the other hand, several papers and paperboards on the market, such as those used for packaging, do not require bleached cellulosic pulp. Therefore, the pulp from this aquatic plant could be incorporated into the cellulosic pulp obtained from wood species. Final viscosity (Table 7) was also low compared to the 8.4 cP value reported for Pinus douglasiana pulp in the final bleaching stage (Rutiaga-Quiñones et al. 1998), possibly because bleaching processes affect cellulosic pulp viscosity, as previously described (Rapson 1963; Casey 1990; Suchy and Argyropoulos 2002). Also the viscosity obtained was low compared to the value of 16.7 cP reported for Cymbopogon winterianus (citronella grass) kraft pulp (Sharma et al. 2017).
Table 7. Characterization of Brown Kraft Pulp of Eichhornia crassipes during the Bleaching Process
Ash Microanalysis in Brown Kraft pulp
The most abundant inorganic elements in the kraft cellulosic pulp were calcium, magnesium, phosphorus, and silicon, both for a 10% and a 20% reagent load (Table 8).
Table 8. Inorganic Elements Detected in E. crassipes Brown Kraft Pulp (at.%)
Some of the elements detected in this work have also been found in Melia dubia kraft pulp (Deepika et al. 2018). In general, there were no significant variations in the concentration of the inorganic elements detected. Potassium and chlorine were the least concentrated in the pulp (Table 8), whereas in the unprocessed biomass of E. crassipes, they were reported as the most abundant elements (Pintor-Ibarra et al. 2018). This is supported by Doldan et al. (2011), who indicate that these inorganic elements are highly soluble in alkali. Recently, Pintor-Ibarra et al. (2018) discussed the effects of inorganic elements present in lignocellulosic materials used in the pulp and paper industry, reporting that calcium, magnesium, iron, phosphorus, silicon, aluminum, and chlorine can cause serious problems during the pulping process. It is also known that these chemical elements favor fouling formation on the surfaces of heat exchangers where the combustion of the black liquor takes place in the kraft process and in the digester. In addition, such chemical elements increase the corrosion in evaporator pipes and produce inert lime in the lime recovery cycle (Libby 1980; Grace et al. 1996; Sithole and Allen 2002; Vakkilainen 2005; Tran and Vakkilainen 2007; Doldan et al. 2011).
Table 9. Ash Microanalysis of Bleached Pulp in the Bleaching Sequence (at.%)
Ash Microanalysis of Bleached Kraft Pulp
Table 9 shows the inorganic elements detected in the ash of the bleached pulp after each bleaching stage. The most abundant were calcium, magnesium, phosphorus and silicon, the same as in the brown kraft pulp (Table 8). The presence of silicon, iron, copper, and manganese (approximately 1 microgram per gram of pulp), increase the oxidation speed of cellulose and react with bleaching agents, affecting aging and producing a yellowish color in cellulosic pulp (Rapson 1963; Macdonald and Franklin 1969; Grace et al. 1996), which may cause the low degree of bleaching obtained for the E. crassipes cellulosic pulp (Table 7).
CONCLUSIONS
- Eichhornia crassipes kraft pulp yield is low.
- The highest kraft pulp yield was with the combination of T = 120 °C and t = 30 min.
- The residual lignin content in the brown pulp is high.
- To minimize the residual lignin, the combination of T = 120 °C and t = 10 min should be used.
- It was observed that during the E. crassipes pulping process, the obtained pulp darkened.
- Cellulosic pulp contains a high concentration of inorganic substances.
- Fiber classification of the brown pulp indicates a high amount of fines, which may cause problems in the drainage stage of paper sheet production.
- The final bleaching obtained by the bleaching sequence is low, which may be due to the high content of inorganic substances in E. crassipes biomass.
- Calcium, magnesium, iron, phosphorus and silicon, were the most abundant inorganic elements in ash from both brown and bleached pulp.
- Finally, the biomass of E. crassipes could be a complementary raw material to cellulosic fibers from wood species to produce handmade paper.
ACKNOWLEDGMENTS
L. F. Pintor-Ibarra is grateful for the support of the Consejo Nacional de Ciencia y Tecnología (CONACYT) for its financial support during his studies. Also, thanks to the Wood, Cellulose and Paper Department of University of Guadalajara, where this work was partially carried out. Finally, the authors are grateful for the support of the Scientific Research Coordination of Universidad Michoacana de San Nicolás de Hidalgo.
This paper is dedicated to the memory of Fermín Correa Méndez (Universidad Intercultural Indígena de Michoacán, México).
REFERENCES CITED
Abdel-Fattah, A. F., and Abdel-Naby, M. A. (2012). “Pretreatment and enzymatic saccharification of water hyacinth cellulose,” Carbohyd. Polym. 87, 2109-2113. DOI: 10.1016/j.carbpol.2011.10.033
Anupam, K., Deepika, Swaroop, V., and Lal, P. S. (2018). “Antagonist, synergistic and iteraction effect of process parameters during oxygen delignification of Melia dubia kraft pulp,” J. Clean Prod. DOI: 10.1016/j.jclepro.2018.07.125
Anupam, K., Sharma, A. K., Lal, P. S., and Bist, V. (2016). “Chapter 12. Physicochemical, morphological, and anatomical properties of plant fibers used for pulp and papermaking,” in: Fiber Plants. Sustainable Development and Biodiversity, K.G. Ramawat, M.R. Ahuja (eds), Springer Int. Publishing. Switzerland, pp. 235-248.
Aswathy, M., Sukumaran, R. K., Devi, G. L., Rajarsee, K. P., Singhania, R. R., and Pandey, A. (2010). “Bio-ethanol from water hyacinth biomass: An evaluation of enzymatic saccharification strategy,” Bioresource Tehcnol 101(3), 925-930.
Atchison, J. E. (1996). “Twenty-five years of global progress in nonwood plant fiber repulping,” Tappi J. 79(10), 87-95.
Awasthi, M., Kaur, J., and Rana, S. (2013). “Bioethanol production through water hyacinth, Eichhornia crassipes via optimization of the pretreatment conditions,” Int. Journal of Emerging Technology and Advanced Engineering 3(3), 42-46.
Bagnall, L. O., Furman, T. de S., Hentges, J. F., Jr. Nolan, W. J., and Shirley, R. L. (1974). “Feed and fiber from effluent-grown water hyacinth,” in: Environmental Protection Technology Series, Wastewater Use in the Production of Food and Fiber, Oklahoma City, OK, USA, pp. 116-141.
Barrett, S. C. H. (1980). “Sexual reproduction in Eichhornia crassipes (water hyacinth). II. Seed production in natural populations,” J. Appl. Ecol. 17, 113-124. DOI: 10.2307/2402967
Bergier, I., Salis, S. M., Miranda, C. H. B., Ortega, E., and Luengo, C. A. (2012). “Biofuel production from water hyacinth in the Pantanal wetland,” Ecohydrology and Hydrobiology 12(1), 77-84. DOI: 10.2478/v10104-011-0041-4
Cámara del Papel (2018). “Informe Anual de Cámara del Papel, edición 2018,” (http://camaradelpapel.mx/), Accessed January 09, 2020.
Casey, J. P. (1990). Pulpa y Papel, Química y Tecnología Química [Pulp and Paper, Chemistry and Chemical Technology], Limusa, México.
Das, A. K., Biswas, S. K., and Nazhad, M. (2013). “Pulp quality of Musa sapientum and Eichhornia crassipes, LAP LAMBERT Academic Publishing. Germany.
Deepika, Anupam, K., and Lal, P. S. (2018). “Melia dubia valorization at 4, 5- and 6-year age for pulp and paper production,” International Journal of Science and Research (IJRS) 8(2), 613-623. DOI: 10.21275/ART20195143
Doldán, J., Poukka, O., Salmenoja, K., Battegazzore, M., Fernandez, V., and Eluén, I. (2011). “Evaluation of sources and routers of non-process elements in a modern eucalyptus kraft pulp mill,” O PAPEL 72(7), 47-52.
Escoto García, T., Rodríguez Rivas, A., Contreras Quiñones, H. J., Díaz Ramos, S. G., and Ochoa Ruiz, H. G. (2013). Aprovechamiento integral de recursos forestales no maderables. Investigación y sustentabilidad [Integral use of non-timber forest resources. Research and sustainability], Universidad de Guadalajara. Guadalajara, Jalisco, México.
FAO (2017). “Datos y cifras globales de productos forestales 2017,” (http://www.fao.org/forestry/statistics/es/), Accessed June 25, 2017.
Feng, W., Xiao, K., Zhou, W., Zhu, D., Zhou, Y., Yuan, Y., Xiao, N., Wan, X., Hua, Y., and Zhao, J. (2017). “Analysis of utilization technologies for Eichhornia crassipes biomass harvested after restoration of wastewater,” Bioresource Technol. 223, 287-295. DOI: 10.1016/j.biortech.2016.10.047
Fileto-Pérez, H. A., Rutiaga-Quiñones J. G., Aguilar-González, C. N., Páez, B. J., López, J., and Rutiaga-Quiñones O. M. (2013). “Evaluation of Eichhornia crassipes as an alternative raw material for reducing sugars production,” BioResources 8(4), 5340-5348. DOI: 10.15376/biores.8.4.5340-5348
Fileto-Pérez, H. A., Rutiaga-Quiñones, O. M., Mark, D., Sytsma, Lorne, I. M., Luo, W., Pankow, J. F., and Rutiaga-Quiñones, J. G. (2015). “GC/MS Analysis of some extractives from Eichhornia crassipes,” BioResources 10(4), 7353-7360. DOI: 10.15376/biores.10.4.7353-7360
Gabriel-Parra, R., Rutiaga-Quiñones, J. G., Orihuela-Equihua, R., Rivera-Prado, J. J., Sanjuán-Dueñas, R., and Carrillo-Parra, A. (2018). “Quality of kraft pulp of Pinus pseudostrobus L. obtained from industrial chips,” Madera y Bosques 24(2), e2421816 (1-8p) DOI: 10.21829/myb.2018.2421816
Ganguly, A., Chatterjee, P. K., and Dey, A. (2012). “Studies on ethanol production from water hyacinth – A review,” Renewable and Sustainable Energy Reviews 16(1), 966-972. DOI: 10.1016/j.rser.2011.09.018
Girisuta, B., Dannon, B., Manurung, R., Janssen, L., and Heeres, H. (2008). “Experimental and kinetic modelling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid,” Bioresource Technol. 99(17), 8367-8375.
Grace, T. M., Malcolm, E. W., and Kocurek, M. J. (1996). Alkaline Pulping (pulp and paper manufacture Series, Volume 5), TAPPI Press, Atlanta, GA, USA. pp. 23-25.
Gutiérrez Pulido, H., and De la Vara Salazar, R. (2004). Análisis y Diseño de Experimentos [Analysis and Design of Experiments], McGraw-Hill Interamericana, México.
INEGI (2013). “Estadísticas a propósito de la industria del papel,” (http://internet.contenidos.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/estudios/economico/a_proposi_de/Papel.pdf), Accessed January 09, 2020.
Istirokhatun, T., Rokhati, N., Rachmawaty, R., Meriyani, M., Priyanto, S., and Susato, H. (2015). “Cellulose isolation from tropical water hyacinth for membrane preparation,” Procedia Environ. Sci. 23, 274-281. DOI: 10.1016/j.proenv.2015.01.041
Jahan, M. S., Sabina, R., and Rubaiyat, A. (2008). “Alkaline pulping and bleaching of Acacia auriculiformis grown in Banglasdesh,” Turk J. Agric. For. 32, 339-347.
Joedodibroto, R., Widyanto, L. S., and Soerjani, M. (1983). “Potential uses of some aquatic weeds as paper pulp,” J. Aquat. Plant Manage 21, 29-32.
Juacida, P. R., Rodriguez, S. S., and Torres, U. M. (2002). “Composición química, obtención de pulpa Kraft y su evaluación papelera en castaño, ciprés y encino,” Bosque 23(1), 125-130. DOI: 10.4206/bosque.2002.v23n1-12
Kumar Das, A., Nakagawa-Izumi, A., and Ohi, H. (2015). “Evaluation of pulp quality of three non-wood species as alternative raw materials for paper production,” Japan Tappi Journal 69(5), 80-86. DOI: 10.2524/jtappij.1501
Lara-Serrano, J. S., Rutiaga-Quiñones, O. M., López-Miranda, J., Fileto-Pérez, H. A., Pedraza-Bucio, F. E., Rico-Cerda, J. L., and Rutiaga-Quiñones, J. G. (2016). “Physicochemical characterization of water hyacinth (Eichhornia crassipes (Mart.) Solms.,” BioResources 11(3), 7214-7223. DOI: 10.15376/biores.11.3.7214-7223
Libby, C. E. (1980). Ciencia y Tecnología sobre Pulpa y Papel [Science and technology on pulp and paper], Tomo 1: Pulpa, C.E.C.S.A., México.
MacDonald, R. G., and Franklin, J. N. (1969). The Pulping of Wood, McGraw-Hill, New York, USA. pp. 34-35.
MacLeod, M. (2007). “The top ten factors in kraft pulp yield,” Paperi ja Puu – Paper and Timber 89(4), 1-7.
Mahamadi, C. (2011). “Water hyacinth as a biosorbent: A review,” Afr. J. of Environ. Sci. Technol. 5(13), 1137-1145. DOI: 10.5897/AJESTX11.007
Mahmood, Q., Zheng, P., Siddiqi, M. R., Islam, E. U., Azim, M. R., and Hayat, Y. (2005). “Anatomical studies on water hyacinth (Eichhornia crassipes (Mart.) Solms) under the influence of textile wastewater,” J. Zhejiang Univ. Sci. B. 6(10), 991-998. DOI: 10.1007/BF02888490
Mako, A. A., Babayemi, O. J., and Akinsoyinu, A. O. (2011). “An evaluation of nutritive value of water hyacinth (Eichhornia crassipes Mart. Solms-Laubach) harvested from different water sources as animal feed,” Livestock Research for Rural Development 23, 106.
Malik, A. (2007). “Environmental challenge vis a vis opportunity: the case of water hyacinth,” Environ. Int. 33(1), 122-138. DOI: 10.1016/j.envint.2006.08.004
Manivannan, A., Jayarani, P. H., and Narendhirakannan, R. T. (2012). “Enhanced acid hydrolysis for bioethanol production from water hyacinth (Eichhornia crassipes) using fermentating yeast Candida intermedia NRRLY-981,” Journal of Scientific and Industrial Research 71(January), 51-56.
Manivannan, A., and Narendhirakannan, R. T. (2014). “Biodegradation of lignocellulosic residues of water hyacinth (Eichhornia crassipes) and response surface methodological approach to optimize bioethanol production using fermenting yeast Pachysolen tannophilus NRRL Y-2460,” International Journal of Biological, Veterinary, Agricultural and Food Engineering 8(2), 157-162.
Masami, G. O. O., Yukinari, I., and Urano, N. (2008). “Ethanol production from the water hyacinth Eichhornia crassipes by yeast isolated from various hydrospheres,” African Journal of Microbioogy Research 2(5), 110-113.
Murithi, G., Onindo, C. O., Wambu, E. W., and Muthakia, G. K. (2014). “Removal of cadmium (II) Ions from water by adsorption using water hyacinth (Eichhornia crass-ipes) biomass,” BioResources 9(2), 3613-3631. DOI: 10.15376/biores.9.2.3613-3631
Nguyen, T. H. T., Boetsa, P., Locka K., Damanik Ambarita M. N., Eurie Forioa, M. A., Sashaa, P., Dominguez Granda, L. E., Thi Hoangd, T. H., Everaerta, G., and Goethals, P. L. M. (2015). “Habitat suitability of the invasive water hyacinth and its relation to water quality and macroinvertebrate diversity in a tropical reservoir,” Limnologica 52, 67-74. DOI: 10.1016/j.limno.2015.03.006
Nigam, J. N. (2002). “Bioconversion of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose-fermenting yeast,” J. Biotechnol. 97(2), 107-116. DOI: 10.1016/S0168-1656(02)00013-5
Nolan, W. J., and Kirmse, D. W. (1974). “The paper marketing properties of water hyacinth,” J. Aquat. Plant Manag. 12, 90-97.
Pintor-Ibarra, L. F., Rivera-Prado, J. J., Ngangyo-Heya, M., and Rutiaga-Quiñones, J. G. (2018). “Evaluation of the chemical components of Eichhornia crassipes as an alternative raw material for pulp and paper,” BioResources 13(2), 2800-2813. DOI: 10.15376/biores.13.2.2800-2813
Poukka, O., Isotalo, I., and Gullichsen, J. E. (1999). “Optimal delignification degrees of cooking and oxygen/alkali stage in production of ECF bleached softwood kraft,” Paperi ja Puu. 81(4), 316-324
Promdee, K., Vitidsant, T., and Vanpetch, S. (2012). “Comparative study of some physical and chemical properties of bio-oil from manila grass and water hyacinth transformed by pyrolysis process,” International Journal of Chemical Engineering and Applications 3(1), 72-75. DOI: 10.7763/IJCEA.2012.V3.163
Rapson, W. H. (1963). The Bleaching of Pulp, TAPPI Monograph Series No. 27. New York, pp. 245-296.
Reales-Alfaro, J. G., Trujillo, L. T., Arzuaga, G., Castaño, H., and Polo, A. (2013). “Acid hydrolysis of water hyacinth to obtain fermentable sugars,” CT&F – Ciencia, Tecnología y Futuro 5(2), 101-112.
Rodriguez, L. F. (2006). “Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur,” Biol Invasions 8(4), 927-939. DOI: 10.1007/s10530-005-5103-3
Rutiaga Quiñones, J. G., Rivera Prado, J. J., and Sanjuán Dueñas, R. (1998). “Evaluación físicomecánica de la pulpa kraft blanqueada de la madera de Pinus douglasiana Martínez,” Ciencia Forestal en México 23(83), 17-31
Saha, S., and Ray, A. K. (2011). “Evaluation of nutritive value of water hyacinth (Eichhornia crassipes) leaf meal in compound diets for Rohu, Labeo rohita (Hamilton, 1822) fingerlings after fermentation with two bacterial strains isolated from fish gut,” Turk. J. Fish Aquat. Sc. 11, 199-207. DOI: 10.4194/trjfas.2011.0204
Satyanagalakshmi, K., Sindhu, R., Binod, P., Ushua, K., Sukumaran, R. K., and Pandey, A. (2011). “Bioethanol production from acid pretreated water hyacinth by separate hydrolysis and fermentation,” J. Sci. Ind. Res. 70(1), 156-161.
SCAN-CM 15:88. (1988). “Viscosity in cupri-ethylenediamin (CDE) solution,” SCAN-CM 15:88. SCAN-test standards. Scandinavian Pulp, Paper and Board testing committee, Stockholm, Sweden.
Sharma, N., Bhawana, Godiyal, R. D., Thapliyal, B. P., and Anupam, K. (2020). “Morphological and anatomical characterization of bleached soda, soda-AQ and kraft pulps from essential oil isolated citronella grass,” Materials Today: Proceedings. In Press, Corrected Proof. DOI: 10.1016/j.matpr.2020.01.373
Sharma, N., Godiyal, R. D., Bhawana, Thapliyal, B. P., and Anupam, K. (2017). “Pulping and bleaching of hydro destillation waste of citronella grass (Cymbopogon winterianus Jowitt) for papermaking,” Waste Biomass Valor. DOI: 101007/s12649-016-9791-y
Shoyakubov, R. S., and Aitmetova, K. I. (1999). “Chemical composition of Eichhornia crassipes and Pistia stratiotes,” Chem. Nat. Compd. 35(2), 227-228. DOI: 10.1007/BF02234946
Singh, A., and Bishnoi, N. R. (2013). “Comparative study of various pretreatment techniques for ethanol production from water hyacinth,” Indust. Crop. Prod. 44(1), 283-289. DOI: 10.1016/j.indcrop.2012.11.026
Sithole, B. B., and Allen, L. (2002). “The effects of wood extractives on system closure,” Tappsa J. 105(7), 22-30.
Stiers, I., Crohain, N., Josens, G., and Triest, L. (2011). “Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds,” Biological Invasions 13(12), 2715-2726. DOI: 10.1007/s10530-011-9942-9
Suchy, M., and Argyropoulos, D. S. (2002). “Catalysis and activation of oxygen and peroxide delignification of chemical pulps: A review,” Tappi J. 1(2), 1-18
Swanson, J. W., and Steber, A. J. (1959). “Fiber surface area and bonded area,” Tappi J. 42(12), 986-994
Tan, S. J., Supri, A. G., and Chong, K. M. (2015). “Properties of recycled high-density polyethylene/water hyacinth fiber composites: The effect of different concentration of compatibilizer,” Polym. Bull. 72(8), 2019-2031. DOI: 10.1007/s00289-015-1387-3
TAPPI T 205 sp-02 (2002). “Forming handsheets for physical tests of pulp,” TAPPI Press, Atlanta, GA.
TAPPI T 233 cm-06 (2006). “Fiber length of pulp by classification,” TAPPI Press, Atlanta, GA.
TAPPI T 236 cm-99 (1999). “Kappa number of pulp,” TAPPI Press, Atlanta, GA.
TAPPI T 412 om-06 (2006). “Moisture in Pulp, Paper and Paperboard,” TAPPI Press, Atlanta, GA.
TAPPI T 413 om-93 (1993). “Ash in wood, pulp, paper and paperboard: combustion at 900°C,” TAPPI Press, Atlanta, GA.
TAPPI T 452 om-02 (2002). “Brightness of pulp, paper, and paperboard (directional Reflectance at 457 nm),” TAPPI Press, Atlanta, GA.
TAPPI T 519 om-02 (2002). “Diffuse opacity of paper (d/0 paper backing),” TAPPI Press, Atlanta, GA.
TAPPI T 625 cm-85 (1984). “Analysis of soda and sulfate black liquor,” TAPPI Press, Atlanta, GA.
Téllez-Sánchez, C., Ochoa-Ruíz, H. G., Sanjuán-Dueñas, R., and Rutiaga-Quiñones, J. G. (2010). “Componentes químicos del duramen de Andira inermis (W. Wright) DC. (Leguminosae),” Revista Chapingo Serie Ciencias Forestales y del Ambiente 16(1), 87-93. DOI: 10.5154/r.rchscfa.2009.11.046
Thamaraiselvi, P. L., and Jayanthi, P. (2012). “Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Eichhornia crassipes (Mart.) Solms.,” Asian J. Plant Sci. Res. 2(2), 115-122.
Torres, L. F., Melo, R., and Colodette, J. L. (2005). “Pulpa kraft blanqueada a partir de Pinus tecunumanii,” Bosque 26(2), 115-122. DOI: 10.4067/S0717-92002005000200014
Tran, H., and Vakkilainnen, E. K. (2007). “Advances in the kraft chemical recovery process, in: 3rd ICEP (International Colloquium on Eucalyptus). Belo, Horizonte, Brazil, pp. 4-7. http://www.eucalyptus.com.br/icep03/200Tran.text.pdf.pdf
Vakkilainen, E. K. (2005). “Kraft recovery boilers – Principles and practice,” The Finnish Recovery Boiler Committée, Valopaino Oy, Helsinki, Finland.
Vijetha, P., Kumaraswamy, K., Prasanna Kumar, Y., Satyasree, N., and Siva Prasad, K. (2014). “Biosorption of Cu, Zn and Pb by Eichhornia crassipes: Thermodynamic and isotherm studies,” International Journal of Scientific & Technology Research 3(3), 439-443.
Villamagna, A. M., and Murphy, B. R. (2010). “Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes),” Freshw. Biol. 55, 282-298. DOI: 10.1111/j.1365-2427.2009.02294.x
Wan Daud, W. R., Mazlan, I., and Law, K. N. (2009). “Effects of kraft pulping variables on pulp and paper properties of Acacia mangium kraft pulp,” Cellulose Chem. Technol. 43(1-3), 9-15.
Widyanto, L. S., Sopannata, A., and Friend U. S. (1983). “Water hyacinth as a potential plant in a paper factory,” J. Aquat. Plant Manage 21, 32-35.
Young, J. H. (1991). “Preparación de la fibra y flujo de alimentación de pasta,” in: Pulpa y Papel: Química y Tecnología Química, J. P. Casey (ed.), Volumen II, LIMUSA. México, pp. 25-136.
Article submitted: February 22, 2020; Peer review completed: April 18, 2020; Revised version received and accepted: October 14, 2020; Published: October 22, 2020.
DOI: 10.15376/biores.15.4.9243-9264
