Abstract
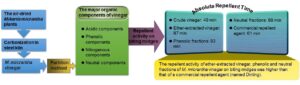
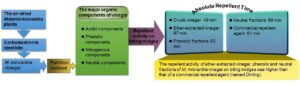
Crude vinegar was prepared from Mikania micrantha plants using a steel kiln. The ether-extracted vinegar and acidic, phenolic, and neutral fractions were obtained by the partition method. The fundamental properties of crude vinegar, including its fractions applied to repel biting midges (Forcipomyia taiwana), were investigated. Results indicated that the crude vinegar had a moisture content of 91%, Gardner color value of 11.2, a reddish-brown color, specific gravity of 1.0164, pH of 5.36, organic acid content of 2.50%, and soluble tar content of 0.78%. In ether-extracted vinegar of M. micrantha, the acidic component was the major ingredient, followed by the neutral, phenolic, and nitrogenous components. The main organic compounds of the acidic, phenolic, nitrogenous, and neutral components were acetic acid, phenol, 3-pyridinol, and 2-furanmethanol, respectively. The results also demonstrated that the crude vinegar, ether-extracted vinegar, and the phenolic and neutral fractions effectively repelled biting midges, with absolute repellent times of 49, 87, 83, and 99 min, respectively. The repellent activity of ether-extracted vinegar and the phenolic and neutral fractions of M. micrantha vinegar on biting midges was higher than that of a commercial repellent agent (named Dinling) with absolute repellent time of 61 min.
Download PDF
Full Article
Manufacture of Mikania micrantha Vinegar and Investigation of its Repellent Activity for Forcipomyia taiwana
Suling Liu,a Chenghsin Hu,b and Kuntsung Lu b,*
Crude vinegar was prepared from Mikania micrantha plants using a steel kiln. The ether-extracted vinegar and acidic, phenolic, and neutral fractions were obtained by the partition method. The fundamental properties of crude vinegar, including its fractions applied to repel biting midges (Forcipomyia taiwana), were investigated. Results indicated that the crude vinegar had a moisture content of 91%, Gardner color value of 11.2, a reddish-brown color, specific gravity of 1.0164, pH of 5.36, organic acid content of 2.50%, and soluble tar content of 0.78%. In ether-extracted vinegar of M. micrantha, the acidic component was the major ingredient, followed by the neutral, phenolic, and nitrogenous components. The main organic compounds of the acidic, phenolic, nitrogenous, and neutral components were acetic acid, phenol, 3-pyridinol, and 2-furanmethanol, respectively. The results also demonstrated that the crude vinegar, ether-extracted vinegar, and the phenolic and neutral fractions effectively repelled biting midges, with absolute repellent times of 49, 87, 83, and 99 min, respectively. The repellent activity of ether-extracted vinegar and the phenolic and neutral fractions of M. micrantha vinegar on biting midges was higher than that of a commercial repellent agent (named Dinling) with absolute repellent time of 61 min.
Keywords: Mikania micrantha; Vinegar; Partition method; Forcipomyia taiwana (biting midges); Repellent activity
Contact information: a: Experimental Forest, National Taiwan University, No. 12, Section 1, Chien-Shan Road, Chu-Shan, Nantou Hsien, 55750, Taiwan; b: Department of Forestry, National Chung Hsing University, 145 Xingda Rd., South Dist., Taichung, 40227, Taiwan;
* Corresponding author: lukt@dragon.nchu.edu.tw
GRAPHICAL ABSTRACT
INTRODUCTION
Mikania micrantha, a perennial broad-leaved vine belonging to Genus Mikania, Family Asteraceae, and Order Asterales. It is mainly distributed in tropical-to-subtropical regions. It has good asexual and sexual reproduction abilities, and all plants in the areas invaded by M. micrantha will be entangled and covered, resulting in their death, causing serious harm to vegetation, ecology, and species diversity. The International Union for Conservation of Nature (IUCN) has listed it as one of the 100 most harmful invasive alien species in the world. The hillsides, low-altitude woodlands, and abandoned agricultural lands or orchards in Taiwan are covered by M. micrantha plants on the ground or tree crowns. The Forestry Bureau, Council of Agriculture, Executive Yuan began to purchase M. micrantha plants in 2009, hoping to cooperate with non-governmental partners to remove M. micrantha. Up until now, the growth area has decreased from 51,852 ha in 2001 to 4,736 ha in 2020. The Forestry Bureau of Taiwan buys about 800 tons every year, and the acquired M. micrantha plants are disposed of by burning or burying; it is a drawback that this natural lignocellulosic biomass cannot be utilized. If M. micrantha plant could be used as a raw material for commodity development, it would not only increase people’s willingness to utilize M. micrantha plants, thereby eradicating it, but also achieve the goal of forestry recycling.
Due to the gradual shortage of fossil resources, countries worldwide have been devoted to research on replacing fossil resources with natural renewable biomass as energy and chemicals. Wood and bamboo vinegar is a liquid collected by condensing the wet smoke generated by pyrolysis while preparing wood and bamboo charcoal. The main components of such vinegar are water, accounting for 80 to 90%, with other organic components accounting for 10 to 20%. There are more than 200 organic components, among which acetic acid has the highest content (Mu et al. 2003; Sulaiman et al. 2005). The vinegar also contains ketones, phenols, aldehydes, and other compounds (Lu et al. 2007). Wood vinegar contains 10% or more of acetic acid, along with dozens of other organic compounds, such as propionic acid, formic acid, and phenols (Grewal et al. 2018; Li et al. 2018; Feng et al. 2020). According to the literature, wood and bamboo vinegar has a wide range of applications as plant growth promoter (Mu et al. 2003, 2004), antibacterial agent (Lu et al. 2007; Jin et al. 2012), anti-termite agent (Yatagai et al. 2002), rubber coagulant (Baimark and Niamsa 2009), deodorant (Akakabe et al. 2006), medicines, incense, etc.; therefore, this vinegar is multifunctional and has great economic value. M. micrantha plants are similar to wood and bamboo in chemical composition. They are mainly composed of cellulose, hemicellulose, lignin, and small amounts of extractive components; thus, the plants can also be utilized as biomass. Hagner et al. (2015) pointed out that wood vinegar can effectively inhibit the growth of various weeds and can be used as pesticides for insects and molluscs. Acetic acid, furfural, and ether extract of wood vinegar induced a clear repellent effect on snails. Marra et al. (2018) found that olive vinegar can effectively prevent Meloidogyne in soil.
Biting midge (Forcipomyia taiwana) is an insect belonging to the Subgenus Lasiohelea, Genus Forcipomyia, Family Ceratopogonidae, Order Diptera, and is commonly known as the biting midge. Twenty-four species in the Subgenus Lasiohelea, Genus Forcipomyia have been recorded in Taiwan, and only F. taiwana and F. anabaenae ingest human blood, between which F. taiwana causes more harm (Lien 1989, 1991). From June to August every year, the weather is mild, and rainfall is abundant, and the threat from F. taiwana is the greatest (Chuang et al. 2000). When the temperature rises to 30 °C during the day, humans are relatively vulnerable to F. taiwana bites, especially on the exposed parts of the body; people with an allergic predisposition will have severe itching or blisters. It will take several days for the scratched wounds to heal, and even longer if infection occurs (Sun 1967). Generally, to prevent biting midge bites, spraying chemical agents such as chlorpyrifos or pirimiphos-methyl is relatively quick-acting, but it is not environment-friendly and will destroy the ecosystem in the long run. Therefore, it is necessary to find ways or products to prevent and control biting midges while taking the environment into account. Mu et al. (2003) produced vinegar from Phyllostachys bambusoides and Phyllostachys pubescens and then separated these two types of bamboo vinegar into acidic substances, phenolic substances, and neutral substances by the partition method to investigate the effects of the vinegar and the separated substances at different dilution ratios on seed germination and radicle development. Therefore, in the present experimental work, firstly, the chemical compositions, elemental analysis, and the proximate analysis of M. micrantha plant were examined. Secondly, the preparation and basic properties of the crude M. micrantha vinegar were conducted. Finally, the organic components of M. micrantha vinegar were separated into acidic, phenolic, neutral, and other substances by the partition method, and the repellent effects of ether-extracted vinegar and the three groups of separated substances at different concentrations were investigated on biting midges. M. micrantha vinegar is expected to function as a hygienic drug against biting midges, and the goal of reusing forestry cycle products will be fulfilled.
EXPERIMENTAL
Materials
M. micrantha plants
The M. micrantha plants obtained from Nantou Forest District Office of Taiwan at September in 2013 were air-dried to a water content of 15% for preparing vinegars as shown in Fig. 1. The plants including leaves, vines, and a small amount of roots were used together for analyzing compositions and preparing vinegars.
Fig. 1. The (a) fresh, (b) air-dried M. micrantha plants
Biting midges (F. taiwana)
The experiment site for biting midge repellence was near the Earth God Shrine in Zhuyuan Lane, Dakeng District, Taichung City, Taiwan.
Methods
Composition analysis of M. micrantha plants
The air-dried M. micrantha plants were ground into powder with a pulverizer, and a particle size of 40- to 60-mesh (425 to 250 μm) was selected to analyze the following basic properties.
Chemical composition
Alcohol-toluene extractives content was determined according to the national standard CNS 4713 (2005). Holocellulose content was determined according to the CNS 3085 (2004). Additionally, α-cellulose content was determined according to the CNS 10865 (2002). Klason lignin content was determined according to the CNS 2721 (2010). Ash content was determined according to the national standard CNS 3084 (2004).
Elemental analysis
An elemental ratio EL III Heraeus CHNOS Rapid F002 elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany) was used to determine the contents of C, H, N, S, and the weight percentage of oxygen content was calculated according to the following Eq. 1:
O (%) = 100 – (C + H + N + S + Ash) (1)
Proximate analysis
Referring to the standard ASTM D7582-12 (2012), a PerkinElmer STA6000 thermogravimetric analyzer (PerkinElmer Inc., Waltham, MA, USA) was used to analyze the contents of pyrolytic volatiles, fixed carbon, and ash.
Production and collection of the crude M. micrantha vinegar
Approximately 5 kg of air-dried M. micrantha plants (water content: 15%) were taken, put into an electrothermal steel kiln (Fig. 2), and heated at a rate of 100 °C h-1 to 600 °C, with a holding time of 1 h. When the temperature had risen to 150 °C, the non-return valve was adjusted to stably release the pyrolytic gas. The crude M. micrantha vinegar was collected as shown in Fig. 3, and the yield was calculated according to the absolute dry weight of the test material. The electrothermal steel kiln consisted of an electrothermal furnace with a height of 1 m and a diameter of 1 m, an electrically controlled heating panel setting instrument (Cheng Sang Scientific Co., Ltd., Changhua, Taiwan) and a water-cooled condensing tower (Cheng Sang Scientific Co., Ltd., Changhua, Taiwan). The electric furnace body consisted of an inner pot with a capacity of 0.064 m3 and an outer furnace with a heat-resistant Grade A1 nickel-chromium wire heater, and the temperature was controlled by the electrically controlled heating panel setting instrument.
Fig. 2. Collecting and cooling system of steel kiln: (1) Steel kiln, (2) inner pot, (3) non-return valve, (4) first collecting barrel, (5) second collecting barrel, (6) cooling tower, (7) third collecting barrel, (8) fourth collecting barrel, (9) fifth collecting barrel, (10) enlarged collecting tank (filter screen), and (11) exhaust outlet (including the exhaust equipment)
Fig. 3. The crude M. micrantha vinegar
Separation of the crude M. micrantha vinegar components
The partition methods of salting-out, solvent extraction, and acid-base neutralization for wood vinegar were adopted in this study (Sugiura 1995), as shown in Fig. 4.
Fig. 4. Partition of crude wood vinegar (Sugiura 1995)
The crude M. micrantha vinegar was first extracted with ether and sodium chloride to divide the vinegar into an ether layer and a water layer. The ether layer was extracted with 5% NaHCO3(aq) to obtain an ether layer and a water layer. The water layer was further extracted with ether and 30% sulfuric acid to obtain an acidic fraction, while the ether layer was extracted with 2 N NaOH(aq) to obtain an ether layer and a water layer. The ether layer was a neutral fraction. The water layer was extracted with ether and 30% sulfuric acid to obtain a phenolic fraction, and then ether was recovered by atmospheric distillation to finally obtain the acidic, phenolic, and neutral fractions.
Analysis of the basic properties of the crude M. micrantha vinegar
Water content was measured using a Metrohm 795 KFT Titrino Karl Fischer volumetric moisture meter (ManualShelf, Herisau, Switzerland), and the deviation of the test should be less than 3%.
Color was measured using a DrLange Lico 100 liquid colorimeter (Dr. Lange, Lengerich, Germany), the Gardner Color Scale, with 0 to 18 grades, was adopted; a larger value meant a darker color.
The pH was measured using a Suntex SP-701 pH meter (Suntex Instruments Co., Ltd., New Taipei, Taiwan) at room temperature (27 °C). Specific gravity was measured using a pycnometer (Starek Scientific Co., Ltd., Taipei, Taiwan) at room temperature (27 °C).
Organic acid content
Next, 1 to 2 g of the vinegar was placed in a 250-mL conical flask and diluted 100 times with distilled water. A few drops of phenolphthalein indicator were added, followed by titration with 0.1 N NaOH solution. A blank test was also carried out. The organic acid content was calculated according to the following Eq. 2,
(2)
where A is the amount of NaOH(aq) (mL) added in the titration of M. micrantha vinegar, B is the amount of NaOH(aq) (mL) added in the blank test, N is the equivalent concentration of NaOH(aq), and S is the mass of M. micrantha vinegar (g).
Soluble tar content
Next, 1 g of the vinegar was added to a sample bottle, which was placed in an oven at 200 °C for 2 h. The residue in the bottle was weighed after cooling, and the soluble tar content was calculated according to the following Eq. 3,
(3)
where T is the residue mass after heating at 200 °C (g) and S is the mass of M. micrantha vinegar (g).
Analysis of organic components
The ether-extracted vinegar and its fractions were filtered through Nylon 0.45-μm filter membranes. Then, the upper ether-soluble part was separated, and its organic components were analyzed using a Perkin-Elmer Clarus 600D gas chromatograph-mass spectrometer (GC-MS) (PerkinElmer, Inc., Waltham, MA, USA). Briefly, 1.0 μL of the ether-extracted vinegar and its acidic, phenolic, and neutral fractions were separately injected into the gas chromatograph. The temperature at the injection port was 250 °C, the separation column was a Stabilwax-DA capillary column (30 m × 0.25 mm ID, film thickness = 0.25 μm), and the flow rate of the carrier gas (helium) was 1.4 mL min-1. The initial temperature was 40 °C, and after maintaining this temperature for 5 min, the temperature was increased at a rate of 5 °C/min to 110 °C at the first stage, at a rate of 3 °C min-1 to 150 °C at the second stage, and at a rate of 5 °C min-1 to 220 °C at the third stage, which was maintained for 5 min; the temperature of the detector was 280 °C, and the scanning mode was 40 to 425 m z-1. The obtained GC-MS chromatograms were compared with a National Institute of Standards and Technology library search.
Repellence experiment of biting midges
In this experiment, Zhuyuan Lane, Dakeng District, North District, Taichung City was selected as the experiment site, and the highest density of biting midges detected in this area was approximately 300 biting midges within 20 min. Human subjects were volunteers and all treatments were safe with no allergic reaction. The experimental method was reviewed and approved by an oral exam committee that oversaw the research.
An area with dimensions of 3 cm × 3 cm, corresponding to the dorsum of the hand, was cut off from each rubber glove. The research subject put on the treated gloves on both hands (Fig. 5). The left hand was smeared with 0.1 mL test solution as the test group, and the right hand was used as the blank group. The time when the left and right hands were bitten by the first biting midge (absolute repellent time) and the number of biting midge bites within 20 min were recorded. The test was performed in triplicate. The commercially available Dinling biting midge-repellent liquid without DEET was selected as the control group. In addition, before each test, the test site was cleaned with neutral soap without perfume to avoid the residual odor from the previous test affecting the test results.
Fig. 5. Biting midge collection by research subjects; (a) control group, (b) test group, and (c) the black dots in the red circle are biting midges.
RESULTS AND DISCUSSION
Basic Properties of M. micrantha Plants
Chemical composition
The chemical composition of M. micrantha plants is shown in Table 1. The contents of holocellulose, α-cellulose, Klason lignin, alcohol-toluene extractives, and ash were 52.03%, 26.20%, 23.77%, 16.98%, and 6.94%, respectively. Compared with the results of softwood, hardwood, and wheat straws obtained by de Wild et al. (2009), it can be found that the α-cellulose content in M. micrantha plants was lower those in softwood (40.40%) and hardwood (44.50 to 49.00%), and was also slightly lower than that in wheat straws (34.00%). Its Klason lignin content was lower than that in softwood (28.20%), but close to those in hardwood (22.20 to 23.10%) and wheat straws (20.00%). Its alcohol-toluene extractives content was noticeably higher than those in softwood (0.13%) and hardwood (1.25 to 2.38%), but close to that in wheat straws (15.54%). Its ash content was higher than those in softwood and hardwood (0.17 to 1.05%), but similar to that in wheat straws (7.46%). In addition, comparison with the composition of M. micrantha plants reported by Lin and Lu (2012) showed that the contents of α-cellulose, Klason lignin, and ash in this study were all lower than their results, which were 35.32%, 25.22%, and 9.36%, respectively, but the content of the alcohol-toluene extractives in this study was noticeably higher than theirs (7.44%), which might be due to the difference in the environment of the M. micrantha plant growth sites.
Table 1. Chemical Compositions of M. micrantha Plants
± Represents standard deviation.
Proximate Analysis
The proximate analysis results of M. micrantha plants are listed in Table 2. The contents of pyrolytic volatiles, fixed carbon, and ash were 71.87%, 18.17%, and 8.37%, respectively. Compared with the proximate analysis of bamboo, softwood, and hardwood studied by Xiao et al. (2007) and de Wild et al. (2009), the results showed that the content of pyrolytic volatiles in M. micrantha plants was noticeably lower than those in softwood and hardwood (83.84 to 84.76%), but similar to that in bamboo (73.92%). Its fixed-carbon content was higher than those in softwood and hardwood (14.96 to 15.44%), as well as that in bamboo (15.30%); its ash content was higher than those in softwood and hardwood (0.48 to 0.83%), as well as that in bamboo (1.63%).
Table 2. Proximate Analysis of M. micrantha Plants
Elemental Analysis
The elemental composition of M. micrantha plants is shown in Table 3. Its carbon, hydrogen, oxygen, nitrogen, and sulfur contents were 42.97, 6.20, 40.54, 1.71, and 0.21%, respectively. Yang et al. (2007) reported the contents of carbon (48.54 to 48.86%), hydrogen (6.21 to 6.24%), oxygen (44.91 to 45.24%), and nitrogen (0.12 to 0.20%) in softwood Larix olgensis, as well as hardwood Populus canadensis and Eucalyptus grandi x E. urophylla. The results showed that the carbon and oxygen contents of M. micrantha plants were lower than those of softwood and hardwood; there was little difference in hydrogen content, while their nitrogen content was noticeably higher than those in softwood and hardwood. In addition, the carbon content in this study was slightly lower than that in bamboo (45.88%), as reported by Xiao et al. (2007), while the hydrogen, oxygen, and nitrogen elements were higher than those in bamboo (5.36, 37.40, and 0.32%, respectively), and there was no noticeable difference in sulfur content.
Table 3. Elemental Compositions of M. micrantha Plants
1: Based on oven dry weight
2: By difference
Fundamental Properties of the Crude M. micrantha Vinegar
Table 4 shows the fundamental properties of the crude M. micrantha vinegar. The yield was approximately 35.29%, the water content was 91.23%, the Gardner color value was about 11.2 (corresponding to a slightly transparent reddish-brown color), the specific gravity was slightly greater than 1, the pH was 5.36, the organic acid content was 2.50%, and the soluble tar content was 0.78%. The vinegar of Phyllostachys bambusoides and Phyllostachys pubescens prepared by Mu et al. (2003) had specific gravities of 1.0246 and 1.0257, organic acid contents of 5.88% and 6.95%, and acidic pH of 2.8.
Table 4. Fundamental Properties of the Crude M. micrantha Vinegar
± Represents standard deviation.
Compared with M. micrantha vinegars, the specific gravity was similar, but the pH of M. micrantha vinegar was noticeably higher than that of bamboo vinegar, showing weak acidity, and the organic acid content was noticeably lower than that in bamboo vinegar. Compared with the fundamental properties of M. micrantha vinegar obtained by Lin and Lu (2012) in the temperature range 150 to 600 °C, in this study, the water content and Gardner color value were higher, the specific gravity, pH, and organic acid content were similar, and the soluble tar content was lower. According to the above results, there were still some slight differences in water content, color, and soluble tar content of vinegar prepared with M. micrantha from different growing environments. From the results mentioned above, comparing with the bamboo vinegar, the higher pH value and the lower organic acid content of the M. micrantha vinegar, which exhibit a marvelous message for the organic components and the repellent activity against biting midges of the vinegar would be discussed in the next sections.
Organic Components of M. micrantha Vinegars
In this study, the pyrolysis temperature for preparing vinegar was increased at a heating rate of 100 °C h-1 to 600 °C, with a holding time of 1 h. The GC-MS analysis results of the organic components of M. micrantha vinegar including ether-extracted vinegar and acidic, phenolic, and neutral fractions are shown in Fig. 6, and the organic compounds are listed in Table 5. According to the analysis results, the organic components in M. micrantha vinegars were classified into four major categories, namely as acidic components, phenolic components, nitrogenous compounds, and neutral components. Among the organic components in ether-extracted M. micrantha vinegar, acidic components had the highest content of 38.94%, followed by neutral components (19.56%), and the contents of phenolic components and nitrogenous compounds were 18.96% and 18.48%, respectively.
Among the acidic components, acetic acid had the highest content of 27.27%, followed by propionic acid (5.39%) and butyric acid (2.61%). Among the phenolic components, phenol had the highest content of 9.04%. Among the nitrogenous compounds, 3-hydroxypyridine had the highest content of 4.16%, followed by pyridine (2.97%). The neutral compounds were mainly furans and cyclic ketone derivatives, with 2-furfuryl alcohol having the highest content of 9.25%, followed by 2-acetylfuran (2.35%).
Table 5 also shows that the ether-extracted vinegar from M. micrantha vinegar was further separated into three fractions, namely acidic, phenolic, and neutral fractions, by the partition method. Among them, the content of acidic compounds in the acidic fraction increased from 38.94% to 85.89% in ether-extracted vinegar, among which acetic acid still had the highest content of 47.32%, followed by propionic acid and butyric acid, which increased to 21.07% and 8.15%, respectively. However, the acidic fraction still contained a small amount of neutral compounds, such as acetonitrile and 2-furfuryl alcohol (1.10% and 4.10%, respectively). The content of phenolic compounds in the phenolic fraction increased from 18.96% to 88.66%, among which the main components were phenol, 2-methoxyphenol, and 2,6-dimethoxyphenol (48.33%, 16.20%, and 7.65%, respectively). The neutral fraction included nitrogenous and neutral compounds. The contents of nitrogenous and neutral compounds increased from 18.48% and 19.56% in ether-extracted vinegar to 43.71% and 40.98%, respectively. The nitrogenous compounds were mainly pyridine and pyridine derivatives, among which pyridine, 2-methylpyridine, and 2,5-dimethylpyridine had relatively high contents of 7.92%, 5.71%, and 5.91%, respectively, which resulted the higher pH value of M. micrantha vinegar. Among the neutral compounds, 2-furfuryl alcohol still had the highest content of 21.01%, followed by 2,3-dimethyl-2-cyclopenten-1-one and 2-acetylfuran, with contents of 6.18% and 5.56%, respectively.
Fig. 6. GC-MS chromatography of ether-extracted vinegar (a), acidic (b), phenolic (c), and neutral (d) fractions of M. micrantha vinegars
Table 5. Organic Components of Ether-extracted Vinegar, Acidic, Phenolic, and Neutral Fractions of M. micrantha Vinegars
*: Figures in rows are sum of percents of components.
-: Represents a trace of components can’t be detected
Repellent Activity of M. micrantha Vinegar Against F. taiwana
In this study, the method of human luring was used, and the experiment site was Zhuyuan Lane, North District, Taichung City, Taiwan. The M. micrantha vinegar and various fractions at different concentrations were used to conduct biting midge-repelling experiments. The time of biting by the first biting midge (absolute repellent time) was recorded during the experiment to evaluate the biting midge-repelling performance. Reportedly, temperature affects the overall density and activity of biting midges, and the blood-sucking activity in daytime gradually increases from 8:00 a.m., reaches its peak at 2:00 p.m., then decreases, and disappears completely from 6 to 7 p.m. (Chen et al. 1981). This experiment was conducted between 10 a.m. and 2 p.m. every day, and the number of biting midge bites within 20 min was recorded to evaluate the density of biting midges on the day of the experiment. The results are listed in Table 6.
The experimental results showed that the absolute repellent time of ether-extracted M. micrantha vinegar, and phenolic and neutral fractions were 87, 83, and 99 min, respectively. However, that of the crude vinegar was 49 min, while that of the acidic fraction was only 14 min. The results indicated that the crude M. micrantha vinegar, ether-extracted vinegar, and phenolic and neutral fractions all had good repellent activities against biting midges. In terms of the density of biting midges, the number of biting midge bites within 20 min was more than 50 in the control group under the same experimental conditions, with the highest number reaching 207. When the acidic fraction was applied, there were 7 biting midge bites within 20 min, and there were no biting midge bites for the other samples.
The temperature and humidity of the experimental station affect the density and activity of biting midges. The experiment were performed for several days, in the control group, the absolute repellent times were within 3 min for all of the test, which did not effect a change of climate. However, with regard to the density of biting midge bites within 20 min, the numbers of biting midge were quite different in each test day, which were from 25 biting midges of alcohol test to 207 biting midges of acidic fractions test.
The crude M. micrantha vinegar, ether-extracted vinegar, and phenolic and neutral fractions with relatively good repellent activities were diluted 5 times. The crude M. micrantha vinegar was diluted with distilled water, while ether-extracted vinegar and the phenolic and neutral fractions were diluted with 95% alcohol. The experimental results of biting midge repellence are also shown in Table 6. The results showed that the absolute repellent times of the experimental and the control groups were the same as 3 min when the experiment was conducted with 95% alcohol, and the number of biting midge in 20 min was approximately 30. Therefore, it can be confirmed that alcohol as solvent had no effect on the biting midge repellence results in each fraction. The absolute repellent time of crude M. micrantha vinegar, ether-extracted vinegar, and the phenolic and neutral fractions diluted 5 times was approximately 30 min or more, among which the neutral fraction had the best effect (absolute repellant time of 45 min), followed by the ether-extracted vinegar (38 min). With regard to the density of biting midges, when applying crude M. micrantha vinegar, ether-extracted vinegar, and the phenolic and neutral fractions diluted 5 times, there were no biting midge bites within 20 min, but the control group suffered about 74 biting midge bites within 20 min under the same conditions, with the highest number being 138. According to Moore et al. (2006), plant-based repellent agents contain phenolic compounds like phenol and its derivatives, and nitrogenous compounds such as pyridine and its derivatives, can be used as pesticides and pest repellent agents, respectively. According to the results in this study, the ether-extracted vinegar, and phenolic and neutral fractions all had good repellent activities against biting midges. The organic components in ether-extracted vinegar contained acidic compounds (38.94%), phenolic compounds (18.96%), nitrogenous compounds (18.48%), and other neutral compounds (19.56%). The phenolic fraction contained phenolic compounds (88.66%) and 2-furanmethanol (3.79%). The neutral fraction contained nitrogenous compounds (43.71%) and other neutral compounds (40.98%). In the neutral fraction, 22 kinds of nitrogenous compounds such as pyridine and pyrazine derivatives had been detected, which emitted pungent stink odor, and the absolute repellent time was 99 min more than the phenolic components of 83 min. It is speculated that the repellent effect of M. micrantha vinegar for biting midges mainly arises from the nitrogenous compounds in the neutral fraction.
Comparing the results of crude M. micrantha vinegar, ether-extracted vinegar, and the three fractions with those of a commercially available anti-biting midge liquid (Dinling) revealed that though the repellent activity of the samples diluted 5 times was slightly lower than that of Dinling (absolute repellent time of 61 min), the repellent activity of undiluted ether-extracted vinegar and the phenolic and neutral fractions was significantly higher than that of the commercial one, and among which the repellent time of the neutral fraction was 1.5 times than that of the commercial Dinling. However, for the practical application, the ether-extracted M. micrantha vinegar can be used as a repellant agent in the future, as it has a good repellent activity against biting midges, and the preparation cost is lower than that of the neutral fraction.
Table 6. Repellent Activity of F. taiwana of M. micrantha Vinegar, Acidic, Phenolic, and Neutral Fractions
± Represents standard deviation.
CONCLUSIONS
- The contents of holocellulose, α-cellulose, Klason lignin, alcohol-toluene extractives, and ash in M. micrantha plants were 52.03, 26.20, 23.77, 16.98, and 6.94%, respectively. Elemental analysis showed that the contents of carbon, hydrogen, oxygen, nitrogen, and sulfur were 42.97, 6.20, 40.54, 1.71, and 0.21%, respectively. Compared with other published literature, the alcohol-toluene extractives, ash, and nitrogen element contents of M. micrantha plant were noticeable higher than those in wood and bamboo.
- The yield of crude M. micrantha vinegar was 35%, and the water content was 91.23%. The color was transparent reddish-brown, and the Gardner color value was 11.2. The specific gravity was slightly greater than 1. The pH value was 5.36, showing weak acidity. The organic acid content was 2.50% and the soluble tar content was 0.78%. Compared with other published literatures, the pH value and the nitrogenous components in the M. micrantha vinegar were higher than those in the wood and bamboo vinegar.
- Among the organic components of the vinegar, the content of acidic components was as high as 38.94%, followed by neutral, phenolic, and nitrogenous components (19.56, 18.96, and 18.48%, respectively). Acetic acid had the highest content among acidic components. The phenolic compounds were mainly phenol. Among the nitrogenous components, the content of 3-hydroxypyridine was the highest. Among the neutral components, the content of 2-furfuryl alcohol was the highest.
- The repellence experiment showed that undiluted crude M. micrantha vinegar, ether-extracted vinegar, and the phenolic and neutral fractions showed good repellent activities against biting midges, and the absolute repellent times were 49, 87, 83, and 99 min, respectively. It was also found that the repellent activities of ether-extracted vinegar and the phenolic and neutral fractions were higher than that of a general commercially available anti-biting midge agent.
REFERENCES CITED
Akakabe, Y., Tamura, Y., Iwanoto, S., Takabayashi, M., and Nyuugaku, T. (2006). “Volatile organic compounds with characteristic odor in bamboo vinegar,” Biosci. Biotechnol. Biochem. 70(11), 2797-2799. DOI: 10.1271/bbb.60317
ASTM D7582-12 (2012). “Standard test methods for proximate analysis of coal and coke by macro thermogravimetric analysis,” ASTM International, West Conshohocken, PA, USA.
Baimark, Y., and Niamsa, N. (2009). “Study on wood vinegars for use as coagulating and antifungal agents on the production of natural rubber sheets,” Biomass Bioenergy 33(6-7), 994-998. DOI: 10.1016/j.biombioe.2009.04.001
Chen, C. S., Lien, J. C., Lin, Y. N., and Hsu, S. J. (1981). “The diurnal biting pattern of a bloodsucking midge Forcipomyia (Lasiohelea) taiwana (Shiraki) (Diptera, Ceratopogonidae),” Chinese J. Microbiol. Immun. 14(1), 54-56. DOI: 10.6661/TESFE.2009006
Chuang, Y., Lin, C., Wang, C., and Yeh, C. (2000). “Distribution and seasonal occurrence of Forcipomyia taiwana (Diptera: Ceratopogonidae) in the Nantou area in Taiwan,” J. Med. Entomol. 37(2), 205-209. DOI: 10.1603/0022-2585-37.2.205
CNS 2721 (2010). “Method of test for determination of acid-insoluble lignin in pulp,” Chinese National Standards, Republic of China, Taiwan.
CNS 3084 (2004). “Method of test for ash in wood,” Chinese National Standards, Republic of China, Taiwan.
CNS 3085 (2004). “Method of test for holocellulose in wood,” Chinese National Standards, Republic of China, Taiwan.
CNS 4713 (2005). “Method of test for ethanol-toluene extractives in wood,” Chinese National Standards, Republic of China, Taiwan.
CNS 10865 (2002). “Method of test for α-, β-, and γ- cellulose in pulp,” Chinese National Standards, Republic of China, Taiwan.
Feng, Y., Li, D., Sun, H., Xue, L., Zhou, B., Yang, L., Liu, J., and Xing, B. (2020). “Wood vinegar and biochar co-application mitigates nitrous oxide and methane emissions from rice paddy soil: A two-year experiment,” Environ. Pollut. 267, Article ID 115403. DOI: 10.1016/j.envpol.2020.115403
Grewal, A., Abbey, L., and Gunupuru, L. R. (2018). “Production, prospects and potential application of pyroligneous acid in agriculture,” J. Anal. Appl. Pyrolysis 135, 152-159. DOI: 10.1016/j.jaap.2018.09.008
Hagner, M., Kuoppala, E., Fagernäs, L., Tiilikkala, K., and Setälä, H. (2015). “Using the copse snail Arianta arbustorum (Linnaeus) to detect repellent compounds and the quality of wood vinegar,” Int. J. Environ. Res. 9(1), 53-60. DOI: 10.22059/IJER.2015.873
Jin, T., Wu, Y., and Wang, Q. (2012). “The inhibitory effects of bamboo vinegar against bacteria and fungi,” Adv. Intel. Soft. Compu. 134, 451-457. DOI: 10.1007/978-3-642-27537-1_55
Li, R., Narita, R., Nishimura, H., Marumoto, S., Yamamoto, S. P., Ouda, R., Yatagai, M., Fujita, T., and Watanabe, T. (2018). “Antiviral activity of phenolic derivatives in pyroligneous acid from hardwood, softwood, and bamboo,” ACS Sustain. Chem. Eng. 6(1), 119-126. DOI: 10.1021/acssuschemeng.7b01265
Lien, J. C. (1989). “Taxonomic and ecological studies on the biting midges of the subgenus Lasiohelea, genus Forcipomyia from Taiwan,” J. Taiwan Museum 42(1), 37-77. DOI: 10.6532/JTM.198906_42(1).0006
Lien, J. C. (1991). “Seven new species and four new record of Forcipomyia subgenus Lasiohelea from Taiwan (Diptera: Ceratopogonide),” J. Taiwan Museum 44(1), 83-116. DOI: 10.6532/JTM.199106_44(1).0003
Lin, T. W., and Lu, K. T. (2012). “Fundamental properties of Mikania micrantha crude vinegars collected from different pyrolysis temperature ranges (in Chinese),” Quarterly J. Chinese Forestry 45(2), 201-220. DOI: 10.30064/QJCF.201206.0005
Lu, K., Kuo, C., and Liu, C. (2007). “Inhibition efficiency of mixed solution of bamboo vinegar and chitosan against Ralstonia solanacearum,” Taiwan J. Forest Science 22(3), 329-338. DOI: 10.7075/TJFS.200709.0329
Marra, R., Vinale, F., Cesarano, G., Lombardi, N., d’Errico, G., Crasto, A., Mazzei, P., Piccolo, A., Incerti, G., Woo, S. L., et al. (2018). “Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes,” PLoS ONE 13(6), Article ID e0198728. DOI: 10.1371/journal.pone.0198728
Moore, S. J., Lenglet, A., and Hill, N. (2006). “Plant-based insect repellents,” in: Insect Repellents: Principles, Methods, and Uses, M. Debboun, S. P. Frances, and D. Strickman (eds.), Taylor Francis, Boca Raton, FL, USA, pp. 278-280. DOI: 10.1201/9781420006650
Mu, J., Uehara, T., and Furuno, T. (2003). “Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants,” J. Wood Science 49(3), 262-270. DOI: 10.1007/s10086-002-0472-z
Mu, J., Uehara, T., and Furuno, T. (2004). “Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants II: Composition of moso bamboo vinegar at different collection temperature and its effects,” J. Wood Science 50, 470-476. DOI: 10.1007/s10086-003-0586-y
Sugiura, G. (1995). Mystery of Wood Vinegar, The National Forestry Improvement and Extension Association (Zenrinkyo), Tokyo, Japan, pp. 130-164. (In Japanese)
Sulaiman, Q., Murphy, R. J., Hashim, R., and Gritsch, C. S. (2005). “The inhibition of microbial growth by bamboo vinegar,” J. Bamboo and Rattan 4(1), 71-80. DOI: 10.1163/1569159053444635
Sun, W. K. C. (1967). “Study of a biting midge, Forcipomyia (Lasiohelea) taiwana (Shiraki) (Diptera: Cerapogonidae) I. Description of the complete life cycle of the midge reared in the laboratory,” Defense Technical Information Center, (https://apps.dtic.mil/sti/pdfs/AD0661925.pdf), Accessed 30 Jan 2021.
de Wild, P. J., den Uil, H., Reith, J. H., Kiel, J. H. A., and Heeres, H. J. (2009). “Biomass valorisation by staged degasification: A new pyrolysis-base thermochemical conversion option to produce value-added chemicals from lignocellulosic biomass,” J. Anal. Appl. Pyrolysis 85(1-2), 124-133. DOI: 10.1016/j.jaap.2008.08.008
Xiao, G., Ni, M., Huang, H., Chi, Y., Xiao, R., Zhong, Z., and Cen, K. (2007). “Fluidized-bed pyrolysis of waste bamboo,” J. Zhejiang University – Science A: Applied Physics & Engineering 8(9), 1495-1499. DOI: 10.1631/jzus. 2007.A1495
Yang, H., Yan, R., Chen, H., Lee, D. H., and Zheng, C. (2007). “Characteristics of hemicellulose, cellulose and lignin pyrolysis,” Fuel 86(12-13), 1781-1788. DOI: 10.1016/j.fuel.2006.12.013
Yatagai, M., Nishimoto, M., Hori, K., Ohira, T., and Shibata, A. (2002). “Termiticidal activity of wood vinegar, its components and their homologues,” J. Wood Science 48, 338-342. DOI: 10.1007/BF00831357
Article submitted: May 6, 2021; Peer review completed: August 1, 2021; Revised version received and accepted: August 17, 2021; Published: August 26, 2021.
DOI: 10.15376/biores.16.4.6831-6849
