Abstract
Lignocellulolytic white-rot fungi allow the bioconversion of agricultural wastes into value-added products that are used in a myriad of applications. The aim of this work was to use corn residues (Zea mays L.) to produce valuable products under solid-state fermentation (SSF) with Pleurotus ostreatus. White-rot fungus P. ostreatus was isolated from maize silage (MS) and thereafter it was inoculated on MS as substrate and compared with maize stover (MSt) and maize cobs (MC) to determine the best lignocellulosic substrate for the production of lignocellulolytic enzymes and extracellular protein. The MS gave the highest productivity of CMCase (368.2 U/mL), FPase (170.5 U/mL), laccase (11.4 U/mL), and MnPase (6.6 U/mL). This is compared to productivity on MSt of 222 U/mL, 50.2 U/mL, 4.55 U/mL, and 2.57 U/mL, respectively; and productivity on MC at the same incubation period as 150.5 U/mL, 48.2 U/mL, 3.58 U/mL, and 2.5 U/mL, respectively. The levels of enzyme production declined with increasing incubation period after 15 and 20 days using MS and MC, respectively, as substrates. Maximum liberated extracellular protein content (754 to 878 µg/mL) was recorded using MS, while a low amount (343 to 408 µg/mL) was liberated with using MSt and MC.
Download PDF
Full Article
Lignocellulolytic Activity of Pleurotus ostreatus under Solid State Fermentation Using Silage, Stover, and Cobs of Maize
Magdah Ganash,a Tarek M. Abdel Ghany,b,* Mohamed A. Al Abboud,c Mohamed M. Alawlaqi,c Husam Qanash,d and Basma H. Amin f
Lignocellulolytic white-rot fungi allow the bioconversion of agricultural wastes into value-added products that are used in a myriad of applications. The aim of this work was to use corn residues (Zea mays L.) to produce valuable products under solid-state fermentation (SSF) with Pleurotus ostreatus. White-rot fungus P. ostreatus was isolated from maize silage (MS) and thereafter it was inoculated on MS as substrate and compared with maize stover (MSt) and maize cobs (MC) to determine the best lignocellulosic substrate for the production of lignocellulolytic enzymes and extracellular protein. The MS gave the highest productivity of CMCase (368.2 U/mL), FPase (170.5 U/mL), laccase (11.4 U/mL), and MnPase (6.6 U/mL). This is compared to productivity on MSt of 222 U/mL, 50.2 U/mL, 4.55 U/mL, and 2.57 U/mL, respectively; and productivity on MC at the same incubation period as 150.5 U/mL, 48.2 U/mL, 3.58 U/mL, and 2.5 U/mL, respectively. The levels of enzyme production declined with increasing incubation period after 15 and 20 days using MS and MC, respectively, as substrates. Maximum liberated extracellular protein content (754 to 878 µg/mL) was recorded using MS, while a low amount (343 to 408 µg/mL) was liberated with using MSt and MC.
Keywords: Pleurotus ostreatus; Lignocellulolytic enzymes; Silage; Stover; Cobs; Maize
Contact information: a: Biology Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia; b: Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt 11725; c: Biology Department, Faculty of Science, Jazan University, Jazan, Saudi Arabia; d: Medical Laboratory Science Department, College of Applied Medical Sciences, University of Hail, Hail, Saudi Arabia; e: Molecular Diagnostics and Personalized Therapeutics Unit, University of Hail, Hail, Saudi Arabia; f: The Regional Centre for Mycology and Biotechnology ( RCMB), Al-Azhar University, Cairo, Egypt,*Corresponding author: tabdelghany.201@azhar.edu.eg
GRAPHICAL ABSTRACT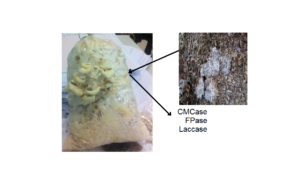
INTRODUCTION
Lignocellulosic biomass is one of the most abundant renewable natural resources in the world (Dong et al. 2020). Among the different cereal crops that are cultivated worldwide, corn (Zea mays) is one of the most abundant crops (Abdel Ghany 2012). Therefore, large amounts of lignocellulosic materials as waste are collected from corn fields (Rahman et al. 2020). Agricultural and agro-industrial activities also produce tons of by-products such as sugarcane bagasse, citrus bagasse, fruit peel, corn straw, and corncobs. The existence of these wastes in the field leads to a serious disposal problem for the agroindustries. Because these residues are nutritious, they have ample potential for being used directly as feed or as components for industrially formulated animal diets (Yang et al. 2001; Rahman et al. 2020). Intensive agriculture practices, particularly in the recent decades, has resulted in an increase in lignocellulosic biomass and its wastes, besides the wastes of food manufacturing (Mtui and Nakamura 2005; Peng et al. 2020). The abundance of these wastes either pre-harvest or post-harvest, has encouraged scientists to re-purpose these wastes and create vital products (Foyle et al. 2007; Abdel Ghany and Bakri 2019; Abdel Ghany et al. 2020; Rahman et al. 2020). Various studies have shown that lignocellulolytic fungi, particularly white-rot fungi (WRF), such as Pleurotus ostreatus, P. cornucopiae, and P. kumm, play a critical role for degrading lignocellulosic wastes and assist its conversion into an attractive bioproduct in many fields (Moya et al. 2016; Peng et al. 2020). Recently, Han et al. (2020) mentioned the potential ability of P. ostreatus for lignocellulolytic enzymes production under solid-state fermentation (SSF) using variable lignocellulosic sources.
The main value-added products recovered include enzymes, reducing sugars, furfural, ethanol, protein and amino acids, carbohydrates, lipids, organic acids, phenols, activated carbon, degradable plastic composites, cosmetics, biosorbent, resins, medicines, foods and feeds, methane, biopesticides, biopromoters, secondary metabolites, surfactants, fertilizer, and other miscellaneous products (Demirbas 2008; Rodríguez-Luna et al. 2020). The biotransformation of lignocellulosic wastes by WRF was achieved by the generation of extracellular ligninolytic enzymes such as laccase, versatile peroxidases, benzoquinone reductases, lytic polysaccharide monooxygenases, etc. (Gupta et al. 2018). Previously, DeSouza et al. (2006) mentioned that lignin peroxidases (LiPase), manganese-dependent peroxidases (MnPase), and laccase are the three major lignin-degrading enzymes with great potential in industrial applications. These enzymes are responsible for the degradation of the major constituents, i.e., cellulose, hemicellulose, and lignin, into low molecular weight molecules that can be assimilated for fungal nutrition (Abdel Ghany and Bakri 2019). White-rot fungi secrete one or more of the three enzymes essential for lignin degradation. The same unique nonspecific mechanisms that give these fungi the ability to degrade lignin also allow them to degrade a wide range of pollutants, including polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), explosives, pesticides, and dyes (Ganash et al. 2016; Elisashvili et al. 2020). The aim of the present study was to explore the use of corn residues (Zea mays L.) including silage as well as stover and cobs under SSF conditions as an eco-friendly manner to produce valuable products of lignocellulolytic enzymes.
EXPERIMENTAL
Materials
Fungi and maize wastes used
One fungus was isolated from maize silage (MS) (Zea mays L.) that is made out of whole ensiled maize plants. The isolate was purified and identified morphologically as Pleurotus ostreatus according to taxonomic keys of Sharma (1989). The fungus was cultivated on potato dextrose agar (PDA) plates for 12 d at 30 °C. Mycelium agar plugs 0.5 mm in diameter (cut along the edge of an actively growing colony) were used as inoculum. Different samples of maize wastes including MS, maize stover (MSt) consisting of leaves and stems without grains and cobs, maize cobs (MC) after removal of grains were dried, cut to different small lengths, ground in an electric grinder, and passed through a sieve (mesh size 5 mm) to obtain uniform sized particles.
Enzymes preparation
Five grams of each sample under solid-state fermentation (SSF) conditions was placed in a 250-mL conical flask. The substrate was moistened with 20 mL moisture agent (distilled water) and then autoclaved at 121 °C for 30 min followed by inoculation by 5 discs (0.5 mm) of growing fungus on PDA, and then homogenized, followed by incubation at 30 °C for different incubation periods up to 25 d. Distilled water (50 mL) was added to each flask after the incubation period and mixed for 30 min on a shaker (300 rpm). The content of the flask was clarified through muslin cloth confined in a glass funnel into a clean dry flask. The filtrates were centrifuged at 80,000 rpm for 10 min. The obtained supernatant was used as crude enzymes.
Protein and reducing sugar assays
Approximately 1.0 mL of 3,5-dinitrosalicylic acid (DNS) reagent was mixed with 1.0 mL of sugar sample, and 1.0 mL of distilled water was used a blank. The tubes containing the reaction mixture were placed in boiling water bath for 10 min, followed by cooling at room temperature, then 5 mL of distilled water was added to each tube. The change in intensity of yellow to orange color was recorded by measuring the absorbance at 540 nm. Glucose was used as a standard (Miller 1959). Estimation of the total soluble protein in the fungal filtrate (supernatant) was carried out following Lowry et al. (1951) method, using bovine serum albumin as a standard.
Carboxymethyl cellulase (CMCase) assay
Carboxymethyl cellulose (CMC) as a substrate of CMCase was prepared by the addition of 1.0 g of CMC to 100 mL of sodium acetate buffer with pH 5.0. Then, 1.0 mL of the supernatant (enzyme) was added to substrate in test tube, followed by incubation at 63 °C for 30 min; then the liberated reducing sugar was estimated by using DNS reagent (Miller 1959), through recording the absorbance at 540 nm. Distilled water was added instead of enzyme for blank experiments. Reducing sugar content was determined via glucose standard curve. One unit of CMCase is considered as the micromoles of glucose liberated per mL of supernatant per minute (Wang et al. 1988).
Manganase peroxidase (MnPase) assay
For MnPase assay, 100 µL of the supernatants were added to 1.0 mL of 2 mM solution of 2,2-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS) and 1.0 mM MnSO4 in Mcllvaine buffer (pH 5.0) followed by the addition 0.4 mM H2O2 for peroxidase activity initiation (Field et al. 1996; Garzillo et al. 2001). The enzymatic activity was estimated in IU by monitoring of the change in absorbance at 420 nm (ABTS), Є420 = 36 Mm-1cm-1 at 30 °C.
Laccase assay.
For laccase assay, 100 µL of the supernatants were added to 1.0 mL of 2 mM ABTS in Mcllvaine buffer (pH 5.0). Then the enzymatic activity was recorded according to Garzillo et al. (2001). The enzymatic activity was estimated in IU by monitoring the absorbance change at 420 nm (ABTS), Є420 = 36 Mm-1cm-1 at 30 °C.
Filter-paperase (FPase) assay
According to Gadgil et al. (1995), FPase was assayed by mixing 1.0 mL of the supernatant with 2 mL of 0.1 M citrate buffer (pH 4.8) containing 0.04 g filter paper (Whatman No. 1) in a clean dry tube. Then, the reaction mixture was incubated at 50 °C for 1 h. Then, the liberated reducing sugar was measured by the DNS method by measuring the absorbance at 540 nm. Distilled water was used instead of enzyme in blank sample. Reducing sugar was determined via glucose standard curve. One unit of FPase, is the micromole of glucose liberated per mL of supernatant per min.
Statistical Analysis
The mean ± SD (standard deviation) of three results were calculated by computer using SPSS ver. 22.0 software. Error bars in all figures referred to SD of three values of treatments
RESULTS
In this study, P. ostreatus, a white rot-fungus was isolated from MS (Fig. 1A), applied for maize wastes degradation, and production of lignocellulolytic enzymes. PDA medium was suitable for sub-culture and growing the inoculum of P. ostreatus (Fig. 1B), followed by appearance of fruiting bodies using maize silage (Fig. 1C). Lignocellulose degradation by P. ostreatus was further studied on maize residues including MS, MSt, and MC.
Fig. 1. Maize silage showing P. ostreatus growth (A) where arrows refer to growth, P. ostreatus growth on PDA (B), and fruiting stage of P. ostreatus on maize silage (C)
The production amounts of CMCase by P. ostreatus were recorded after 5 d on MS (100.25 U/mL), MSt (76.42 U/mL), and MC (44.54 U/mL). The CMCase production increased with increasing incubation period of 15 d in MS, and of 20 d in MSt and MC utilizations (Fig. 2).
The highest amount of CMCase (368.24 U/mL) was recorded using MS compared with using MC or MSt as substrates. The highest FPase were recorded after 10 d (170.50, 50.22, and 48.20 U/mL) on MS, MSt, and MC, respectively. Surprisingly, at 25 d of incubation period, the FPase production of 90.34 U/mL was achieved, silage produced a lesser amount (150.45 U/mL) at 5 d of incubation period, unlike leaves with stems or MC (Fig. 2).
Fig. 2. Production of CMCase by P. ostreatus on cobs, stover, and silage of maize under SSF
Fig. 3. Production of FPase by P. ostreatus on cobs, stover, and silage of maize under SSF
The results revealed that the highest laccase production (11.45 and 4.55 U/mL) was obtained after 15 d of incubation period on MS and MSt substrates, while 20 d was the optimum period for laccase production (3.77 U/mL) on cobs substrate (Fig. 4). As the incubation period was increased, the MnPase production also increased to reach the highest productivity (6.58 U/mL and 2.58 U/mL) after 15 d on MS and MSt, respectively (Fig. 5). Meanwhile, it reached the highest production of 3.77 U/mL at 20 d of incubation period using the MC substrate.
Fig. 4. Production of Laccase by P. ostreatus on cobs, stover, and silage of maize under SSF
Fig. 5. Production of MnPase by P. ostreatus on cobs, stover, and silage of maize under SSF
The highest extracellular protein content was recorded on MS within the range of 754 to 878 µg/mL, while the lowest extracellular protein was recorded on MSt and MC substrates within the range 343 to 408 µg/mL (Fig. 6).
Fig. 6. Extracellular protein production by P. ostreatus on cobs, stover, and silage of maize
DISCUSSION
Various lignocellulosic wastes that are generated worldwide result in serious environmental pollution and loss of valuable materials. Therefore, its bioconversion to several value-added products such as enzymes is important. The production of some lignocellulolytic enzymes by P. ostreatus was evaluated in the current study under SSF conditions using MS, MSt, and MC as substrates. The three substrates are from the same plant but differ in their contents; therefore, it is important to evaluate and compare them as potential sources of lignocellulolytic enzymes. Fungi compared with other microorganisms are able spread across the surface and penetrate inside spaces of substrates under SSF conditions. Early and recent studies mentioned that P. ostreatus contributes to the disintegration of cellulose, hemicellulose, and lignin via the synthesis of enzymes (Cohen et al. 2002; Alfaro et al. 2020). Several studies have reported that white-rot fungi are capable of breaking down all constituents of lignocellulose (Huang et al. 2008; Rahman et al. 2020; Alvarez and Bautista 2021). As a result of this degradation, some of the important products of the biotechnological applications were obtained. Propagation of P. ostreatus on lignocellulosic wastes plays an important role in maintaining natural resources and ecosystems via reutilizing these wastes (Hultberg et al. 2020). According to Han et al. (2020), the mode of cultivation of P. ostreatus and types of substrates play a critical role in enzyme productivity.
P. ostreatus produced CMCase, FPase, MnPase, laccase, etc. using MS, MSt, and MC substrates. Trejo-López et al. (2020) obtained laccases and cellulases using P. ostreatus by degrading agricultural wastes and applied these substances to enhance goat milk products. These studies confirmed the safe applications of P. ostreatus. Currently MS has become the major feed constituent in the ration of dairy animals in many countries.
The results obtained in this study showed the production of variable enzymes depending on the substrate type. For example, the amount of CMCase produced was 368.2, 222.5, and 150.5 U/mL utilizing MS, MSt, and MC (Fig. 2), respectively, beside other enzymes. These findings were in agreement with Vladimir et al. (2009) who mentioned that lignocellulosic substrate type had the greatest impact on enzyme secretion. The greatest amount of enzymes using MS might be attributed to the constituents of MS that may contain proteins, minerals, and fats. These constituents encourage fungal growth and ligninolytic enzymes activity. The MSt and MC were lacking higher contents of these constituents. Although they come from the same plant, the MSt, MC, and MS were found to differ in chemical structure, as well as in mechanical and physical properties that influence the enzyme production.
With increases in the incubation period, the productivity of enzymes increased initially, but at 15 d or at 20 d of incubation the productivity began to decline (Figs. 2 to 5). The early rate of higher production of enzymes activity on maize substrates may be due to mycelial growth, which then decreased at fruiting stage that started after two weeks. The MSt and MC indicated the highest productivity of CMCase up to 20 d of incubation, unlike MS. This phenomenon might be attributed to the hemicellulose and cellulose degradation that were faster for MS than MSt and MC. Furthermore, the difference in enzyme activity in the current study could also be attributed to the ingredients of applied substrate, as reported partially by Ozcirak and Ozturk (2017).
According to Sharma and Arora (2010), the maximum laccase production (0.72 U/L) was obtained after 21 d incubation of wheat straw inoculated by Phlebia floridensis at 27 °C. From the obtained results, P. ostreatus has drawn considerable attention as an appropriate fungus for the creation of lignin-degrading enzymes or direct application in lignocellulose bioconversion processes.
The highest protein contents were obtained in MS compared to MSt and MC (Fig. 6). This observation may be explained on the basis of substrate constituents, and also that P. ostreatus was able to secrete a large quantity of extracellular protein from silage. However, these proteins as indicated from the results for lignocellulolytic enzymes, may be due to production of another lignocellulolytic enzymes or proteolytic enzymes. These findings agree with previous studies (Bosco et al. 1999; Ruggeri and Sassi 2003)
CONCLUSIONS
- The best substrates from corn residues for lignocellulase production were determined in this study and followed the order: silage > stover > cobs of corn (Zea mays L.) (MS > MSt > MC).
- The highest productivity for lignocellulases was recorded at 15 d of incubation period for most enzymes.
- Current findings designate that maize waste was evaluated for its potential application in the creation of lignocellulosic enzymes. However, the variability of waste constitution affects enzymes production and its amounts.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
REFERENCES CITED
Abdel Ghany, T. M. (2012). “Fungal leaf spot of maize: Pathogen isolation, identification and host biochemical characterization,” Mycopath. 10(2), 41-49
Abdel Ghany, T. M., and Bakri, M. M. (2019). “Effectiveness of a biological agent (Trichoderma harzianum and its culture filtrate) and a fungicide (methyl benzimacold-2-ylcarbamate) on the tomato rotting activity (growth, cellulolytic, and pectinolytic activities) of Alternaria solani,” BioResources 14(1), 1591-1602. DOI: 10.15376/biores.14.1.1591-1602
Abdel Ghany, T. M., Bakri, M. M., Al-Rajhi, A. M., Al Abboud, M. A., Alawlaqi, M. M., and Shater, A. R. M. (2020). “Impact of copper and its nanoparticles on growth, ultrastructure, and laccase production of Aspergillus niger using corn cobs wastes,” BioResources 15(2), 3289-3306. DOI: 10.15376/biores.15.2.3289-3306
Alfaro, M., Majcherczyk, A., Kües, U., Ramírez, L., and Pisabarro, A. G. (2020). “Glucose counteracts wood-dependent induction of lignocellulolytic enzyme secretion in monokaryon and dikaryon submerged cultures of the white-rot basidiomycete Pleurotus ostreatus,” Sci. Rep. 10, Article Number 12421. DOI: 10.1038/s41598-020-68969-1
Alvarez, L. V., and Bautista, A.B. (2021). “Growth and yield performance of Pleurotus on selected lignocellulosic wastes in the vicinity of PUP main campus, Philippines,” Indian Journal of Science and Technology 14(3), 259-269. DOI: 10.17485/IJST/v14i3.389
An, Q., Wu, X. J., Han, M. L., Cui, B. K., He, S. H., Dai, Y. C., and Si, J. (2016). “Sequential solid-state and submerged cultivation of white rot fungus Pleurotus ostreatus on lignocellulosic biomass for the activity of lignocellulolytic enzymes,” BioResources 11(4), 8791-8805. DOI: 10.15376/biores.11.4.8791-8805
Bosco, F., Ruggeri, B., and Sassi, G. (1999). “Performances of a trickle-bed reactor (TBR) for exoenzymes production by Phanerochaete chrysosporium: Influence of superfacial liquid velocity,” Chem. Eng. Sci. 54(15-16), 3163-3169. DOI: 10.1016/S0009-2509(98)00365-0
Cohen, R., Persky, L., and Hadar, Y. (2002). “Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus,” Appl. Microbiol. Biotechnol. 58(5), 582-594. DOI: 10.1007/s00253-002-0930-y
Demirbas, A. (2008). “Heavy metal adsorption onto agro-based waste materials: A review,” J. Hazard. Mater. 157(2-3), 220-229. DOI: 10.1016/j.jhazmat.2008.01.024
DeSouza, D. T., Tiwari, R., Sah, A. K., and Raghukumar, C. (2006). “Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes,” Enzyme Microb. Technol. 38(3-4), 504-511. DOI: 10.1016/j.enzmictec.2005.07.005
Dong, H., Zheng, L., Yu, P., Jiang, Q., Wu, Y., Huang, C., and Yin, B. (2020). “Characterization and application of lignin-carbohydrate complexes from lignocellulosic materials as antioxidants for scavenging in vitro and in vivo reactive oxygen species,” ACS Sustain. Chem. Eng. 8(1), 256-266. DOI: 10.1021/acssuschemeng.9b05290
Elisashvili, V., Asatiani, M. D., and Kachlishvili, E. (2020). “Revealing the features of the oxidative enzyme production by white-rot Basidiomycetes during fermentation of plant raw materials,” in: Microbial Enzymes and Biotechniques, P. Shukla (ed), Springer, New York, NY, USA. DOI: 10.1007/978-981-15-6895-4_7
Foyle, T., Jennings, L., and Mulcahy, P. (2007). “Compositional analysis of lignocellulosic materials: Evaluation of methods used for sugar analysis of waste paper and straw,” Bioresource Technol. 98(16), 3026-3036. DOI: 10.1016/j.biortech.2006.10.013
Gadgil, N. J., Daginawala, H. F., Chakakrabarti, T., and Khanna, P. (1995). “Enhanced cellulose production by mutant of Trichoderma reesei,” Enzyme Microb. Technol. 17(10), 942-946. DOI: 10.1016/0141-0229(94)00131-A
Ganash, M. A., Abdel Ghany, T. M., and Reyad, A. M. (2016). “Pleurotus ostreatus as a biodegradator for organophosphorus insecticide Malathion,” J. Environ. Anal. Toxicol. 6(3), Article Number 369. DOI: 10.4172/2161-0525.1000369
Garzillo, A. M., Colao, M. C., Buonocore, V., Oliva, R., Falcigno, L., Saviano, M., Santoro, A. M., Zappala, R., Bonomo, R. P.,Bianco, C., Giardina, P., Palmieri, G., and Sannia, G. (2001). “Structural and kinetic characterization of native laccases from Pleurotus ostreatus, Rigidoporus lignosus, and Trametes trogii,” Journal of Protein Chemistry 20(3), 191-201. DOI: 10.1023/A:1010954812955
Gupta, D. K., Rühl, M., Mishra, B., Kleofas, V., Hofrichter, M., Herzog, R., Pecyna, M. J., Sharma, R., Kellner, H., and Hennicke, F. (2018). “The genome sequence of the commercially cultivated mushroom Agrocybe aegerita reveals a conserved repertoire of fruiting-related genes and a versatile suite of biopolymer-degrading enzymes,” BMC Genom. 19(1), 1-13. DOI: 10.1186/s12864-017-4430-y
Han, M., An, Q., He, S., Zhang, X., Zhang, M., Gao, X., Wu, Q., and Bian, L. (2020). “Solid-state fermentation on poplar sawdust and corncob wastes for lignocellulolytic enzymes by different Pleurotus ostreatus strains,” BioResources 15(3), 4982-4995. DOI: 10.15376/biores.15.3.4982-4995
Huang, D. L., Zeng, G. M., Feng, C. L., Hu, S., Jiang, X. Y., Tang, L., Su, F. F., Zhang, Y., Zeng, W., and Liu, H. L. (2008). “Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity,” Environm. Sci. Technol. 42(13), 4946-4951. DOI: 10.1021/es800072c
Hultberg, M., Ahrens, L., and Golovko, O. (2020). “Use of lignocellulosic substrate colonized by oyster mushroom (Pleurotus ostreatus) for removal of organic micropollutants from water,” J. Environ. Manage. 272, Article Number 111087. DOI: 10.1016/j.jenvman.2020.111087
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). “Protein measurement with folin phenol reagent,” J. Biol. Chem. 193(1), 265-275.
Miller, G. L. (1959). “Use of dinitrosalicylic acid reagent for determination of reducing sugar,” Anal. Chem. 31(3), 426-428. DOI: 10.1021/ac60147a030
Moya, R., Berrocal, A., Rodríguez-Zúñiga, A., Rodriguez-Solis, M., Villalobos-Barquero, V., Starbird, R., and Vega-Baudrit, J. (2016).”Biopulp from pineapple leaf fiber produced by colonization with two white-rot fungi: Trametes versicolor and Pleurotus ostreatus,” BioResources 11(4), 8756-8776. DOI: 10.15376/biores.11.4.8756-8776
Mtui, G., and Nakamura, Y. (2005). “Bioconversion of lignocellulosic waste from selected dumping sites in Dar es Salaam,” Tanzania Biodegrad. 16, article no. 493. DOI: 10.1007/s10532-004-5826-3
Ozcirak, E. S., and Ozturk, R. (2017). “Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus,” Annals of Agrarian Science 15(2), 273-77. DOI: 10.1016/j.aasci.2017.04.003
Peng, W., Lü, F., Hao, L., Zhang, H., Shao, L., and He, P. (2020). “Digestate management for high-solid anaerobic digestion of organic wastes: A review,” Bioresource Technol. 297, Article ID 122485. DOI: 10.1016/j.biortech.2019.122485
Rahman, S., Mondal, I. H., Yeasmin, M. S., Abu Sayeed, M., Hossain, A., and Ahmed, M. B. (2020). “Conversion of lignocellulosic corn agro-waste into cellulose derivative and its potential application as pharmaceutical excipient,” Processes 8(6), Article Number 711. DOI: 10.3390/pr8060711
Rodríguez-Luna, D., Ruiz, H. A., González-Morales, S., Sandoval-Rangel, A., Cabrera de la Fuente, M., Charles-Rodríguez, A. V., and Robledo-Olivo, A. (2020). “Recovery of melon residues (Cucumis melo) to produce lignocellulolytic enzymes,” Biomass Convers. Biorefin. DOI: 10.1007/s13399-020-01055-8
Ruggeri, B., and Sassi G. (2003). “Experimental sensitivity analysis of a trickle bed bioreactor for lignin peroxidases production by Phanerochaete chrysosporium,” Process Biochemistry 38, 1669-1676. DOI: 10.1016/S0032-9592(02)00199-1
Sharma, O. P. (1989). Textbook of Fungi, 5th Ed., Tata McGraw-Hill, New Delhi.
Sharma, R., and Arora, D. (2010). “Changes in biochemical constituents of paddy straw during degradation by white rot fungi and its impact on in vitro digestibility,” J. Appl. Microbiol. 109(2), 679-686. DOI: 10.111/j.1365-2672.2010.04695.x
Trejo-López, M. T., Ayala-Martínez, M., Zepeda-Bastida, A., Franco-Fernández, M. J., and Soto-Simental, S. (2020). “Using spent Pleurotus ostreatus substrate to supplemented goats to increase fresh cheese yields,” Small Ruminant Research 195, Article ID 106297. DOI: 10.1016/j.smallrumres.2020.106297
Vladimir, E., Eva, K., Nino, T., Eka, M., Tamar, K., and Spiros, N. A. (2009). “Lignocellulose-degrading enzyme production by white-rot basidiomycetes isolated from the forests of Georgia,” World J. Microbiol. Biotechnol. 25(2), 331-339. DOI: 10.1007/s11274-008-9897-x
Wang, C., Hseu, T., and Huang, C. (1988). “Induction of cellulose by cello-oligosaccharides in Trichoderma koningii G-39,” J. Biotechnol. 9(1), 47-60. DOI: 10.1016/0168-1656(88)90014-4
Yang, X. X., Chen, H. Z., Gao, H. L., and Li, Z. H. (2001). “Bioconversion of corn straw by coupling ensiling and solid-state fermentation,” Bioresource Technol. 78(3), 277-280. DOI: 10.1016/S0960-8524(01)00024-4
Article submitted: December 31, 2020; Peer-review completed: March 27, 2021; Revised version received and accepted: March 29, 2021; Published: April 8, 2021.
DOI: 10.15376/biores.16.2.3797-3807
