Abstract
The production of ethanol from lignocellulosic biomass provides an alternative to fossil fuels. In this study, liquid hot water (LHW)-pretreated Miscanthus x giganteus (MxG) was used to produce bioethanol through simultaneous saccharification and fermentation (SSF). MxG was pretreated at temperatures between 170 and 200 °C, for 5 to 15 min. The pretreatment was able to remove between 68.3% and 77.0% of the lignin present in the biomass. The highest percentage yields of sugars from pretreated MxG after enzymatic saccharification (32 °C, pH 4.5, 48 h), by a cocktail of two enzymes were 44.0% glucose and 42.0% xylose of theoretical. Ethanol concentrations between 0.780 and 3.715 g/L, and a high ethanol yield of 71.8% of theoretical were obtained using Saccharomyces cerevisiae (ATCC 24858) for fermentation. A comparison of scanning electron micrographs of the pretreated biomass showed morphological changes that enhanced the release of glucose and bioethanol yield.
Download PDF
Full Article
Liquid Hot Water Pretreatment of Miscanthus X giganteus for the Sustainable Production of Bioethanol
Nana Abayie Boakye-Boaten, Shuangning Xiu,* Abolghasem Shahbazi, and Jonathan Fabish
The production of ethanol from lignocellulosic biomass provides an alternative to fossil fuels. In this study, liquid hot water (LHW)-pretreated Miscanthus x giganteus (MxG) was used to produce bioethanol through simultaneous saccharification and fermentation (SSF). MxG was pretreated at temperatures between 170 and 200 °C, for 5 to 15 min. The pretreatment was able to remove between 68.3% and 77.0% of the lignin present in the biomass. The highest percentage yields of sugars from pretreated MxG after enzymatic saccharification (32 °C, pH 4.5, 48 h), by a cocktail of two enzymes were 44.0% glucose and 42.0% xylose of theoretical. Ethanol concentrations between 0.780 and 3.715 g/L, and a high ethanol yield of 71.8% of theoretical were obtained using Saccharomyces cerevisiae (ATCC 24858) for fermentation. A comparison of scanning electron micrographs of the pretreated biomass showed morphological changes that enhanced the release of glucose and bioethanol yield.
Keywords: Bioethanol; Liquid hot water pretreatment; Simultaneous saccharification and fermentation; Miscanthus x giganteus
Contact information: Department of Natural Resources and Environmental Design, Biological Engineering Program, Sockwell Hall, NC A&T State University, 1601 E. Market St. Greensboro, NC, 27411;
* Corresponding author: xshuangn@ag.ncat.edu
INTRODUCTION
Presently, the world greatly depends on fossil fuels for its energy needs. Our strong dependence on fossil fuels comes from the intensive use and consumption of petroleum derivatives, which, combined with diminishing petroleum resources, causes environmental and political concerns (Cherubini 2010). According to the International Energy Agency (IEA), fossil fuels currently contribute about 80% of the global energy demand. This demand is projected to increase by 40% by 2035, with fossil fuels contributing 75%. This has necessitated the need to search for new energy sources.
Over the last several decades, biofuels, in particular ethanol, have gathered increasing attention as fuels for transportation (Kim et al. 2013). Bioethanol is used as a partial replacement for gasoline because of its high octane number and the ability to provide oxygen to fuel, resulting in reduced CO emissions (Galbe and Zacchi 2002).
There are important differences between biofuel crops with regards to the impacts they have on the environment and the economic incentives and consequences of their uses for bioenergy development. For instance first-generation biofuel biomass, such as corn require costly inputs and management, whereas second-generation biofuels materials, like energy grasses including giant Miscanthus can produce larger yields and have little to no requirements for fertilizer use (Smith et al. 2013). Second-generation biofuels from perennial crops have the potential to meet bioenergy goals with less land, thereby reducing risks of increased food prices and land use change (Smith et al. 2013). As a result, the use of lignocellulosic biomass for bioethanol production has become one of the major research and development foci in the biofuel industry. Using this abundant and renewable carbohydrate source in place of fossil fuels has been characterized as the most effective way to fight both the energy crisis and environmental problems caused by biowaste accumulation and carbon dioxide emissions from the petrochemical industries (Limayem and Ricke 2012).
Lignocellulosics consist primarily of cellulose, hemicellulose, and lignin, which make up about 90% of its dry matter. Cellulose and hemicellulose, which make up about two thirds of the cell wall dry matter, are polysaccharides that can be hydrolyzed to sugars and then fermented to bioethanol. Process performance, i.e., bioethanol yield from biomass, is directly related to cellulose, hemicellulose, and individual sugar concentrations in the feedstock (Balat 2011). Cellulosic materials are particularly attractive as feedstock for biofuel production because of their relatively low cost, great abundance, and sustainable supply. Cellulose is the most abundant biopolymer on earth, and biofuel production from cellulosic biomass has become the major focus of intensive research and development (Agbor et al. 2011). Lignocellulosic biomass contains approximately 40% to 50% cellulose, a glucose polymer; approximately 25% to 35% hemicellulose, a sugar heteropolymer; and approximately 15% to 20% lignin, a non-fermentable phenyl-propene unit; plus lesser amounts of minerals, oils, soluble sugars, and other components (Wyman et al. 2005). These polymers are associated with each other in a hetero-matrix to different degrees and varying relative composition depending on the type, species, and source of the biomass (Agbor et al. 2011).
One type of lignocellulosic biomass that has received much attention recently is Miscanthus x giganteus (MxG). MxG has attracted considerable attention as a dedicated energy crop. It is a non-invasive perennial grass (sterile hybrid) that requires little or no herbicide and nitrogen for its cultivation (El Hage et al. 2010). The species MxG is a large perennial hybrid resulting from a cross between Miscanthus sinensis and Miscanthus sacchariflorus (Parveen et al. 2011). It is a C4 perennial plant that can be cultivated on-site for between 15 to 20 years and grows to a height of up to 4 m (Jeżowski 2008). The remarkable adaptability of Miscanthus to different environments makes this novel crop suitable for establishment and distribution under a range of European and North American climatic conditions (Lewandowski et al. 2000).
To enhance the competitiveness of lignocellulosic ethanol, the need to develop cheaper raw materials such as energy crops for ethanol production is considered essential (Guo et al. 2008). Producing ethanol from lignocellulosic materials through biological conversion involves three main steps: (1) lignocellulose pretreatment, which converts the recalcitrant lignocelluloses structure to reactive cellulosic intermediates; (2) enzymatic hydrolysis, by which enzymes such as cellulases and hemicellulases, hydrolyze reactive intermediates to fermentable sugars (e.g., glucose and xylose); and (3) fermentation using microorganisms, which produces cellulosic ethanol or other bio-based chemicals (e.g., lactic acid and succinic acid) (Singh et al. 2014).
Pretreatment is one of the most expensive steps in the biological conversion of cellulosic biomass (Haghighi et al. 2013). It is essential for the removal of lignin, reduction of cellulose crystallinity, and increase in material porosity. An effective pretreatment should produce a significant percent of cellulose, support the lesser production of inhibitors, and be cost effective (Singh et al. 2014).
Liquid hot water (LHW) pretreatment is often employed in bioethanol production from lignocellulosic biomass. This is sometimes referred to as hydrothermolysis, aquasolv, uncatalyzed solvolysis, or aqueous fractionation. LHW pretreatment can efficiently hydrolyze the hemicelluloses (partially), modify the lignin, increase the surface area, and decrease the cellulose crystallinity and degree of polymerization (Singh et al. 2014). This method is generally conducted at elevated temperatures (120 to 260 °C) for a period of time (5 to 40 min) (Wan and Li 2011). Water and acetyl groups within hemicelluloses, which act as acids at around 200 °C, are believed to catalyze extensive hydrolysis of hemicellulose to its component sugars, primarily xylose (Wan and Li 2011). Beyond solubilization of lignin and hemicellulose within the biomass, hot water pretreatment is designed to avoid or lessen the formation of inhibitors that affect the subsequent downstream ethanol production processes of hydrolysis and fermentation. Liquid hot water pretreatment results in re-localization of lignin on the surface of the lignocellulosic material, producing a good performance in the enzymatic hydrolysis and fermentation.
In ethanol production from lignocellulosic materials, enzymatic hydrolysis and fermentation can be done separately or simultaneously. However, comparatively simultaneous saccharification and fermentation (SSF) is more favored than separate hydrolysis and fermentation (SHF). This is because in SSF, glucose released by the action of cellulase is converted quickly to ethanol by the fermenting microorganism, thus minimizing the end-product inhibition to cellulase caused by glucose and cellobiose accumulation (Zhao and Xia 2009). Furthermore, in SSF, the use of a single vessel for saccharification and fermentation, thus obviating the need for two operating vessels, increasing the simplicity of operation, lowering capital investment, and improving the process economics, is considered a useful advantage over SHF (Oberoi et al. 2011).
A few research works have been undertaken with Miscanthus as feedstock via various pretreatment and fermentation methods to produce ethanol. Using MxG as feedstock and alkali-extrusion pretreatment, SSF was used to optimize enzyme and biomass dosage, giving a maximized ethanol concentration of 67.0 g/L and an ethanol conversion rate of 88.1% for 25% loading of pretreated Miscanthus with 30 filter paper unit (FPU)/g glucose of enzyme (Kang et al. 2013). Oxalic acid pretreatment and a SSF process has also been used to achieve a maximum ethanol concentration of 20.2 g L-1 and a volumetric ethanol productivity of 0.28 g L-1 h-1 from Miscanthus (Scordia et al. 2013). Another study assessed the hydrolysis of hemicellulose in Miscanthus for ethanol production using a combined biomimetic and inorganic acid hydrolysis through a SSF process (Guo et al. 2012). A review of works on hydrothermal processing of biomass has also been conducted, with a focus on modelling, separation and application of the components of lignocellulosic biomass for value-added products (Ruiz et al. 2013)
This study investigated the possibility of enhancing ethanol production using LHW-pretreated MxG, assessing the impact of pretreatment conditions (temperature and residence time) on ethanol concentration and yield, employing a cocktail of enzymes for enzymatic hydrolysis, through a SSF process. To the best of our knowledge, no information is yet available that reports on the SSF of LHW-pretreated MxG for ethanol production. Since giant Miscanthus is a promising biomass with one of the highest energy-use efficiency (EUE) among various potential energy crops and since it is expected to play an important role in sustainable biomass fuel production in the new future (Głowacka 2011), this research is timely. Furthermore, it is important to study the use of water as a pretreatment agent, as it is readily available, cheaper than other pretreatment agents, and has the likelihood to result in fewer residual chemicals further downstream in the bioethanol production process.
EXPERIMENTAL
Sample Preparation
MxG was harvested from the North Carolina Agricultural and Technical State University farm, in the city of Greensboro, between May 2014 and August 2014 using a Tanaka TPH 270s-pole hedge trimmer to achieve consistent cuts. MxG was grown using various fertilizer application amounts (T1: 0 lbs/ac, T2: 60 lbs/ac, T3: 120 lbs/ac, T5: 280 lbs/ac). NPK complex 17-17-17 was used for growing the Miscanthus. The freshly cut MxG was immediately shredded into smaller sizes using a DR wood chipper/shredder (14.50 Pro Manual Start, DR Power Equipment, Vergennes, Vermont) and bagged in plain polythene. The harvested biomass was then pressed and separated into green juice and solid cake, using a Carver laboratory press (#2094 cage equipment, Carver Inc., Wabash, IN) at an optimized force of 133447 N for a period of 15 min to allow for effective separation and stored at a temperature of 4 oC to maintain its freshness for further processing. The green juice was stored in a freezer for use in further downstream processes as part of a biorefinery platform. The pressed solid cake was dried in an isotemp oven (Fisher Scientific, USA) at a temperature of 105 oC for a period of 24 h to completely remove any moisture from the solid MxG cake. Using a rotary knife mill (Thomas Model 4 Wiley mill, Thomas Scientific, Swedesboro, NJ) the dried MxG was ground to particle sizes between 0.3 and 0.6 mm for further analyses and downstream processing. T5 was used for pretreatment and SSF experimentations.
Biomass Analytical Procedures
Compositional analysis of the biomass was performed using the laboratory analytical procedures (LAPs) of the National Renewable Energy Laboratory. The moisture content of the biomass was determined by the method of LAP #001 (Sluiter et al. 2008a). The ash content of the biomass was determined by the method of LAP #005 (Sluiter et al. 2008b). Structural analyses were carried out according to the methods using LAP #003 for acid-insoluble lignin and the determination of carbohydrates in biomass using HPLC (Sluiter et al. 2011)
Liquid Hot Water (LHW) Pretreatment
This was carried out in a high-pressure continuous stirred tank reactor (Model 4570, Parr instrument company, Moline, IL). The reactions were run at final temperatures of 170, 180, 190, and 200 °C, with residence times of 5, 10, and 15 min. A slurry of 10% biomass and 90% aqueous solution was prepared by stirring 25 g of biomass into 250 g of water. The batch reactor is rated up to a working pressure of 3.45 MPa and working temperature of 500 °C. A heavy-duty magnetic drive stirrer associated with the reactor was used for the mixing. A J-type thermocouple was inserted into the reactor to measure the temperature of the reaction media. A standard pressure gauge was installed on the reactor head. A PID controller was used to control and indicate the temperature of the reactor. The cylindrical reactor was placed in a tubular electric heater. The biomass slurry in the reactor was heated to its final temperature and held there for the duration of the residence time under consideration. The reactor was then allowed to cool to below 50 °C. The pretreated samples were then washed with distilled water, centrifuged at 5510 revolutions per minute (RPM) for 15 min, and the supernatants were decanted. Compositional analysis and SSF processes were then carried out on the remaining samples.
Scanning Electron Microscopy (SEM) Imagery
The morphologies of the LHW-pretreated MxG and untreated MxG were examined using a Carl Zeiss Auriga Bu FIB field emission scanning electron microscope (FESEM). Thin layers of the samples were mounted on a copper sample holder, using carbon tape.
Simultaneous Saccharification and Fermentation (SSF)
The biomass loading was 5 g (wet basis), with a total working volume for the reaction of 50 mL and a pH adjusted to 4.5 by the addition of 0.05 M citric buffer. Wheaton septum glass bottles (125 mL) were used as the reaction vessels. Enzymatic hydrolysis was achieved by the addition of a cocktail of enzymes including Cellic® Ctec (Novozyme CZP0005) using a loading of 60 FPU/g-1 glucan and hemicellulase enzyme (Novozyme NS22002), at a loading of 2.5 FBG/g-1 glucan. Saccharomyces cerevisiae (ATCC 24858) was then inoculated into the reaction vessel to commence the SSF process.
Saccharomyces cerevisiae (ATCC 24858) was the yeast used to ferment the enzymatically generated sugars. For ethanol production through the SSF process, 10 mL of seed culture was used to inoculate 200 mL of yeast-peptone-dextrose (YPD) broth in a 1-L Erlenmeyer flask. The cultures were incubated in a shaker at 30 °C and 150 rpm and grown aerobically overnight. The suspended yeast cultures were transferred into 50-mL capped centrifugation tubes and were harvested by centrifugation at 4818 RPM for 15 min at room temperature. The supernatant was discarded, and the cells were transferred into 125-mL Wheaton septum glass bottles containing 50 mL of pretreated hydrolyzate already inoculated with the enzymes. The bottles were then tightly capped to allow fermentation to occur largely under anaerobic conditions. The cultures were placed in a rotary shaker and incubated at 32 °C and 150 rpm. Aliquots of the fermentation broth were collected at designated times: 0, 3, 6, 9, 12, 24, and 42 h. The aliquot samples were analyzed for sugars and ethanol concentrations using HPLC. Untreated MxG was also taken through the same process to serve as a control and allow for comparison.
The percent saccharification was calculated as follows (Krishna and Chowdary 2000):
![]() The ethanol yield was expressed as the percentage of the theoretical yield using the following formula,
The ethanol yield was expressed as the percentage of the theoretical yield using the following formula,
![]()
where Cethanol,f is the ethanol concentration at the end of the fermentation (g/L), Cethanol,i is the ethanol concentration at the beginning of the fermentation (g/L), Cbiomass is the dry biomass concentration at the beginning of the fermentation (g/L), f is the cellulose fraction of the dry biomass (g/g), and 0.568 is the conversion factor from cellulose to ethanol.
Chemical Analyses
The elemental composition (C, H, N, S) of the untreated and pretreated MxG was determined using a PE 2400 II CHNS/O analyzer (Perkin Elmer Japan Co., Ltd.). The oxygen content was calculated by difference: O%=100-(C+H+N+S)%. The amount of sugar monomers (cellobiose, glucose, arabinose, and xylose) in all liquid fractions and the ethanol and acetic acid concentrations were determined by HPLC (Waters, Milford, MA) with a KC-811 ion exclusion column and a Waters 410 refractive index detector (RID). The mobile phase was 0.1% H3PO4 solution at a flow rate of 1 mL/min. The temperatures of the detector and column were maintained at 35 and 60 °C, respectively.
Statistical Analyses
To determine the reliability and reproducibility of the results of the SSF process, all experiments were carried out in duplicate. Data are reported as averages and were analyzed using one-way analysis of variance (ANOVA), with significant differences between the means determined at p < 0.05 using the statistical software StatPlus: Mac professional version 5. MATLAB R2012b was used to assess the responses of various dependent parameters to independent parameters.
RESULTS AND DISCUSSION
Mass Flows into Juice and Solid Cake by Mechanical Pressing
The mechanical press was largely effective in separating freshly harvested MxG into juice and cake, with a mass distribution of between 445.5 g/kg and 593 g/kg juice and 407 g/kg and 554.5 g/kg solid cake (Fig. 1).
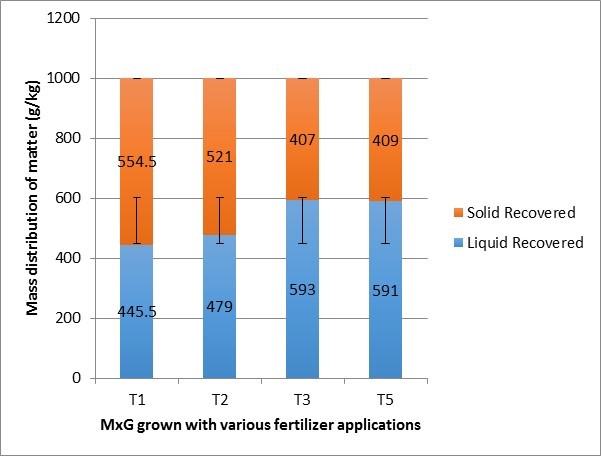
Fig. 1. Separation of MxG into solid cake and juice
LHW Pretreatment
The purpose of the pretreatment was to remove lignin and/or hemicellulose, to disrupt the crystalline structure of cellulose, and to increase the porosity of the material, making it more accessible to enzymatic attack (Oberoi et al. 2011). Water at high temperatures behaves like a mild acid, leading to partial hydrolysis of cellulose and hemicellulose (Mosier et al. 2005). Untreated MxG contained approximately 45% cellulose, 39.1% hemicellulose, and 5.5% lignin (Table 1). LHW pretreatment was able to break down approximately 68.1% to 77.0% of the lignin at various temperatures and retention times (Table 1). However, it was not very effective in the partial hydrolysis of the hemicellulose, as expected, but still resulted in an increase in cellulose content. Temperature and time were significant (p < 0.05) parameters in the LHW-pretreated biomass composition. There were no measurable amounts of inhibitors (acetic acid, furfural, and 5-hydromethylfurfural) in the hydrolysate of the pretreated samples. To prevent the formation of inhibitors, the pH was kept between 4 and 7 during pretreatment. Keeping the pH between 4 and 7 minimizes the formation of degradation products that can further catalyze hydrolysis of cellulosic materials during pretreatment (Hendriks and Zeeman 2009). If catalytic degradation of sugars occurs it results in a series of reactions that are difficult to control and result in undesirable side products. By keeping the pH between 4 and 7 the autocatalytic formation of fermentation inhibitors are avoided during the pretreatment (Hendriks and Zeeman 2009).
Table 1. Composition of MxG Before and After LHW Pretreatment
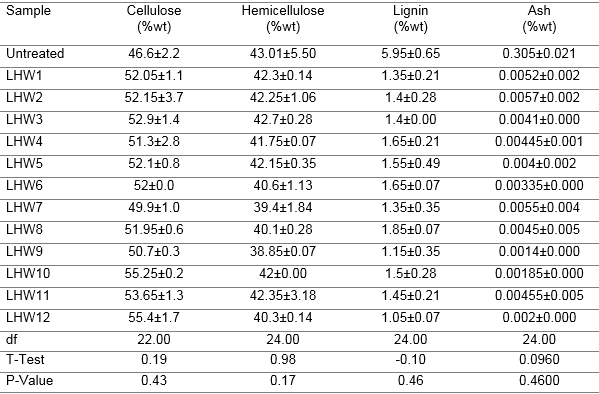
Generally, the increase in cellulose and hemicellulose content did not occur necessarily with increasing temperature or residence time. The increases were fairly constant within the temperatures considered. Perhaps the conditions were too mild for the material, and higher temperatures and retention times should be considered. Because of the presence of lignin in low amounts, MxG offers a good potential for enzymatic conversion of cellulose and hemicellulose into simple sugars. The increase in sugar concentration in the pretreated MxG could be because of release of some of the sugars bound to the insoluble polysaccharide fractions of MxG. Furthermore, some of the sugars were strongly bonded to the insoluble polysaccharide fractions and thus could not be extracted into ethanol. However, because of the LHW pretreatment, these sugars were released and could be extracted in the form of ethanol.
Elemental Composition of Untreated and Pretreated Biomass
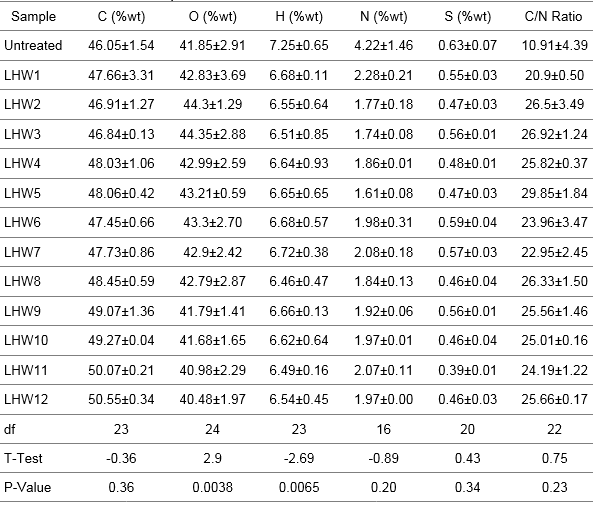
MxG has been noted as a good candidate for bioethanol production because of its high carbon content. The carbon content of MxG appeared to generally increase with increasing pretreatment temperature and retention time (Table 2). There was also a decrease in the amount of nitrogen and sulfur in the pretreated biomass compared to the untreated biomass (Table 2). This could have had a positive effect on ethanol concentration and yield percentage. It has been reported that the concentration of ethanol produced increases with increasing carbon to nitrogen (C/N) ratio of a substrate (Imamoglu and Sukan 2014), reaching a maximum at the maximum C/N ratio. These researchers concluded that C/N ratio was a parameter influencing the yield of ethanol from biomass, with a linear correlation between the two parameters. The C/N ratio of MxG pretreated at 200 °C for 15 min was 25.65, which corresponded to the highest ethanol concentration and yield; the C/N ratio for untreated biomass was 10.91 (Table 2).
Table 2. Elemental Composition of MxG Before and After Pretreatment
Morphological Changes after LHW Pretreatment
Electron micrographs showing morphological features of untreated and LHW-pretreated MxG are provided in Fig. 2.

Fig. 2a. SEM image of untreated MxG

Fig. 2b. SEM image of MxG pretreated at 170 °C for 15 min

Fig. 2c. SEM image of MxG pretreated at 200 °C for 15 min
After pretreatment, some morphological changes were observed, indicative of partial damage to the biomass structure. The untreated biomass exhibited a more robust structure, which might include hemicellulose, lignin and other binding materials (Fig. 2a). Figure 2b shows a slight disruption of the robust structure of the untreated MxG when it was treated with hot water at 170 °C for 15 min. Pretreatment at 200 °C for 15 min led to the further breakdown of the robust structure of MxG (Fig. 2c).
Figure 2c shows that the surface layer was removed and broken or made loose during LHW treatment, resulting in the exposure of the internal structure and fibers (Saini et al. 2013). In comparing the images, it can be said that the rigidity of the cellulose fibers was lost, as it became distorted after pretreatment, which can be attributed to the removal of the lignin and hydrolysis of hemicellulose.
Saccharification Ratio, Ethanol Concentration, and Ethanol Yield
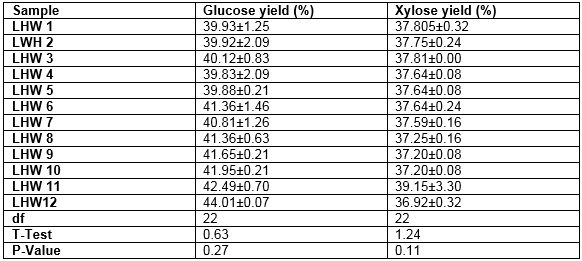
The resulting glucose and xylose yield percentages (saccharification ratio) were slightly below 50% for samples treated at higher temperatures and retention time (Table 3). The pretreatment temperature and time were found to have significant effects on the saccharification ratio (p < 0.05). Generally, the saccharification ratio increased with increasing temperature and time, which was consistent with the ethanol concentrations and yield observed. Achieving a higher glucose saccharification positively improved the ethanol yield. Park et al. (2010) achieved saccharification ratios of newspaper prints for ethanol production of 32%, 34%, and 37% at 30, 37, and 40 °C, respectively.
Table 3. Saccharification Ratio or Sugar Yield of Pretreated MxG
Both ethanol concentration and yield showed high linear correlation with increasing pretreatment temperature and retention time of LHW pretreatment. The theoretical SSF ethanol yield was calculated by assuming that all the potential glucose in pretreated material was available for fermentation (Cuevas et al. 2010). Ethanol concentration increased from 0.780 to 3.715 g/L with increasing temperature and time, with a corresponding increase in ethanol yield from 15.1% to 71.8% of theoretical, where a temperature of 200 °C for 15 min resulted in an ethanol yield of 71.8% of theoretical (Fig. 3). This figure, which shows the curve fitting analyses using a linear model polynomial, resulted in a model with a coefficient of determination (R2) of 0.7676. The pretreatment time and temperature were found to be significant (p < 0.05) parameters affecting ethanol concentration and yield. The concentration of acetic acid, a fermentation inhibitor increased from 0.73 to 0.845 g/L during the SSF process. No detection of HMF and furfural was observed during the fermentation process.
The ethanol yield of untreated MxG was calculated to be 27.4% of theoretical. This is comparable to ethanol yield calculated for pretreatment at 170 °C (15% to 34%). Higher pretreatment temperatures, i.e., 190 and 200 °C, gave higher ethanol concentrations and yields, most likely as a result of the higher porosity of the crystalline structure of the cellulose and lower lignin amounts of samples treated at these temperatures. The effectiveness of LHW pretreatment on cellulose digestibility and the subsequent production of ethanol is strongly related to pretreatment severity (Wan and Li 2011). A study of rice hulls, for instance, showed that enzymatic digestibility increased to greater than 70% when the severity of the pretreatment was increased by increasing the temperature and treatment time (Ko et al. 2009). As a result of using an SSF process, the increase in ethanol concentration and yield could be attributed to the presence of yeast, together with cellulases, which reduces the accumulation of glucose, thereby increasing the saccharification rate and ethanol yield (Cuevas et al. 2010). Furthermore, the removal of lignin observed in this study may have resulted in the enhanced enzymatic hydrolysis by reducing the cellulose-lignin binding and increasing the accessibility of cellulase to the enzyme (Ko et al. 2009).
A correlation analysis to determine a model for the predictability of glucose response to pretreatment temperature and retention time (Fig. 4) resulted in an R2 of 0.9508, indicating that only about 5% of all variation for the response (glucose concentration) could not be explained. This expresses a sufficient fit. Normally, a model with R2 > 0.90 is considered to have a very high correlation (Saini et al. 2013).
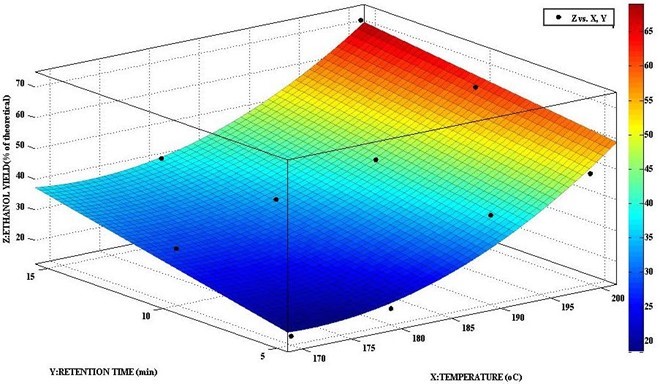
Fig. 3. Response of ethanol yield to pretreatment temperature and retention time
Ethanol concentration was also higher for the pretreated MxG compared to the untreated MxG in most cases (Fig. 5). The time series shows that ethanol production increased rapidly from time 0 to 3 h and kept increasing steadily throughout the period to 42 h. The experiment was carried out for only 42 h because the SSF process has been reported to improve the speed and yield of hydrolysis when compared to other methods involving separate hydrolysis and fermentation steps (Park et al. 2010). Producing a high ethanol concentration and yield in a relatively shorter amount of time is economically desirable.
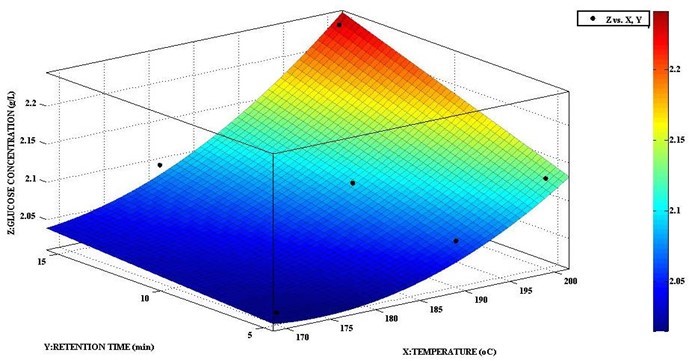
Fig. 4. Response of glucose concentration (Z) to pretreatment temperature (X) and retention time (Y)
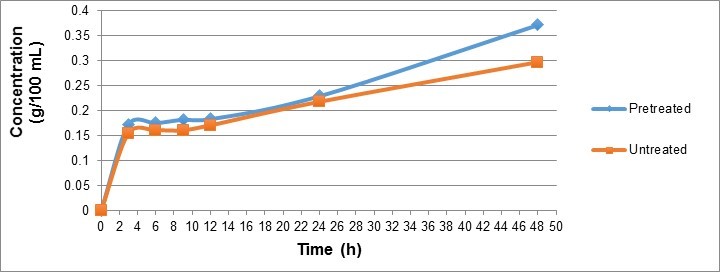
Fig. 5. A comparison of ethanol concentration versus time for untreated MxG and MxG pretreated at 200 °C for 15 min
The temperature of 30 °C used for the SSF process might have also had a positive effect on the high ethanol yield and concentration observed after 24 h. Normally, prolonged fermentation at higher temperatures can adversely affect the fermentation ability of the cells. Yeast cells perform best in the pH and temperature vicinity of 5.0 and 30 °C, respectively (Oberoi et al. 2011). In a study of banana peel at a SSF temperature of 35 °C, an increase in fermentation time beyond 14 h did not result in a significant increase in ethanol concentration, which was attributed to stress on the yeast cells possibly caused by low pH, high temperature, and ethanol accumulation beyond a certain percentage in the reactor (Oberoi et al. 2011).
High ethanol concentration and yield for the process can also be attributed to the cocktail of enzymes employed in the SSF process. Cellic® Ctec (Novozyme CZ0005) is a cellulase complex suitable for the degradation of cellulose into fermentable sugars. It is a blend of aggressive cellulases and high levels of beta-glucosidase. Beta-glucosidase for instance can reduce cellobiose inhibition during SSF by converting cellobiose into glucose, leading to increased hydrolysis (Boonsawang et al. 2012).
There was also a negative correlation between glucose and ethanol concentrations. As expected, the glucose concentration decreased with increasing ethanol concentration over time (Fig. 6). The xylose concentration remained unchanged, as Saccharomyces cerevisiae does not metabolize pentose sugars such as xylose.
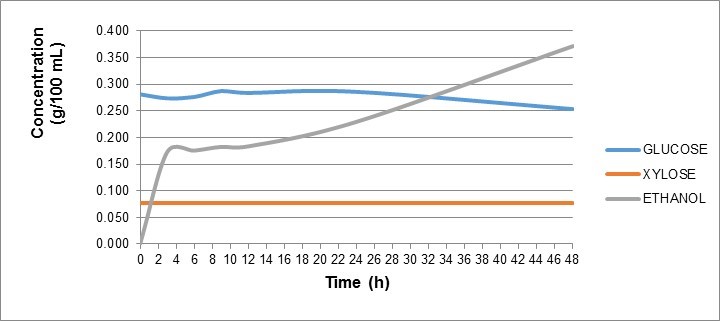
Fig. 6. Glucose, xylose, and ethanol concentrations with time
CONCLUSIONS
- Liquid hot water pretreatment was effective in the delignification of MxG, resulting in an increase in the monomeric sugar glucose, allowing for effective production of ethanol from the process.
- The pretreatment severity was significant in the saccharification ratio, ethanol concentration, and rate of production, as well as the ethanol yield. Generally, the glucose and ethanol concentration and ethanol yield increased with increasing pretreatment temperature and residence time.
- The use of a cocktail of enzymes worked synergistically to produce more monomeric sugars from their polymeric forms, which aided in the fermentation process, leading to high ethanol yields.
- Saccharomyces cerevisiae remains a very viable microorganism for the fermentation of hexose sugar glucose into ethanol at moderate reaction temperatures.
- A combination of factors most likely led to the high amount of ethanol produced in the short period of time considered in this research, which is desirable because being able to restrict the fermentation period while increasing the ethanol yield will be a boost to the industrial growth and development of this bioethanol processing system.
ACKNOWLEDGMENTS
The authors are grateful for the support of the USDA-CSREES-Evans-Allen Project, Grant No. NCX-272-5-13-130-1.
REFERENCES CITED
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., and Levin, D. B. (2011). “Biomass pretreatment: Fundamentals toward application,” Biotechnology Advances 29(6), 675-685. DOI: 10.1016/j.biotechadv.2011.05.005
Balat, M. (2011). “Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review,” Energy Conversion and Management 52(2), 858-875. DOI: 10.1016/j.enconman.2010.08.013
Boonsawang, P., Subkaree, Y., and Srinorakutara, T. (2012). “Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF),” Biomass and Bioenergy 40, 127-132. http://doi.org/10.1016/j.biombioe.2012.02.009
Cherubini, F. (2010). “The biorefinery concept: Using biomass instead of oil for producing energy and chemicals,”Energy Conversion and Management 51(7), 1412–1421. DOI: 10.1016/j.enconman.2010.01.015
Cuevas, M., Sánchez, S., Bravo, V., García, J. F., Baeza, J., Parra, C., and Freer, J. (2010). “Determination of optimal pre-treatment conditions for ethanol production from olive-pruning debris by simultaneous saccharification and fermentation,” Fuel 89(10), 2891-2896. DOI: 10.1016/j.fuel.2010.02.005
El Hage, R., Chrusciel, L., Desharnais, L., and Brosse, N. (2010). “Effect of autohydrolysis of Miscanthus x giganteus on lignin structure and organosolv delignification,” Bioresource Technology 101(23), 9321–9329. DOI: 10.1016/j.biortech.2010.06.143
Galbe, M., and Zacchi, G. (2002). “A review of ethanol production from softwood,” Appl Microbiol Biotechnol 59, 618-628. DOI: 10.007/s00253-002-1058-9
Głowacka, K. (2011). “A review of the genetic study of the energy crop Miscanthus,” Biomass and Bioenergy 35(7), 2445-2454. DOI: 10.1016/j.biombioe.2011.01.041
Guo, B., Zhang, Y., Ha, S.-J., Jin, Y.-S., and Morgenroth, E. (2012). “Combined biomimetic and inorganic acids hydrolysis of hemicellulose in Miscanthus for bioethanol production,” Bioresource Technology 110, 278-287. DOI: 10.1016/j.biortech.2012.01.133
Guo, G.-L., Chen, W.-H., Chen, W.-H., Men, L.-C., and Hwang, W.-S. (2008). “Characterization of dilute acid pretreatment of silvergrass for ethanol production,” Bioresource Technology 99(14), 6046-6053. DOI: 10.1016/j.biortech.2007.12.047
Haghighi Mood, S., Hossein Golfeshan, A., Tabatabaei, M., Salehi Jouzani, G., Najafi, G. H., Gholami, M., and Ardjmand, M. (2013). “Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment,” Renewable and Sustainable Energy Reviews 27, 77-93. DOI: 10.1016/j.rser.2013.06.033
Hendriks, A. T. W. M., and Zeeman, G. (2009). “Pretreatments to enhance the digestibility of lignocellulosic biomass,” Bioresource Technology 100(1), 10-18. DOI: 10.1016/j.biortech.2008.05.027
Imamoglu, E., and Sukan, F. V. (2014). “The effects of single and combined cellulosic agrowaste substrates on bioethanol production,” Fuel 134, 477-484. DOI: 10.1016/j.fuel.2014.05.087
Jeżowski, S. (2008). “Yield traits of six clones of Miscanthus in the first 3 years following planting in Poland,” Industrial Crops and Products 27(1), 65-68. DOI: 10.1016/j.indcrop.2007.07.013
Kang, K. E., Han, M., Moon, S.-K., Kang, H.-W., Kim, Y., Cha, Y.-L., and Choi, G.-W. (2013). “Optimization of alkali-extrusion pretreatment with twin-screw for bioethanol production from Miscanthus,” Fuel 109, 520-526. DOI: 10.1016/j.fuel.2013.03.026
Kim, T. H., Choi, C. H., and Oh, K. K. (2013). “Bioconversion of sawdust into ethanol using dilute sulfuric acid-assisted continuous twin screw-driven reactor pretreatment and fed-batch simultaneous saccharification and fermentation,” Bioresource Technology 130, 306-313. DOI: 10.1016/j.biortech.2012.11.125
Ko, J. K., Bak, J. S., Jung, M. W., Lee, H. J., Choi, I.-G., Kim, T. H., and Kim, K. H. (2009). “Ethanol production from rice straw using optimized aqueous-ammonia soaking pretreatment and simultaneous saccharification and fermentation processes,” Bioresource Technology 100(19), 4374-4380. DOI: 10.1016/j.biortech.2009.04.026
Krishna, H. S., and Chowdary, G. V. (2000). “Optimization of simultaneous saccharification and fermentation for the production of ethanol from lignocellulosic biomass,” 48(5), 1971-1976. DOI: 10.1021/jf991296z
Lewandowski, I., Clifton-Brown, J. C., Scurlock, J. M. O., and Huisman, W. (2000). “Miscanthus: European experience with a novel energy crop,” Biomass and Bioenergy 19(4), 209-227. DOI: 10.1016/S0961-9534(00)00032-5
Limayem, A., and Ricke, S. C. (2012). “Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects,” Progress in Energy and Combustion Science 38(4), 449-467. DOI: 10.1016/j.pecs.2012.03.002
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pretreatment of lignocellulosic biomass,” Bioresource Technology 96(6), 673-686. DOI: 10.1016/j.biortech.2004.06.025
Oberoi, H. S., Vadlani, P. V., Saida, L., Bansal, S., and Hughes, J. D. (2011). “Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process,” Waste Management 31(7), 1576-1584. DOI: 10.1016/j.wasman.2011.02.007
Park, I., Kim, I., Kang, K., Sohn, H., Rhee, I., Jin, I., and Jang, H. (2010). “Cellulose ethanol production from waste newsprint by simultaneous saccharification and fermentation using Saccharomyces cerevisiae KNU5377,” Process Biochemistry 45(4), 487-492. DOI: 10.1016/j.procbio.2009.11.006
Parveen, I., Threadgill, M. D., Hauck, B., Donnison, I., and Winters, A. (2011). “Isolation, identification and quantitation of hydroxycinnamic acid conjugates, potential platform chemicals, in the leaves and stems of Miscanthus × giganteus using LC–ESI-MSn,” Phytochemistry 72(18), 2376-2384. DOI: 10.1016/j.phytochem.2011.08.015
Ruiz, H. A., Rodríguez-Jasso, R. M., Fernandes, B. D., Vicente, A. A., and Teixeira, J. A. (2013). “Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review,” Renewable and Sustainable Energy Reviews 21, 35-51. DOI: 10.1016/j.rser.2012.11.069
Saini, J. K., Anurag, R. K., Arya, A., Kumbhar, B. K., and Tewari, L. (2013). “Optimization of saccharification of sweet sorghum bagasse using response surface methodology,” Industrial Crops and Products 44, 211-219. DOI: 10.1016/j.indcrop.2012.11.011
Scordia, D., Cosentino, S. L., and Jeffries, T. W. (2013). “Effectiveness of dilute oxalic acid pretreatment of Miscanthus × giganteus biomass for ethanol production,” Biomass and Bioenergy 59, 540-548. DOI: 10.1016/j.biombioe.2013.09.011
Singh, R., Shukla, A., Tiwari, S., and Srivastava, M. (2014). “A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential,” Renewable and Sustainable Energy Reviews 32, 713-728. DOI: 10.1016/j.rser.2014.01.051
Sluiter, A., Hames, B., Christian, D., Payne, C., Ruiz, R., Scarlata, C., … Wolfe, J. (2008). “Determination of total solids in biomass and total dissolved solids in liquid process samples,” National Renewable Energy Laboratory (NREL), Golden, CO.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008). “Determination of ash in biomass,” National Renewable Energy Laboratory (NREL), Golden, CO.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, J., Sluiter, J., Templeton, D., and Christian, D. (2011). “Determination of structural carbohydrates and lignin in biomass,” National Renewable Energy Laboratory (NREL), Golden, CO.
Smith, C., David, M., Mitchell, C., Masters, M., Anderson-Teixeira, K., Bernacchi, C., and DeLucia, E. (2013). “Reduced nitrogen losses after conversion of row crop agriculture to perennial biofuel crops,” Journal of Environmental Quality 42, 219-288.
Wan, C., and Li, Y. (2011). “Effect of hot water extraction and liquid hot water pretreatment on the fungal degradation of biomass feedstocks,” Bioresource Technology 102(20), 9788-9793. DOI: 10.1016/j.biortech.2011.08.004
Wyman, C. E., Dale, B. E., Elander, R. T., Holtzapple, M., Ladisch, M. R., and Lee, Y. Y. (2005). “Coordinated development of leading biomass pretreatment technologies,” Bioresource Technology 96(18), 1959-1966. DOI: 10.1016/j.biortech.2005.01.010
Zhao, J., and Xia, L. (2009). “Simultaneous saccharification and fermentation of alkaline-pretreated corn stover to ethanol using a recombinant yeast strain,” Fuel Processing Technology 90(10), 1193-1197. DOI: 10.1016/j.fuproc.2009.05.018
Article submitted: March 24, 2015; Peer review completed: July 10, 2015; Revised version received and accepted: July 22, 2015; Published: July 30, 2015.
DOI: 10.15376/biores.10.3.5890-5905
