Abstract
The prospects of biofuel production from microalgal carbohydrates and lipids coupled with greenhouse gas mitigation due to photosynthetic assimilation of CO2 have ushered in a renewed interest in algal feedstock. Furthermore, microalgae (including cyanobacteria) have become established as commercial sources of value-added biochemicals such as polyunsaturated fatty acids and carotenoid pigments used as antioxidants in nutritional supplements and cosmetics. This article presents a comprehensive synopsis of the metabolic basis for accumulating lipids as well as applicable methods of lipid and cellulose bioconversion and final applications of these natural or refined products from microalgal biomass. For lipids, one-step in situ transesterification offers a new and more accurate approach to quantify oil content. As a complement to microalgal oil fractions, the utilization of cellulosic biomass from microalgae to produce bioethanol by fermentation, biogas by anaerobic digestion, and bio-oil by hydrothermal liquefaction are discussed. Collectively, a compendium of information spanning green renewable fuels and value-added nutritional compounds is provided.
Download PDF
Full Article
Microalgae as a Feedstock for Biofuel Precursors and Value-Added Products: Green Fuels and Golden Opportunities
Yuting Tang,a Julian N. Rosenberg,b Pavlo Bohutskyi,b,c Geng Yu,b Michael J. Betenbaugh,b and Fei Wang a,*
The prospects of biofuel production from microalgal carbohydrates and lipids coupled with greenhouse gas mitigation due to photosynthetic assimilation of CO2 have ushered in a renewed interest in algal feedstock. Furthermore, microalgae (including cyanobacteria) have become established as commercial sources of value-added biochemicals such as polyunsaturated fatty acids and carotenoid pigments used as antioxidants in nutritional supplements and cosmetics. This article presents a comprehensive synopsis of the metabolic basis for accumulating lipids as well as applicable methods of lipid and cellulose bioconversion and final applications of these natural or refined products from microalgal biomass. For lipids, one-step in situ transesterification offers a new and more accurate approach to quantify oil content. As a complement to microalgal oil fractions, the utilization of cellulosic biomass from microalgae to produce bioethanol by fermentation, biogas by anaerobic digestion, and bio-oil by hydrothermal liquefaction are discussed. Collectively, a compendium of information spanning green renewable fuels and value-added nutritional compounds is provided.
Keywords: Microalgae; Biofuels; Biochemicals; Lipid Profiles; Algal Strain Development
Contact information: a: College of Chemical Engineering, Nanjing Forestry University, Jiangsu Key Lab of Biomass-based Green Fuels and Chemicals, NO. 159 Longpan Street, Nanjing, JS 210037 PR China; b: Department of Chemical & Biomolecular Engineering, Johns Hopkins University, 3400 N. Charles St, Baltimore, MD 21218 USA; c: Current address – Biological Sciences Division, Fundamental & Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA USA;
* Corresponding author: hgwf@njfu.edu.cn
INTRODUCTION
Increased carbon dioxide release coupled with declines in non-renewable crude oil resources has resulted in the consideration of liquid biofuels derived from plant materials as a potential valuable alternative source of energy. Moreover, liquid biofuels can be regarded as a form of solar energy stored in plant organisms, in contrast to other renewable energy sources such as tidal and wind, and they are also compatible with existing engines within the transportation infrastructure. Currently, first generation bioethanol feedstocks such as sugar-based crops of sugarcane (Singh et al. 2013), sugar beet (Balcerek et al. 2011), starch-based crop of cassava (Osei et al. 2013), and biodiesel feedstocks such as rapeseed (Iriarte et al. 2011), soybeans (Alcantara et al. 2000), palm oil (Kansedo et al. 2009) as well as sunflower (Buratti et al. 2012) have been criticized on account of their direct competition with food resources. To make the situation worse, the growth of oil crops for transportation fuel production may occupy arable land that can instead be used for food crops cultivation (Ahmad et al. 2011). To eliminate those drawbacks, a second-generation of non-food crops has been developed for bioenergy production. These non-food energy crops are reed (Kuhlman et al. 2013), switchgrass (Bansal et al. 2013), corn stover (Leboreiro and Hilaly 2013), giant Miscanthus (Lewandowski et al. 2003) for bioethanol along with jatropha (Hailegiorgis et al. 2013; Pramanik 2003), Euphorbia antisyphilitica (Padmaja et al. 2009), and waste cooking oils (Canakci 2007). However, the technology for conversion of these crops to biofuels still needs to be improved for profitable commercial utilization (FAO 2008). A recent report indicated that production costs of the second biofuels are estimated to be 17-26 $/GJ in 2020 and 14-23 $/GJ in 2030, compared to 20–30 $/GJ for fossil fuels. The conversion process, supply chain logistic, local labor costs, and agricultural efficiency are key factors in economic analysis (van Eijck et al. 2014).
Microalgae are a class of third-generation biofuel feedstocks that, compared to conventional carbohydrate or oil crops, present a number of unique advantages. While whole algal biomass may serve as feedstock for biofuel production, the energy-dense algal reserves such as polysaccharides and especially lipids (about 17 and 38 kJ g-1, respectively (Berg et al. 2002) are especially appealing. Generation of biomass with high-level of energy reserves, which is a prerequisite for efficient biofuel production, requires minimizing of energy-intensive cellular processes while maximizing the energy and carbon flow into biofuel precursors.
In addition to serving as an alternative fuel source, microalgae are also an important source of high-value chemicals, including carotenoids (Borowitzka 2010), omega-3 polyunsaturated fatty acids, such as eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) (Mendes et al. 2009), phycobilins (Singh et al. 2005), and astaxanthin (Liu et al. 2013). Thus, microalgae can serve as feedstock for value-added biochemicals. This paper summarizes the chemical products derived from microalgae considered to have the greatest potential. It aims to provide a comprehensive synopsis of the metabolic basis for accumulating these valuable biomolecules, applicable methods of bioconversion to generate biofuel, and applications of the natural or refined products from microalgae, ranging from green renewable fuels to value-added nutritional compounds.
CARBOHYDRATES
The microalgal carbohydrate content varies significantly from as low as 5% to as high as 80% and consists of soluble monosaccharides or disaccharides (usually intermediate products), energy storage polymeric carbohydrates, cell envelope structural polysaccharides, and extracellular polysaccharides. While the carbohydrate metabolism in microalgae is still not revealed completely, the energy storage carbohydrates (e.g. starch) are typically synthesized in either chloroplast or cytosol (Klein 1987; Zeeman et al. 2010) and may represent up to 40-55% of the cell’s dry mass (Gonzalez-Fernandez and Ballesteros 2012; John et al. 2011). In contrast, several studies showed that the cell wall structural polysaccharides are synthesized by plasma membrane complexes (cellulose) and by Golgi-localized enzymes (e.g. hemicellulose and pectin) (Lerouxel et al. 2006; Popper et al. 2011; Driouich et al. 2012). While the lack of lignin and a relatively high fraction of structural polysaccharides in cell weight make microalgae a potentially attractive feedstock for biofuels, the starch represents an even more advantageous substrate. Starch does not require costly pretreatment and could be easily processed using the existing bioethanol infrastructure developed for conversion of starches produced by terrestrial plants (e.g. corn). Nevertheless, the carbohydrate and particularly starch productivity has to be improved in order to make algal biofuel production economically efficient and sustainable.
Effect of Environmental Factors on Synthesis and Accumulation of Carbohydrates in Microalgae
Energy (light) and carbon (CO2) abundance, nutrient availability, environmental parameters (temperature, pH, salinity, and inhibiting compounds), and the presence or absence of other organisms may have an impact on algal metabolism and therefore on carbohydrates synthesis and accumulation. Additionally, the recent advances in algal biotechnology allow application of genetic engineering techniques for redirection of the carbon flow towards the desired products (Radakovits et al. 2010; Aikawa et al. 2015). The beneficial effects on algal starch content and productivity through manipulation by some of the factors mentioned above are described below and summarized in Table 1.
Inorganic and organic carbon
Carbon dioxide serves as the only carbon source for photoautotrophically grown microalgae. Several studies report improvements of both biomass growth rate and enhancing of starch accumulation as result of shifting from the ambient level (0.04%) to 2% CO2 in enriched air (Cheng et al. 2015; Tanadul et al. 2014) or increasing the dissolved CO2 concentration from 3 to 190 mmol L-1 in an aqueous medium by pH adjustment (Xia and Gao 2005). However, the algal response to high CO2 content has been found to be species-specific. For example, Cheng et al. (2015) found that starch content was nearly two times higher in Chlorella vulgaris and C. variabilis when cultured with 2% CO2, but it did not increase in C. sorokiniana and C. minutissima. Similarly, the activities of carbonic anhydrase and nitrate reductase enzymes in C. pyrenoidosa and Chlamydomonas reinhardtii exhibited different responses to elevation of dissolved CO2 content (Xia and Gao 2005). Moreover, other studies demonstrated that increasing CO2 content from ambient to 2-5% improves biomass growth but has either negative (up to 2-3 fold reduction) or no effect on starch accumulation when applied without the cell arrest (Thyssen et al. 2001; Izumo et al. 2007; Li et al. 2013). Lowering CO2 concentration can induce carbon dioxide concentrating mechanisms (CCM) that may enhance CO2 supply to ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), and thus improve photosynthesis efficiency and carbon storage (Raven 2010).
A number of microalgae are able to utilize organic carbon substrates (e.g. glucose, acetate, or glycerol) as the only source of carbon and energy (heterotrophy) or together with inorganic carbon and light (mixotrophy). Supplementation with organic carbon is mostly investigated and applied to boost algal lipid content. But a few studies documented enhancement of carbohydrate or starch content in Chlorella species under mixotrophy or heterotrophy (Abreu et al. 2012; Yeh and Chang 2012; Li et al. 2015a). In contrast, switching from photoautotrophy did not benefit starch accumulation in C. reinhardtii without stressing the cells (Ball et al. 1990). Importantly, the application of waste-derived organic carbon for mixotrophic or heterotrophic production of algal biomass, demonstrated by Abreu et al. (2012), is likely to be the only economical substrate source for biofuel production. In fact, a number of species from Chlorella (C. vulgaris (Perez-Garcia et al. 2010; Heredia-Arroyo et al. 2011; Abreu et al. 2012; Mitra et al. 2012; Farooq et al. 2013), C. sorokiniana, C. pyrenoidosa (Hongyang et al. 2011; Wang et al. 2012), C. protothecoides, C. minutissima, C. kessleri), Scenedesmus (S. obliquus (Hodaifa et al. 2009; Zhang et al. 2013), S. dimorphus, S. quadricauda, S. bijuga), Chlamydomonas (C. debaryana, C. globosa), and Micractinium genera have been reported to grow either mixotrophically or heterotrophically in various types of wastewater including municipal, sludge anaerobic digestion effluent, soybean or starch processing, brewery, ethanol thin stillage, piggery, dairy, and poultry media (Bhatnagar et al. 2011; Park et al. 2012; Bohutskyi et al. 2015c). More specifically, the increase of carbohydrate was observed when Scenedesmus obliquus was mixotrophically cultivated in municipal wastewater supplemented with CO2 and food wastewater (Ji et al. 2015). The carbohydrate content of Chlorella vulgaris JSC-6 using 5-fold dilution of swine wastewater was 58.3 and 54.0% of dry weight under mixotrophic and heterotrophic growth respectively (Wang et al. 2015). It has been noted that microalgae show the ability to accumulate carbohydrate in wastewater and the production of carbohydrate from microalgae grown in wastewater could be viable with the advances in cultivation technology. Microalgae in wastewater could be the best source to generate not only biodiesel, the one with high carbohydrate content have the potential to produce the other products such as bioethanol and bio-oil (Abinandan and Shanthakumar 2015).
Light intensity
Photoautotrophic metabolism utilizes light as the only energy source for generation of new cells as well as energy-storage molecules such as starch. Also, several enzymes involved in carbon fixation (e.g. Calvin-Benson cycle) and therefore starch synthesis are light-activated through the ferredoxin/thioredoxin system (Buchanan 1984; Michalska et al. 2009).
In general, low light intensity is not favorable for high-rate accumulation of starch since all energy is utilized to maintain homeostasis and for cell division. Several studies reported that elevation of light intensity from 60-75 to 300-400 mmol m-2 s-1 boosted carbohydrate/starch content from 5-15 to 30-40% (Friedman et al. 1991; De Philippis et al. 1992; Ho et al. 2012).
Similarly, the mean light intensity above 265 mmol m-2 s-1 was found to be required for starch accumulation in C. vulgaris (Brányiková et al. 2011). In contrast, Carvalho et al. (2009) reported absence of strong relation between light intensity and carbohydrate or starch accumulation, suggesting either carbon limitation or elimination of other carbon sinks such as protein synthesis or cell division.
However, oversaturation with light may also cause a decrease in algal growth rate due to formation of harmful reactive oxygen species (Leverenz et al. 1990; Zhu et al. 2008; Stephenson et al. 2011). In addition, irradiation is not applicable for starch induction for outdoor facilities due to the lack of control over the sunlight, which fluctuates along with season and weather conditions impacting algal carbohydrate content (Tredici et al. 1991).
Temperature
The growth rate and therefore productivity of all cell components including carbohydrates increases until it reaches the optimal temperature of around 25 to 30 °C due to increasing activities of carbon-fixing enzymes (e.g. RuBisCO; see Nakamura and Miyachi 1982b). Although the detailed effect of temperature on carbohydrate content remains unclear, a few authors demonstrated a stimulation of carbohydrate synthesis when the temperature was increased to an optimal range (Oliveira et al. 1999; Gigova et al. 2012). On the other hand, Renaud et al. (2002) reported a lack of relation between temperature and carbohydrate contents for Isochrysis sp., Rhodomonas sp., Cryptomonas sp., Chaetoceros sp., and Prymnesiophyte alga. Finally, Nakamura and Miyachi (1982a) observed a maximum starch content in C. vulgaris around 20 to 24 °C and rapid loss of starch along with further elevation of temperature to 38 °C. This reduction may be result of a stronger activation of the starch-degrading enzymes (e.g. α-glucan phosphorylase) than the enzymes responsible for carbon fixation (e.g. RuBisCO) at higher temperatures (Nakamura and Miyachi 1982b). A distinct effect on different enzymes is confirmed by the reduction of amylopectin and increase of amylose fractions in starch with the increases in the temperature from 10 to 38 °C (Nakamura and Imamura 1983).
pH
The pH affects all organisms due to its impact on the chemistry of most inorganic and organic molecules and therefore on kinetics of metabolic reactions. However, pH has an additional influence on photoautotrophs through its relation with CO2 solubility and the carbonic acid protonation state (Eq. 1).
While the optimal pH is species-specific, it is in the range from 7.0 to 9 for many freshwater and marine microalgae. However, since the pH generally increases along with photosynthetic activity and CO2 consumption, many green microalgae and cyanobacteria are able to grow at pH values as high as 10.5 to 11. The change in carbohydrate content in response to pH change is species specific as well. The maximum accumulation of carbohydrate was reported for Skeletonema costatum at pH 7 (Taraldsvik and Myklestad 2000), for Dunaliella bardawil at pH 7.5 and Chlorella ellipsoidea at pH 9 (Khalil et al. 2009).
Enhancing Synthesis and Accumulation of Algal Carbohydrates
As discussed above, while providing a sufficient amount of light and carbon for optimal algal growth is important, it does not guarantee accumulation of energy reserves in large quantity due to the tremendous expenditure of energy and carbon for cell duplication. Therefore, arresting of cell cycle is a typical method for maximizing carbon flow into energy storage molecules.
Application of chemical inhibitors for arresting the cell cycle
Various algal cell processes can be targeted to arrest cell division. For example, Zachleder demonstrated the inhibition of nuclear DNA synthesis in Scenedesmus quadricauda by application of FdUrd (5-fluorodeoxyuridine), specifically inhibiting thymidylate synthase (Zachleder 1994, 1995). While FdUrd-treated cells accumulated higher starch content than the untreated control, the difference was not dramatic because FdUrd did not inhibit biosynthesis of other biomolecules, including proteins and RNA. More promising results were achieved by applying the antibiotic cycloheximide, which boosted the starch content in C. vulgaris to nearly 60% by specifically inhibiting biosynthesis of proteins in cytoplasm (Brányiková et al. 2011). However, industrial application of chemical inhibitors like that may have a detrimental impact on the environment as well.
Table 1. Comparison of Starch Induction Techniques Reported in the Literature
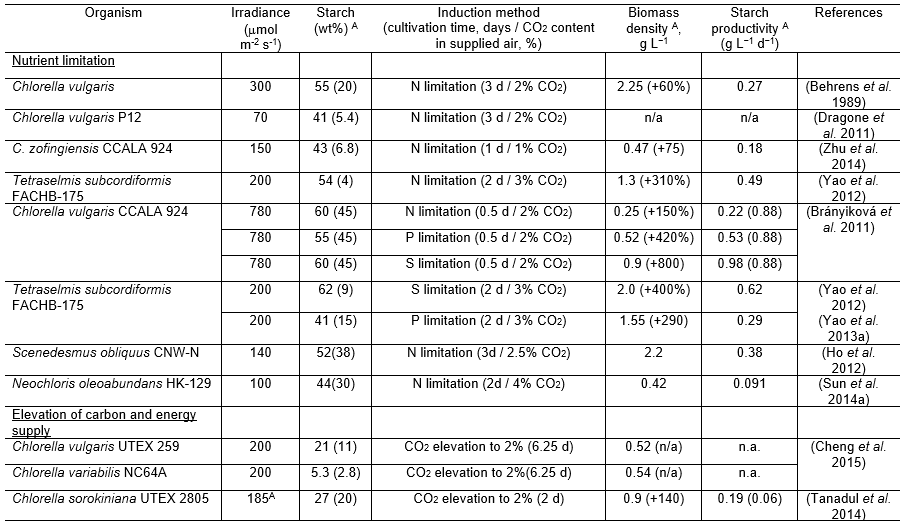
Nutrient limitation
Cultivation of microalgae in nutrient-depleted conditions is a widely applied method to stop the generation of new cells since nutrients are required for the synthesis and function of DNA, RNA, proteins, pigments, and co-enzymes. It is important to keep in mind that elevation of starch content does not automatically mean enhancing its productivity, which is a fraction of both biomass productivity and starch content in biomass. Hence, optimal processing requires providing the optimal supply of carbon and light resources and ensuring the generation of an adequate amount of “lean” algal biomass prior to nutrient depletion.
While the limitation in nitrogen is the most widely studied method for induction of starch synthesis, depletion in other nutrients such as phosphorus or sulfur may be successfully applied as well (Table 1). Importantly, a combination of nitrogen limitations together with sufficient amounts of light (under 50 mmol m-2 s-1) and carbon (2% CO2) were found to be the most efficient way to boost starch content up to 55% of the dry weight in Chlorella vulgaris (Behrens et al. 1989). Starch can also be enhanced up to 50-60% under sulfur deprivation together with sufficient amounts of light during relatively short periods of time (0 to 3 days) in other species (Brányiková et al. 2011; Yao et al. 2012).
LIPIDS
The lipid content of algae often ranges from 20 to 50% under different cultivation conditions, especially in stressed environments. Nitrogen limitations or other stresses, enhance lipid accumulation for almost all microalgal species (Araujo et al. 2013). For fuel production, the lipid content (% lipid per dry weight of biomass) and the lipid productivity (volumetric g L-2 d-1 or areal g m-2 d-1) are pivotal considerations to evaluate the potential of algal strains to produce biofuel. In addition, long-chain omega-3 polyunsaturated fatty acids (PUFAs) are valuable lipids from microalgae. They cannot be synthesized by higher plants or animals and are widely used as nutrition supplements. EPA and DHA have attracted much attention due to their prominent bioactivities. DHA production from microalgae has already been commercially exploited (Spolaore et al. 2006), and the production of EPA from some microalgae may be maximized when the microalgae are heterotrophically cultivated in low-cost medium (Wen and Chen 2003).
Maximizing Neutral Lipid Production in Microalgae
The biomass and the oil content in each cell are two key factors that account for the overall yield of microalgal biodiesel. Neutral lipids, commonly stored as triacylglycerol (TAG), are the principal source of biodiesel. A schematic of the biochemical pathways for TAGs is provided in Fig. 1.
There are several ways to maximize the rate of production of TAGs. Manipulations of the nutrient supply, different light intensity, light wavelength, and temperature have already been established to be associated with the enhancement of TAGs in microalgae. For example, Klok et al. (2013) found that the excess light absorption combined with limitation of nitrogen nutrition will enhance the yield of TAGs in Neochloris oleoabundans from 1.5% to 12.4% (w/w). Guo et al. (2015) observed that a 30% enhancement of lipids of Chlorella sp. was obtained under the light intensity of 320 μmol m-2 s-1, which was higher than at 80, 160 and 240 μmol m-2 s-1.
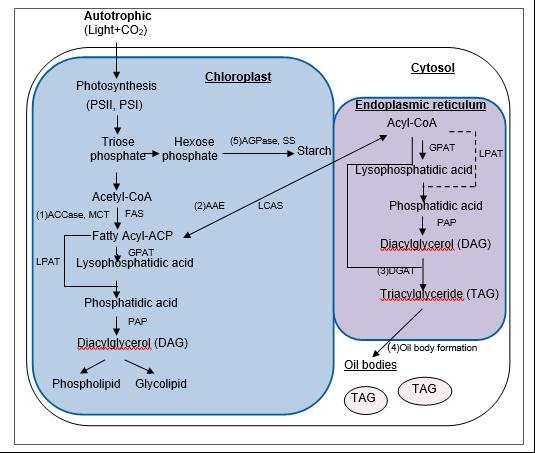
Fig. 1. Basic overview of the pathways of carbon capture and lipid biosynthesis (Klok et al. 2014; Scott et al. 2010). Enzyme abbreviations: ACCase, acetyl-CoA carboxylase; MCT, malonyl-CoA:ACP transacylase; FAS, fatty acid synthase; AAE, acyl-ACP esterase; LCAS, long-chain acyl-CoA synthase; GPAT, glycerol-3-phosphate acyltransferase; LPAT, lysophosphatidic acid acyltransferase; DGAT, diacylglycerol acyltransferase; PAP, phosphatidic acid phosphatase; AGPase, ADP-glucose pyrophosphorylase; SS, starch synthase
Kim et al. (2014) stated that FAME yield of Nannochloropsis gaditana under red light was 21.1% of dry weight, two fold higher than that under white light. The enhanced TAG content was from 18.59 to 31.71% of dry weight in Isochrysis galbana under combined optimal temperature (30 °C) and light intensity (300 μmol m-2 s-1) (Kurpan Nogueira et al. 2015).
Manipulation of lipid biosynthesis in algae could also be achieved through metabolic engineering, especially the overexpression of enzymes (Rosenberg et al. 2015). Combining an improved understanding of the metabolic pathways together with their regulation and interdependence can potentially contribute to improving the oil content (Rosenberg et al. 2008). A number of research studies addressing these challenges are summarized below.
Evaluation of lipid synthesis enzymes influencing TAG production
The production of fatty acids that control the lipid biosynthesis may be regulated by acetyl CoA carboxylase (ACCase) (Bao and Ohlrogge 1999). Different effects on oil content have been observed in microalgae and higher plants with overexpression of acetyl-CoA carboxylase (Step 1). Increasing the activity of ACCase resulted in higher lipid content, which has been demonstrated in higher plants such as rapeseed (Roesler et al. 1997). Unfortunately, a two to three-fold higher ACCase activity in the transformed Cyclotella cryptica and Navicula saprophila did not affect the TAG level (Dunahay et al. 1996). The effects of overexpression of other enzymes, which are related to the synthesis of TAGs, have been explored in several studies. Overexpression of acyl-ACP esterase in Phaeodactylum tricornutum changed the fatty acid profile with cellular lipid content increasing two-fold, demonstrating that biofuel production can be enhanced by the overexpression of thioesterases (Step 2) (Radakovits et al. 2011).
Understanding the regulation of lipid body metabolism in algae, especially how the nitrate depletion induces the lipid accumulation, may contribute to increasing the oil content in the future. Previous studies have provided insights regarding this underlying mechanism. Msanne et al. (2012) reported that increasing transcript abundance was indicated for several enzymes for diacylglycerol:acyl-CoA acyltransferases when the microalga Chlamydomonas was photoautotrophically cultivated under deprivation of nitrogen, while genes encoding enzymes for de novo fatty acid synthesis, such as 3-ketoacyl-ACP synthase I were decreased (Step 3). Li et al. (2012) found that the transcript levels of genes concerning pyruvate and acetyl-CoA synthesis increase dramatically under nitrate depletion condition. It is likely that pyruvate and acetyl-CoA are important to TAG synthesis. A major integral protein was found in Chlorella oil bodies and may play an important role in supporting the stability and structure of lipid storage organelles (Step 4) (Lin et al. 2012).
Elimination of ‘redundant’ pathways to make precursor metabolites available for biofuel production
In order to address metabolic bottlenecks to lipid production, Wang et al. (2009) observed that a deficiency of ADP-glucose pyrophosphorylase, an enzyme essential for production of starch (Fig. 1, step 5), contributes to the accumulation of TAG. Li et al. (2010) reported that the neutral lipid content in the Chlamydomonas reinhardtii starchless mutant that were cultivated under high light and nitrate depletion was dramatically increased when the starch production pathway was blocked. It was found that the neutral and total lipids rose to 32.6% and 46.4% of dry mass, respectively, in the mutant BAFJ5 lacking ADP-glucose pyrophosphorylase (Step 5).
ALGAL BIOMASS CONVERSION INTO BIOFUELS
Production of biodiesel from microalgal lipids provides opportunities to generate biofuel. In addition, microalgae can be used as a cellulosic biomass to produce bioethanol by fermentation, biogas by anaerobic digestion, and bio-oil by hydrothermal liquefaction. Furthermore, these technologies can also use microalgal residues after lipid extraction, and the combined productions of biofuel from microalgae could maximize their overall value.
Bioethanol Production from Microalgal Carbohydrates
Sugar/starch and lignocellulosic biomass are generally used as feedstocks to produce bioethanol. Although sugar is easier to be fermented than starchy or cellulosic materials, it usually comes from agricultural crops and thus is not cheap. In addition, sugar crops are also used as human and animal food (Walker 2011). Starch or lignocellulosic feedstocks are cheaper than sugar, but pretreatment prior to fermentation that converts the starch or lignocellulosic feedstock to fermentable sugars is required. However, lignin, existing in lignocellulosic feedstock, is very hard to degrade and raises the cost of pretreatment (Harun et al. 2010).
Microalgae capable of accumulating high amounts of polysaccharides can also serve as a feedstock for bioethanol production. Although in most circumstances, the polysaccharides are entrapped in the microalgal cell wall, pre-treatment can break the cell wall and convert these complex polysaccharides into simple sugars prior to bioethanol fermentation of the starchy or cellulosic materials. The absence of lignin in microalgae may make the pretreatment and hydrolysis step easier.
Acid pretreatment is widely used. After using 3% (v/v) of sulfuric acid to treat Chlorococcum humicola at 160 °C for 15 min, a 52 wt% (ethanol/microalgae, g/g) maximum production was achieved (Harun and Danquah 2011). Ho et al. (2013) compared enzymatic and acid hydrolysis of Chlorella vulgaris biomass, a strain containing up to 51% carbohydrate and found the glucose yield was 90.4% and 93.6% of microalgal carbohydrates, respectively. These results indicate that the exploitation of carbohydrate-accumulating microalgae as a raw material for bioethanol fermentation is economically viable. The other promising option is alkaline pretreatment. Harun et al. (2011) stated that the bioethanol yield obtained was 26% of algal biomass by using 0.75% (w/v) of NaOH to pretreat Chlorococcum infusionum. The cost of the pretreatment step for ethanol production can be lowered by exploiting the carbohydrate-rich microalgae’s existing enzymatic or anaerobic digestion systems (John et al. 2011).
In addition to using the microalgal biomass to produce bioethanol directly, research has shown that 60% higher ethanol yields can be produced from Chlorococum sp. biomass in which lipids were extracted prior to fermentation compared to the undamaged cells (Harun et al. 2010). John et al. (2011) summarized the algae strains that can be used for bioethanol fermentation after oil extraction. The starch or fermentable biomass in Saccharina latissima, Laminaria hyperborea, Chlamydomonas reinhardtii, and Spirulina fusiformis can present up to 50% of biomass after oil extraction and they are useful as feedstock for producing bioethanol (John et al. 2011). Bioethanol can be produced either before or after lipids extraction and offers an alternative bioenergy end product, and the combination of biodiesel and bioethanol production from the same microalgal biomass can improve their overall value.
Biodiesel Production from Microalgal Lipids
Lipids extraction for biodiesel
Extraction of microalgal oil can be achieved by physical, chemical, or biological procedures as well as a combination of each. The existing methods include mechanical pressing, solvent extraction, solvent extraction in combination with enzymatic hydrolysis, supercritical fluid extraction, microwave-assisted extraction, ultrasonic-assisted extraction, and osmotic shock (Mercer and Armenta 2011). Table 2 shows results from some recent extraction studies and the lipid yields, especially more valuable fatty acids.
Table 2. Common Lipid Extraction Methods and their Efficiency
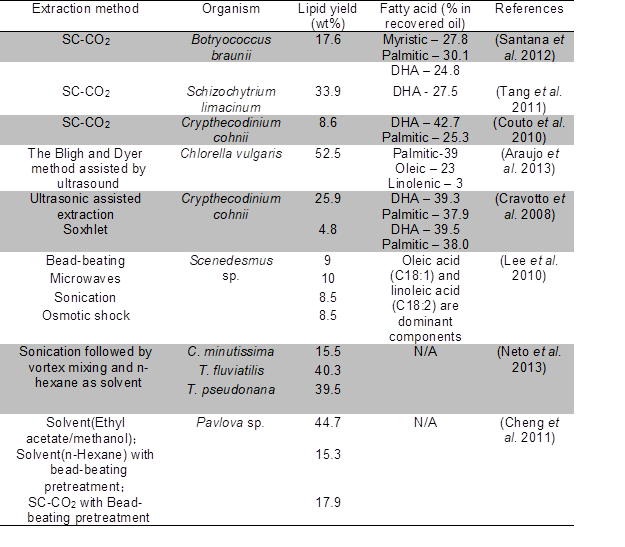
SC-CO2 = supercritical carbon dioxide extraction
As shown in Table 2, the effectiveness in recovering lipids and the lipid compositions vary across different species. The extraction methods also have an impact on lipid yields from algae.
Lipid extraction is a vital downstream step for biodiesel production. Effective lipid extraction procedures that are low energy-intensive and eliminate the negative effects on the environment such as avoiding the usage of large amounts of solvent, have attracted much attention. In most cases, solvent extraction in combination with cell disruption resulted in more effective lipid removal than solvent extraction alone. Moreover, the purity of the extracted oil is important, and other cellular components such as DNA and chlorophyll should be eliminated in case of the contamination. Recently, Goettel et al. (2013) found that prior to lipid extraction, pulse electric field (PEF) treatment for cell wall disruption can enhance the effectiveness of extraction. In a first step, PEF treatment facilitates separation of water-soluble intracellular substances. In a subsequent step, solvent extraction resulted in a higher lipid yield. This selection process might provide the chance to obtain microalgal-based biorefinery by adopting the PEF technology.
Another approach for accessing lipids is to adopt biological methodologies such as using enzymes to decompose the thick cell wall of microalgae. Cellulases have proven to be effective in hydrolyzing the cell wall of microalgae. For example, Fu et al. (2010) used an electrospun polyacrylonitrile (PAN) nanofibrous material to immobilize cellulases. The hydrolyzing conversion rate was 62% during first time use and kept at 40% after reusing it five times. Yin et al. (2010) found the yields of protein, chlorophyll, and peptides were increased after the Chlorella suspensions were treated by cellulases from Cellulomonas sp. YJ5. Cho et al. (2013) also found that compared to no enzymatic hydrolysis process, the lipid extraction yield and the total fatty acid methyl ester (FAME) productivity of Chlorella vulgaris were improved substantially with the treatment of cellulose hydrolysis prior to solvent extraction.
In-situ transesterification
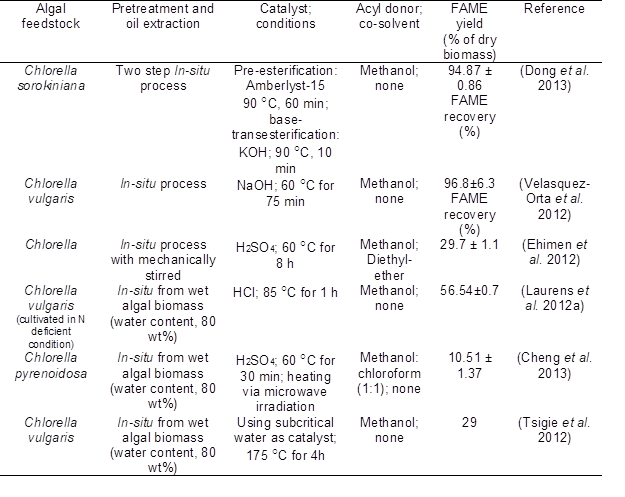
Finally, after extraction, the crude lipids are converted to biodiesel by transesterification. However, this conventional two-step approach to biodiesel production suffers from compounding inefficiencies. Moreover, Laurens et al. (2012b) pointed out that it is hard to standardize the analytical methods that are used for calculation of algal biomass components. Eight researchers at three institutions collected the algal biomass data and found that many factors would affect the measurements of lipid and protein. As such, more simple and accurate approaches to quantify cellular oil content in algae should be developed. To address this, a single-step in-situ transesterification process, particularly for calculation of algal FAMEs, was developed. In-situ transesterification refers to the direct conversion of whole algal biomass into fatty acids using either acid or base catalysts, or other catalysts. No prior lipid extraction procedure is needed (Carrapiso and García 2000). Table 3 contains a compilation of recent results using in-situ transesterification for the production of biodiesel from algae.
Collectively, the results in Table 3 indicate that in-situ transesterification could be simpler and may also achieve high efficiency. Furthermore, gravimetric methods, which calculate crude lipid yield after extraction, have many limitations, which are highly affected by the type of solvent and the components of lipids in algae. For example, some cellular components such as pigments, waxes, proteins, and sterols that cannot be converted to FAME will be co-extracted during the tradition extraction process and thus may lead to overestimates of the lipid yield (Palmquist and Jenkins 2003). Therefore, gravimetric weights can include non-saponifiable lipids, some of which may not be appropriate feedstocks for biodiesel production. Alternatively, in-situ methods provide more reliable and accurate data for the selection of microalgal fuel potential by calculating the FAME yield.
Table 3. In-situ Transesterification for the Production of Biodiesel from Algae
Biogas Production from Whole Algal Cells or Lipid-Extracted Algal Residues through Anaerobic Digestion
Anaerobic digestion (AD) represents another approach for the bioconversion of algal biomass to energy in the form of methane-rich biogas (Bohutskyi and Bouwer 2013). Importantly, the amount of energy produced through AD does not depend strictly on the biomass biochemical composition if compared to biodiesel or bioethanol processes since AD is able to convert most cell fractions into biogas. This advantage makes possible utilization of low-quality algal biomass cultivated in wastewater (Bohutskyi et al. 2015c) or even low-quality algae collected from contaminated lakes and ponds, improving sustainability of coastal ecosystems (Yuan et al. 2011).
In addition, biogas and biodiesel production can be integrated into other bioprocesses in which lipids are extracted first for biodiesel and lipid-extracted algal residues (LEA) utilized through AD, enhancing the energy produced by 30 to 50% (Bohutskyi et al. 2014b). Finally, implementation of AD in the algal conversion process allows recovering some essential nutrients from LEA and their subsequent recycling back for the further cultivation of microalgae. Nearly 50% of the nitrogen, phosphorus, and sulfur, as well as part of trace elements, may be recycled, replacing costly chemical fertilizers and enabling a more sustainable scale-up algal biofuel processing (Bohutskyi et al. 2015a). However, efficient conversion of algal biomass into biogas may require, preliminary pretreatment (e.g. thermal, thermochemical, or enzymatic) because certain algal biochemical constituents including the cell wall may be recalcitrant to biodegradation and limit biogas yields (Bohutskyi et al. 2014a; 2015a). Although biogas production from microalgae still has a number of hurdles, progress in this area in recent years demonstrates its great potential for enhancing the utilization of complete algal biomass for biofuels and bioenergy.
Hydrothermal Liquefaction or Gasification of Microalgal Biomass
Hydrothermal liquefaction is another option to produce bio-oil, along with productions of gaseous, aqueous, and solid by-products (López Barreiro et al. 2013). This HTL-produced bio-oil represents a mix of aromatic hydrocarbons, heterocyclic, phenol, amine, amide, indole, alkane, and nitrile (Chaiwong et al. 2013). Previous research has focused on strain selection in terms of the use of microalgae as a source of whole biomass for hydrothermal conversion. In fact, either the whole microalgal biomass or the microalgal residues after extraction of value-added products including lipids, pigments, polysaccharide or protein, etc., can be utilized for thermochemical conversion (López Barreiro et al. 2013). The combination of value-added constituent extraction and thermochemical conversion may be beneficial to make the process of utilizing microalgae economically viable.
The effect of microalgae biochemical compositions on the bio-oil yield from microalgae is in debate. Biller and Ross (2011) noted that strains with higher lipid content achieved higher conversion efficiency, while other research indicates that high bio-oil yield can also be obtained from microalgae with low lipid content (Ross et al. 2010; Yu et al. 2011). Either way the requirements for raw materials for thermochemical conversion are not so strict compared to biodiesel or bioethanol processes that depend strongly on lipid and carbohydrate algal contents. Overall, the strain that possesses high biomass productivity and can also be used for value-added products may be the best option. For example, Nannochloropsis sp. appears to be a promising raw material for combining biodiesel and bio-oil production. Nannochloropsis sp. has a high lipid content and is a good source of polyunsaturated acid, EPA (Hu and Gao 2006). Research has shown that either bio-oil or gas products can be retrieved from up to 90% of the chemical energy initially existing in the Nannochloropsis sp. (Brown et al. 2010). Furthermore, different heterogeneous catalysts can also affect the efficiency of hydrothermal liquefaction of Nannochloropsis sp. as well (Duan and Savage 2010). Thus, further research on hydrothermal conversion of Nannochloropsis sp. after extraction of value-added contents should be investigated further and it could expand the overall economic balance sheet for algal conversion processes generating oils.
ADDED-VALUE ALGAL PRODUCTS
Polyunsaturated Fatty Acids (PUFAs)
Polyunsaturated fatty acids (PUFAs) play an important role in human health and physiology, and PUFAs have been shown to reduce the risk of cardiovascular disease (Mozaffarian and Wu 2011).
Currently, PUFAs are mainly extracted from fish and fish oils that are likely to become even more prevalent, including cod liver oil (30% EPA + DHA), tuna oils (20 to 24% EPA + DHA), and salmon oils (15 to 20% EPA + DHA). Krill and squid oils may also be used eventually, but in krill, the EPA and DHA are bound to the phospholipids, and thus, further processing is required (Borowitzka 2013). However, the consumption of fish oil has many limitations due to the possible accumulation of toxins, fish odor, and the presence of mixed fatty acids (Pulz and Gross 2004). Furthermore, these oils are not suitable for vegetarian diets.
Microalgae have historically been used to produce long-chain polyunsaturated fatty acids (PUFA) such as γ-linolenic acid, arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (Ratledge 2010). The productivity and yield figures shown in Tables 4 demonstrate that microalgae could potentially replace fish sources to produce EPA and DHA.
Carotenoids
Carotenoids are a class of photosynthetic pigments found in microalgae in addition to chlorophyll. For example, lutein, zeaxanthin, beta-carotene, and astaxanthin represent the major carotenoids observed in plants.
Astaxanthin
Astaxanthin is a keto-carotenoid that possesses strong antioxidant activity. The antioxidant capacity of astaxanthin is observed to be 10-times greater than beta-carotene and 500-times greater than alpha-tocopherol (Dufossé 2007). As a result, astaxanthin can be used for treatment of a number of diseases including cancer, diabetes, cardiovascular, as well as liver, neurodegenerative, and gastrointestinal diseases, etc. (Ambati et al. 2014). It has also been widely used in health supplements, cosmetic products, and in the food industries because of its powerful antioxidant activity and strong pigmentation (Ambati et al. 2014; Guerin et al. 2003).
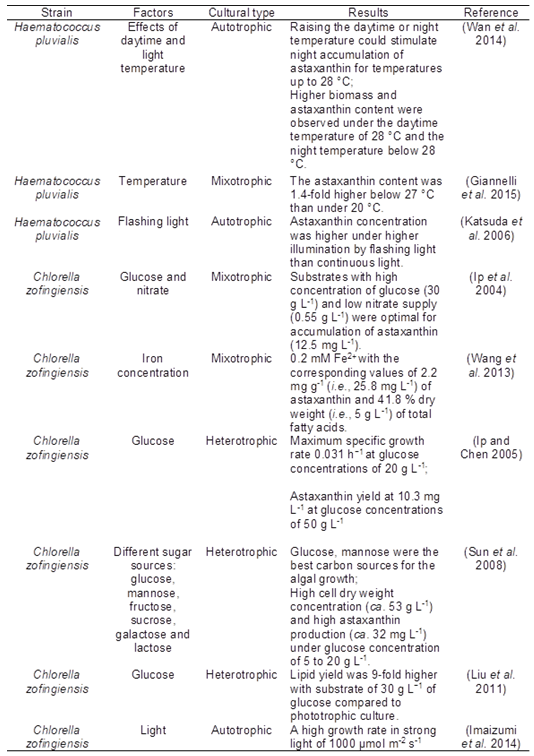
The main natural sources of astaxanthin are microalgae, yeast (Xanthophyllomyces dendrorhous), salmon, trout, Pacific krill (Euphausia pacifica), Antarctic krill (Euphausia superba), shrimp (Pandalus borealis), and crayfish (Ambati et al. 2014; Fassett and Coombes 2011). Haematococcus pluvialis and Chlorella zofingiensis are two prominent strains of microalgae known to produce astaxanthin. Haematococcus pluvialis has been a commercial source of the antioxidant (Cysewski 2004; Rao et al. 2007). The antioxidant activity of H. pluvialis is associated with its carotenoid content, and the antioxidant capacity of astaxanthin di-esters has been observed to be 60% higher than the astaxanthin monoester and twice than free astaxanthin (Cerón et al. 2007). The remarkable accumulation of astaxanthin in Haematococcus pluvialis and Chlorella zofingiensis can often be maximized under stress culture conditions, as shown in Fig. 2 (Ip and Chen 2005; Liu et al. 2014; Sun et al. 2008). Some stress factors that will alter the growth and accumulation of astaxanthin in Haematococcus pluvialis and Chlorella zofingiensis are listed in Table 5.
Table 4. DHA and EPA Production by Microalgae in the Literature
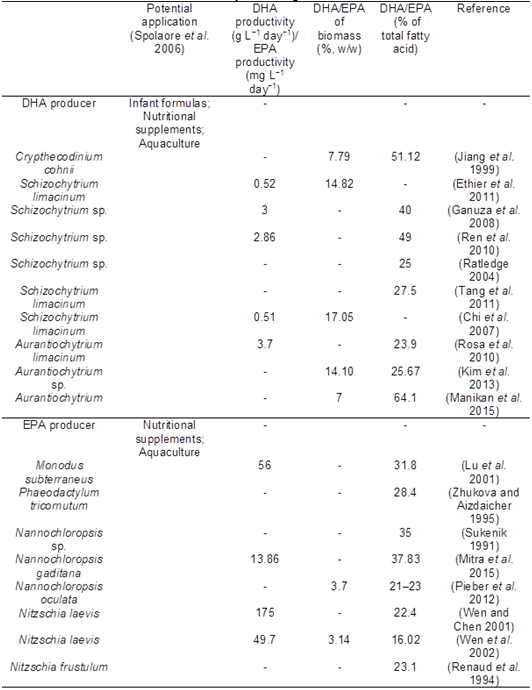
Fig. 2. Natural induction of carotenoid accumulation in Chlorella zofingiensis due to nutrient deprivation in small-scale batch culture. Over the course of weeks to months, a striking change in pigmentation can be observed in liquid culture. This shift from chlorophyll to carotenoid biosynthesis can be stimulated by a variety of stressors including nitrogen deprivation and high light intensity.
Beta-carotene
Like astaxanthin, beta-carotene is widely used in the food, pharmaceutical, and cosmetics industries. The cultivation of the microalgae Dunaliella bardawil for the commercially production of astaxanthin dates back to the 1980s (Borowitzka 2013). Numerous studies have been carried out on the extraction and isolation of beta-carotene from D. salina.
Supercritical fluid extraction (SFE) may be the most widely used technique for beta-carotene extraction. Mendes et al. (2003) found that cis-isomers of synthetic and natural beta-carotene dissolved better than all-trans isomers in supercritical CO2 and resulted in higher yields.
A recent study indicated that efficient extraction of beta-carotene from D. salina yield was achieved through centrifugal partition extraction (CPE). In this case, 65% beta-carotene recovery was achieved using ethyl oleate with 5% dichloromethane as an extraction solvent. The least amount of cells were damaged and the measurement of photosynthetic activity showed that more than 65% of the cells were kept viable. Sustainable growth was achieved by applying this “biocompatible” extraction after dichloromethane evaporation. This approach allows for continuous cultivation of D. salina through application of CPE process coupled with photobioreactor (Marchal et al. 2013).
Table 5. Some Factors Affecting Growth and Astaxanthin Production of Haematococcus pluvialis and Chlorella
Phycobilins
Phycobilins are water-soluble pigments that, once purified, can be used as cosmetics, colorants in food, and as fluorescent labeling reagents in different analytical techniques. Phycobilins consist of four pyrrole rings connected by a single carbon bridge. Water-solubale phycobiliprotein is formed when phycobilins are connected to polypeptides. According to the absorption spectra, phycobilins can be classified into three types: phycoerythrins (PEs) or phycoerythrocyanins (PECs) (480 to 580 nm); phycocyanins (PCs), (600 to 640 nm); and allophycocyanins (APCs, 620 to 660 nm) (Yen et al. 2013).
Spirulina platensis is an excellent source of phycobiliprotein [particularly, allophycocyanin (APC) and c-phycocyanin (CPC)] (Eriksen 2008; Patil et al. 2008). Previous research reported that C-phycocyanin and allophycocyanin can be separated and purified simultaneously by aqueous two-phase extraction (ATPE). Moreover, a superior separation of phase forming components from the products along with an increase in APC purity was achieved through integration of membrane processing in combination with ATPE (Patil et al. 2008).
High-value Polysaccharides
Polysaccharides represent yet another category of high-value products available from microalgae. They have been shown to exhibit and number of health benefits and interesting structural characteristics (Arad and Levy-Ontman 2010). Sulphated exopolysaccharides in particular, which can be released into the medium, have been utilized in health care and food (Raposo et al. 2013). They also display remarkable pharmacological activities and can be used as antioxidant and anti-inflammatory agents (Chen et al. 2010; Matsui et al. 2003). The algal strains that are good sources of sulphated exopolysaccharides include Cylindrotheca closterium (Staats et al. 1999), Chlorella stigmatophora (Guzmán et al. 2003), Cochlodinium polykrikoides (Hasui et al. 1995), Gyrodinium impudicum (Yim et al. 2007), and Isochrysis galbana (Sun et al. 2014b), among others. Arad and Levy-Ontman (2010) found that the cell-wall sulfated polysaccharides in red algae also possess unique structures, composition, fluid dynamics, and high stability. When combined with their useful bioactivities, these structural properties can be useful for various potential biotechnology applications.
CONCLUSIONS
The biochemical composition of microalgae offers a wide diversity of products with a range of biotechnology applications. The application of microalgal carbohydrates for bioethanol production can be an environmentally friendly approach relative to many other options. Biodiesel production from microalgal biomass with high lipid content is even more promising due to an even higher energy density of lipids. Moreover, the carbohydrate and lipid contents may be enhanced in the future through directed engineering efforts. In terms of lipid quantification, in-situ methods may prove to be simpler and more accurate compared to conventional extraction and transesterification methods, thereby aiding in the selection and screening of algal species for desirable biofuel properties. A combination of lipids extraction and the utilization of microalgal polysaccharides may offer a more feasible pathway due to more complete utilization of biomass. However, breakthrough technological innovations are needed to make algal biofuels economically efficient. Further research is required on selection of strains rich in fuel precursors and on genetic engineering methods for expression of enzymes responsible for their biosynthesis. Screening for robust and biodegradable algal strains that can be cultured in wastewater for anaerobic digestion to generate biogas has potential applications. Also a deeper understanding of the effect of the cell composition on the hydrothermal liquefaction or gasification process yields will be useful. All in all, much has been achieved but more research needed to be undertaken in order to make cellulosic microalgal biomass as a successful large-scale and commercialized product.
Microalgae also have a long history and even greater potential as sources of fatty acids, especially the long-chain polyunsaturated fatty acids, such as γ-linolenic acid, arachidonic acid, EPA, and DHA, which may serve as viable sources of precious PUFAs to replace fishmeal. Other valuable biochemical constituents of microalgae offer nutraceutical and pharmaceutical applications including astaxanthin and beta-carotene as antioxidants and food, phycobilins as cosmetics and colorants, and bioactive polysaccharides as anti-inflammatory drugs. In light of these advantageous traits, more in depth and sustained investigations of microalgal biorefinery processes are warranted. Moreover, using microalgae as a feedstock for biofuel in combination with its application as a value-added product will make processes commercially viable sooner and help to realize the great opportunities present in microalgae.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the Natural Science Foundation of Jiangsu Universities (11KJA480001), the national Natural Science Foundation of China (31170537) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Partial support was also provided by grant number NSF-EFRI-1332344 from the National Science Foundation (MJB), DOE DE SC0012658 grant (MJB) and a fellowship to JNR from the Johns Hopkins Environment, Energy, Sustainability & Health Institute (E2SHI).
REFERENCES CITED
Abinandan, S., and Shanthakumar, S. (2015). “Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review,” Renewable and Sustainable Energy Reviews 52, 123-132. DOI:10.1016/j.rser.2015.07.086
Abreu, A. P., Fernandes, B., Vicente, A. A., Teixeira, J., and Dragone, G. (2012). “Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source,” Bioresource Technology 118, 61-66. DOI:10.1016/j.biortech.2012.05.055
Ahmad, A. L., Yasin, N. H. M., Derek, C. J. C., and Lim, J. K. (2011). “Microalgae as a sustainable energy source for biodiesel production: A review,” Renewable and Sustainable Energy Reviews 15(1), 584-593. DOI:10.1016/j.rser.2010.09.018
Aikawa, S., Ho, S.-H., Nakanishi, A., Chang, J.-S., Hasunuma, T., and Kondo, A. (2015). “Improving polyglucan production in cyanobacteria and microalgae via cultivation design and metabolic engineering,” Biotechnology journal 10(6), 886-898. DOI:10.1002/biot.201400344
Alcantara, R., Amores, J., Canoira, L., Fidalgo, E., Franco, M. J., and Navarro, A. (2000). “Catalytic production of biodiesel from soy-bean oil, used frying oil and tallow,” Biomass and Bioenergy 18(6), 515-527. DOI:10.1016/S0961-9534(00)00014-3
Ambati, R. R., Siew Moi, P., Ravi, S., and Aswathanarayana, R. G. (2014). “Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review,” Marine Drugs 12(1), 128-152. DOI:10.3390/md12010128
Arad, S., and Levy-Ontman, O. (2010). “Red microalgal cell-wall polysaccharides: Biotechnological aspects,” Current Opinion in Biotechnology 21(3), 358-364. DOI:10.1016/j.copbio.2010.02.008
Araujo, G. S., Matos, L. J. B. L., Fernandes, J. O., Cartaxo, S. J. M., Gonçalves, L. R. B., Fernandes, F. A. N., and Farias, W. R. L. (2013). “Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method,” Ultrasonics Sonochemistry 20(1), 95-98. DOI:10.1016/j.ultsonch.2012.07.027
Balcerek, M., Pielech-Przybylska, K., and Patelski, P. (2011). “Selection of yeast strains for alcoholic fermentation of sugar beet thick juice and green syrup,” Biomass and Bioenergy 35(12), 4841-4848. DOI:10.1016/j.biombioe.2011.09.024
Ball, S. G., Dirick, L., Decq, A., Martiat, J.-C., and Matagne, R. (1990). “Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii,” Plant Science 66(1), 1-9. DOI:10.1016/0168-9452(90)90162-H
Bansal, A., Illukpitiya, P., Singh, S. P., and Tegegne, F. (2013). “Economic competitiveness of ethanol production from cellulosic feedstock in Tennessee,” Renewable Energy 59(0), 53-57. DOI:10.1016/j.renene.2013.03.017
Bao, X., and Ohlrogge, J. (1999). “Supply of fatty acid is one limiting factor in the accumulation of triacylglycerol in developing embryos,” Plant Physiology 120(4), 1057-1062. DOI:10.1104/pp.120.4.1057
Behrens, P. W., Bingham, S. E., Hoeksema, S. D., Cohoon, D. L., and Cox, J. C. (1989). “Studies on the incorporation of CO2 into starch by Chlorella vulgaris,” Journal of Applied Phycology 1(2), 123-130. DOI:10.1007/bf00003874
Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). “Triacylglycerols are highly concentrated energy stores,” in: Biochemistry, (ed.), W. H. Freeman, New York, 1050
Bhatnagar, A., Chinnasamy, S., Singh, M., and Das, K. C. (2011). “Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters,” Applied Energy 88(10), 3425-3431. DOI:10.1016/j.apenergy.2010.12.064
Biller, P., and Ross, A. B. (2011). “Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content,” Bioresource Technology 102(1), 215-25. DOI:10.1016/j.biortech.2010.06.028
Bohutskyi, P., Betenbaugh, M. J., and Bouwer, E. J. (2014a). “The effects of alternative pretreatment strategies on anaerobic digestion and methane production from different algal strains,” Bioresource Technology 155, 366-372. DOI:10.1016/j.biortech.2013.12.095
Bohutskyi, P., and Bouwer, E. (2013). “Biogas production from algae and cyanobacteria through anaerobic digestion: A review, analysis, and research needs,.” in: Advanced Biofuels and Bioproducts, Lee, J. W. (ed.), Springer New York, 873-975. DOI:10.1007/978-1-4614-3348-4_36
Bohutskyi, P., Chow, S., Ketter, B., Betenbaugh, M. J., and Bouwer, E. J. (2015a). “Prospects for methane production and nutrient recycling from lipid extracted residues and whole Nannochloropsis salina using anaerobic digestion,” Applied Energy 154, 718-731. DOI:10.1016/j.apenergy.2015.05.069
Bohutskyi, P., Ketter, B., Chow, S., Adams, K. J., Betenbaugh, M. J., Allnutt, F. C. T., and Bouwer, E. J. (2015b). “Anaerobic digestion of lipid-extracted Auxenochlorella protothecoides biomass for methane generation and nutrient recovery,” Bioresource Technology 183, 229-239. DOI:10.1016/j.biortech.2015.02.012
Bohutskyi, P., Kula, T., Kessler, B., Hong, Y., Bouwer, E., Betenbaugh, M., and Allnutt, F. C. T. (2014b). “Mixed trophic state production process for microalgal biomass with high lipid content for generating biodiesel and biogas,” BioEnergy Research 7(4), 1174-1185. DOI:10.1007/s12155-014-9453-5
Bohutskyi, P., Liu, K., Nasr, L. K., Byers, N., Rosenberg, J. N., Oyler, G. A., Betenbaugh, M. J., and Bouwer, E. J. (2015c). “Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate,” Applied Microbiology and Biotechnology 99(14), 6139-6154. DOI:10.1007/s00253-015-6603-4
Borowitzka, M. (2013). “High-value products from microalgae—their development and commercialisation,” Journal of Applied Phycology 25(3), 743-756. DOI:10.1007/s10811-013-9983-9
Borowitzka, M. A. (2010). “Carotenoid production using microorganisms,” in: Single Cell Oils: Microbial and Algal Oils, Cohen, Z., and Ratledge, C. (eds.), AOCS Press, Urbana, 225
Brányiková, I., Maršálková, B., Doucha, J., Brányik, T., Bišová, K., Zachleder, V., and Vítová, M. (2011). “Microalgae-novel highly efficient starch producers,” Biotechnology and Bioengineering 108(4), 766-776. DOI:10.1002/bit.23016
Brown, T. M., Duan, P., and Savage, P. E. (2010). “Hydrothermal liquefaction and gasification of Nannochloropsis sp,,” Energy & Fuels 24(6), 3639-3646. DOI:10.1021/ef100203u
Buchanan, B. B. (1984). “The ferredoxin/thioredoxin system: A key element in the regulatory function of light in photosynthesis,” BioScience 34(6), 378-383. DOI:10.2307/1309730
Buratti, C., Barbanera, M., and Fantozzi, F. (2012). “A comparison of the European renewable energy directive default emission values with actual values from operating biodiesel facilities for sunflower, rape and soya oil seeds in Italy,” Biomass and Bioenergy 47(0), 26-36. DOI:10.1016/j.biombioe.2012.10.008
Canakci, M. (2007). “The potential of restaurant waste lipids as biodiesel feedstocks,” Bioresource Technology 98(1), 183-190. DOI:10.1016/j.biortech.2005.11.022
Carrapiso, A., and García, C. (2000). “Development in lipid analysis: Some new extraction techniques and in situ transesterification,” Lipids 35(11), 1167-1177. DOI:10.1007/s11745-000-0633-8
Carvalho, A. P., Monteiro, C. M., and Malcata, F. X. (2009). “Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavlova lutheri,” Journal of applied phycology 21(5), 543-552. DOI: 10.1007/s10811-009-9415-z
Cerón, M. C., García-Malea, M. C., Rivas, J., Acien, F. G., Fernandez, J. M., Del Río, E., Guerrero, M. G., and Molina, E. (2007). “Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content,” Applied Microbiology and Biotechnology 74(5), 1112-1119. DOI:10.1007/s00253-006-0743-5
Chaiwong, K., Kiatsiriroat, T., Vorayos, N., and Thararax, C. (2013). “Study of bio-oil and bio-char production from algae by slow pyrolysis,” Biomass and Bioenergy 56, 600-606. DOI:10.1016/j.biombioe.2013.05.035
Chen, B., You, W., Huang, J., Yu, Y., and Chen, W. (2010). “Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata,” World Journal of Microbiology & Biotechnology 26(5), 833-840. DOI:10.1007/s11274-009-0240-y
Cheng, C.-H., Du, T.-B., Pi, H.-C., Jang, S.-M., Lin, Y.-H., and Lee, H.-T. (2011). “Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2,” Bioresource Technology 102(21), 10151-10153. DOI:10.1016/j.biortech.2011.08.064
Cheng, J., Yu, T., Li, T., Zhou, J., and Cen, K. (2013). “Using wet microalgae for direct biodiesel production via microwave irradiation,” Bioresource Technology 131(0), 531-535. DOI:10.1016/j.biortech.2013.01.045
Cheng, Y. S., Labavitch, J. M., and VanderGheynst, J. S. (2015). “Elevated CO2 concentration impacts cell wall polysaccharide composition of green microalgae of the genus Chlorella,” Letters in Applied Microbiology 60(1), 1-7. DOI:10.1111/lam.12320
Chi, Z., Pyle, D., Wen, Z., Frear, C., and Chen, S. (2007). “A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation,” Process Biochemistry 42(11), 1537-1545. DOI:10.1016/j.procbio.2007.08.008
Cho, H.-S., Oh, Y.-K., Park, S.-C., Lee, J.-W., and Park, J.-Y. (2013). “Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris,” Renewable Energy 54(0), 156-160. DOI:10.1016/j.renene.2012.08.031
Couto, R. M., Simões, P. C., Reis, A., Da Silva, T. L., Martins, V. H., and Sánchez-Vicente, Y. (2010). “Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii,” Engineering in Life Sciences 10(2), 158-164. DOI:10.1002/elsc.200900074
Cravotto, G., Boffa, L., Mantegna, S., Perego, P., Avogadro, M., and Cintas, P. (2008). “Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves,” Ultrasonics Sonochemistry 15(5), 898-902. DOI:10.1016/j.ultsonch.2007.10.009
Cysewski, G. R., Lorenz, R. T. (2004). “Industrial production of microalgal cell-mass and secondary products—species of high potential: Haematococcus,” in: Microalgal Culture: Biotechnology and Applied Phycology, Richmond, A. (ed.), Blackwell Science, Oxford, 281
De Philippis, R., Sili, C., and Vincenzini, M. (1992). “Glycogen and poly-b-hydroxybutyrate synthesis in Spirulina maxima,” Journal of General Microbiology 138(8), 1623-1628. DOI:10.1099/00221287-138-8-1623
Dong, T., Wang, J., Miao, C., Zheng, Y., and Chen, S. (2013). “Two-step in situ biodiesel production from microalgae with high free fatty acid content,” Bioresource Technology 136(0), 8-15. DOI:10.1016/j.biortech.2013.02.105
Dragone, G., Fernandes, B. D., Abreu, A. P., Vicente, A. A., and Teixeira, J. A. (2011). “Nutrient limitation as a strategy for increasing starch accumulation in microalgae,” Applied Energy 88(10), 3331-3335. DOI:10.1016/j.apenergy.2011.03.012
Driouich, A., Follet-Gueye, M.-L., Bernard, S., Kousar, S., Chevalier, L., Vicré-Gibouin, M., and Lerouxel, O. (2012). “Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants,” Frontiers in Plant Science 3. DOI:10.3389/fpls.2012.00079
Duan, P., and Savage, P. E. (2010). “Hydrothermal liquefaction of a microalga with heterogeneous catalysts,” Industrial & Engineering Chemistry Research 50(1), 52-61. DOI:10.1021/ie100758s
Dufossé, L. (2007). “Pigments from microalgae and microorganisms: Sources of food colorants,” in: Food Colorants: Chemical and Functional Properties, Socaciu, C. (ed.), CRC Press, 399. DOI:10.1201/9781420009286.sec5c
Dunahay, T., Jarvis, E., Dais, S., and Roessler, P. (1996). “Manipulation of microalgal lipid production using genetic engineering,” in: Seventeenth Symposium on Biotechnology for Fuels and Chemicals, Wyman, C. and Davison, B. (ed.), Humana Press, 223-231. DOI:10.1007/978-1-4612-0223-3_20
Ehimen, E. A., Sun, Z., and Carrington, G. C. (2012). “Use of ultrasound and co-solvents to improve the in-situ transesterification of microalgae biomass,” Procedia Environmental Sciences 15, 47-55. DOI:10.1016/j.proenv.2012.05.009
Eriksen, N. (2008). “Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine,” Applied Microbiology and Biotechnology 80(1), 1-14. DOI:10.1007/s00253-008-1542-y
Ethier, S., Woisard, K., Vaughan, D., and Wen, Z. (2011). “Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid,” Bioresource Technology 102(1), 88-93. DOI:10.1016/j.biortech.2010.05.021
FAO. (2008). “The state of food and agriculture 2008,” New York: Food and Agriculture Organization.
Farooq, W., Lee, Y.-C., Ryu, B.-G., Kim, B.-H., Kim, H.-S., Choi, Y.-E., and Yang, J.-W. (2013). “Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity,” Bioresource Technology 132, 230-238. DOI:10.1016/j.biortech.2013.01.034
Fassett, R. G., and Coombes, J. S. (2011). “Astaxanthin: A potential therapeutic agent in cardiovascular disease,” Marine Drugs 9(3), 447-465. DOI:10.3390/md9030447
Friedman, O., Dubinsky, Z., and Arad, S. (1991). “Effect of light intensity on growth and polysaccharide production in red and blue-green rhodophyta unicells,” Bioresource Technology 38(2-3), 105-110. DOI:10.1016/0960-8524(91)90139-b
Fu, C.-C., Hung, T.-C., Chen, J.-Y., Su, C.-H., and Wu, W.-T. (2010). “Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction,” Bioresource Technology 101(22), 8750-8754. DOI:10.1016/j.biortech.2010.06.100
Ganuza, E., Anderson, A. J., and Ratledge, C. (2008). “High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system,” Biotechnology Letters 30(9), 1559-1564. DOI:10.1007/s10529-008-9723-4
Giannelli, L., Yamada, H., Katsuda, T., and Yamaji, H. (2015). “Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis,” Journal of Bioscience and Bioengineering 119(3), 345-350. DOI:10.1016/j.jbiosc.2014.09.002
Gigova, L., Ivanova, N., Gacheva, G., Andreeva, R., and Furnadzhieva, S. (2012). “Response of Trachydiscus minutus (Xanthophyceae) to temperature and light,” Journal of Phycology 48(1), 85-93. DOI:10.1111/j.1529-8817.2011.01088.x
Goettel, M., Eing, C., Gusbeth, C., Straessner, R., and Frey, W. (2013). “Pulsed electric field assisted extraction of intracellular valuables from microalgae,” Algal Research 2(4), 401-408. DOI:10.1016/j.algal.2013.07.004
Gonzalez-Fernandez, C., and Ballesteros, M. (2012). “Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation,” Biotechnology Advances 30(6), 1655-1661. DOI:10.1016/j.biotechadv.2012.07.003
Guerin, M., Huntley, M. E., and Olaizola, M. (2003). “Haematococcus astaxanthin: applications for human health and nutrition,” Trends in Biotechnology 21(5), 210-216. DOI:10.1016/S0167-7799(03)00078-7
Guo, X., Su, G., Li, Z., Chang, J., Zeng, X., Sun, Y., Lu, Y., and Lin, L. (2015). “Light intensity and N/P nutrient affect the accumulation of lipid and unsaturated fatty acids by Chlorella sp.,” Bioresource Technology 191, 385-390. DOI:10.1016/j.biortech.2015.04.014
Guzmán, S., Gato, A., Lamela, M., Freire-Garabal, M., and Calleja, J. M. (2003). “Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum,” Phytotherapy Research 17(6), 665-670. DOI:10.1002/ptr.1227
Hailegiorgis, S. M., Mahadzir, S., and Subbarao, D. (2013). “Parametric study and optimization of in situ transesterification of Jatropha curcas L assisted by benzyltrimethylammonium hydroxide as a phase transfer catalyst via response surface methodology,” Biomass and Bioenergy 49(0), 63-73. DOI:10.1016/j.biombioe.2012.12.003
Harun, R., and Danquah, M. K. (2011). “Influence of acid pre-treatment on microalgal biomass for bioethanol production,” Process Biochemistry 46(1), 304-309. DOI:10.1016/j.procbio.2010.08.027
Harun, R., Danquah, M. K., and Forde, G. M. (2010). “Microalgal biomass as a fermentation feedstock for bioethanol production,” Journal of Chemical Technology & Biotechnology 85(2), 199-203. DOI:10.1002/jctb.2287
Harun, R., Jason, W. S. Y., Cherrington, T., and Danquah, M. K. (2011). “Exploring alkaline pre-treatment of microalgal biomass for bioethanol production,” Applied Energy 88(10), 3464-3467. DOI:10.1016/j.apenergy.2010.10.048
Hasui, M., Matsuda, M., Okutani, K., and Shigeta, S. (1995). “In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses,” International Journal of Biological Macromolecules 17(5), 293-297. DOI:10.1016/0141-8130(95)98157-T
Heredia-Arroyo, T., Wei, W., Ruan, R., and Hu, B. (2011). “Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials,” Biomass and Bioenergy 35(5), 2245-2253. DOI:10.1016/j.biombioe.2011.02.036
Ho, S.-H., Chen, C.-Y., and Chang, J.-S. (2012). “Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N,” Bioresource Technology 113, 244-252. DOI:10.1016/j.biortech.2011.11.133
Ho, S.-H., Huang, S.-W., Chen, C.-Y., Hasunuma, T., Kondo, A., and Chang, J.-S. (2013). “Bioethanol production using carbohydrate-rich microalgae biomass as feedstock,” Bioresource Technology 135(0), 191-198. DOI:10.1016/j.biortech.2012.10.015
Hodaifa, G., Martínez, M. E., and Sánchez, S. (2009). “Daily doses of light in relation to the growth of Scenedesmus obliquus in diluted three-phase olive mill wastewater,” Journal of Chemical Technology & Biotechnology 84(10), 1550-1558. DOI:10.1002/jctb.2219
Hongyang, S., Yalei, Z., Chunmin, Z., Xuefei, Z., and Jinpeng, L. (2011). “Cultivation of Chlorella pyrenoidosa in soybean processing wastewater,” Bioresource Technology 102(21), 9884-9890. DOI:10.1016/j.biortech.2011.08.016
Hu, H., and Gao, K. (2006). “Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration,” Biotechnology Letters 28(13), 987-992. DOI:10.1007/s10529-006-9026-6
Imaizumi, Y., Nagao, N., Yusoff, F. M., Taguchi, S., and Toda, T. (2014). “Estimation of optimum specific light intensity per cell on a high-cell-density continuous culture of Chlorella zofingiensis not limited by nutrients or CO2,” Bioresource Technology 162, 53-59. DOI:10.1016/j.biortech.2014.03.123
Ip, P.-F., and Chen, F. (2005). “Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark,” Process Biochemistry 40(2), 733-738. DOI:10.1016/j.procbio.2004.01.039
Ip, P.-F., Wong, K.-H., and Chen, F. (2004). “Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture,” Process Biochemistry 39(11), 1761-1766. DOI:10.1016/j.procbio.2003.08.003
Iriarte, A., Rieradevall, J., and Gabarrell, X. (2011). “Environmental impacts and energy demand of rapeseed as an energy crop in Chile under different fertilization and tillage practices,” Biomass and Bioenergy 35(10), 4305-4315. DOI:10.1016/j.biombioe.2011.07.022
Izumo, A., Fujiwara, S., Oyama, Y., Satoh, A., Fujita, N., Nakamura, Y., and Tsuzuki, M. (2007). “Physicochemical properties of starch in Chlorella change depending on the CO2 concentration during growth: Comparison of structure and properties of pyrenoid and stroma starch,” Plant Science 172(6), 1138-1147. DOI:10.1016/j.plantsci.2007.03.001
Ji, M.-K., Yun, H.-S., Park, Y.-T., Kabra, A. N., Oh, I.-H., and Choi, J. (2015). “Mixotrophic cultivation of a microalga Scenedesmus obliquus in municipal wastewater supplemented with food wastewater and flue gas CO2 for biomass production,” Journal of Environmental Management 159, 115-120. DOI:10.1016/j.jenvman.2015.05.037
Jiang, Y., Chen, F., and Liang, S.-Z. (1999). “Production potential of docosahexaenoic acid by the heterotrophic marine dinoflagellate Crypthecodinium cohnii,” Process Biochemistry 34(6–7), 633-637. DOI:10.1016/S0032-9592(98)00134-4
John, R. P., Anisha, G. S., Nampoothiri, K. M., and Pandey, A. (2011). “Micro and macroalgal biomass: A renewable source for bioethanol,” Bioresource Technology 102(1), 186-193. DOI:10.1016/j.biortech.2010.06.139
Kansedo, J., Lee, K. T., and Bhatia, S. (2009). “Biodiesel production from palm oil via heterogeneous transesterification,” Biomass and Bioenergy 33(2), 271-276. DOI:10.1016/j.biombioe.2008.05.011
Katsuda, T., Shimahara, K., Shiraishi, H., Yamagami, K., Ranjbar, R., and Katoh, S. (2006). “Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis,” Journal of Bioscience and Bioengineering 102(5), 442-446. DOI:10.1263/jbb.102.442
Khalil, Z. I., Asker, M. M. S., El-Sayed, S., and Kobbia, I. A. (2009). “Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea,” World Journal of Microbiology and Biotechnology 26(7), 1225-1231. DOI:10.1007/s11274-009-0292-z
Kim, C. W., Sung, M.-G., Nam, K., Moon, M., Kwon, J.-H., and Yang, J.-W. (2014). “Effect of monochromatic illumination on lipid accumulation of Nannochloropsis gaditana under continuous cultivation,” Bioresource Technology 159, 30-35. DOI:10.1016/j.biortech.2014.02.024
Kim, K., Jung Kim, E., Ryu, B.-G., Park, S., Choi, Y.-E., and Yang, J.-W. (2013). “A novel fed-batch process based on the biology of Aurantiochytrium sp. KRS101 for the production of biodiesel and docosahexaenoic acid,” Bioresource Technology 135, 269-274. DOI:10.1016/j.biortech.2012.10.139
Klein, U. (1987). “Intracellular carbon partitioning in Chlamydomonas reinhardtii,” Plant Physiology 85(4), 892-7. DOI:10.1104/pp.85.4.892
Klok, A. J., Lamers, P. P., Martens, D. E., Draaisma, R. B., and Wijffels, R. H. (2014). “Edible oils from microalgae: insights in TAG accumulation,” Trends in Biotechnology 32(10), 521-528. DOI:10.1016/j.tibtech.2014.07.004
Klok, A. J., Martens, D. E., Wijffels, R. H., and Lamers, P. P. (2013). “Simultaneous growth and neutral lipid accumulation in microalgae,” Bioresource Technology 134(0), 233-243. DOI:10.1016/j.biortech.2013.02.006
Kuhlman, T., Diogo, V., and Koomen, E. (2013). “Exploring the potential of reed as a bioenergy crop in the Netherlands,” Biomass and Bioenergy 55, 41-52. DOI:10.1016/j.biombioe.2012.06.024
Kurpan Nogueira, D. P., Silva, A. F., Araújo, O. Q. F., and Chaloub, R. M. (2015). “Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae,” Biomass and Bioenergy 72, 280-287. DOI:10.1016/j.biombioe.2014.10.017
Laurens, L. L., Quinn, M., Van Wychen, S., Templeton, D., and Wolfrum, E. (2012a). “Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification,” Analytical and Bioanalytical Chemistry 403(1), 167-178. DOI:10.1007/s00216-012-5814-0
Laurens, L. M. L., Dempster, T. A., Jones, H. D. T., Wolfrum, E. J., Van Wychen, S., McAllister, J. S. P., Rencenberger, M., Parchert, K. J., and Gloe, L. M. (2012b). “Algal biomass constituent analysis: Method uncertainties and investigation of the underlying measuring chemistries,” Analytical Chemistry 84(4), 1879-1887. DOI:10.1021/ac202668c
Leboreiro, J., and Hilaly, A. K. (2013). “Analysis of supply chain, scale factor, and optimum plant capacity for the production of ethanol from corn stover,” Biomass and Bioenergy 54(0), 158-169. DOI:10.1016/j.biombioe.2013.03.021
Lee, J.-Y., Yoo, C., Jun, S.-Y., Ahn, C.-Y., and Oh, H.-M. (2010). “Comparison of several methods for effective lipid extraction from microalgae,” Bioresource Technology 101(1, Supplement), S75-S77. DOI:10.1016/j.biortech.2009.03.058
Lerouxel, O., Cavalier, D. M., Liepman, A. H., and Keegstra, K. (2006). “Biosynthesis of plant cell wall polysaccharides — A complex process,” Current Opinion in Plant Biology 9(6), 621-630. DOI:10.1016/j.pbi.2006.09.009
Leverenz, J., W., Falk, S., Pilström, C.-M., and Samuelsson, G. (1990). “The effects of photoinhibition on the photosynthetic light-response curve of green plant cells (Chlamydomonas reinhardtii),” Planta 182(2), 161-168. DOI:10.1007/bf00197105
Lewandowski, I., Scurlock, J. M. O., Lindvall, E., and Christou, M. (2003). “The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe,” Biomass and Bioenergy 25(4), 335-361. DOI:10.1016/S0961-9534(03)00030-8
Li, T., Gargouri, M., Feng, J., Park, J.-J., Gao, D., Miao, C., Dong, T., Gang, D. R., and Chen, S. (2015a). “Regulation of starch and lipid accumulation in a microalga Chlorella sorokiniana,” Bioresource Technology 180, 250-257. DOI:10.1016/j.biortech.2015.01.005
Li, X., Přibyl, P., Bišová, K., Kawano, S., Cepák, V., Zachleder, V., Čížková, M., Brányiková, I., and Vítová, M. (2013). “The microalga Parachlorella kessleri – A novel highly efficient lipid producer,” Biotechnology and Bioengineering 110(1), 97-107. DOI:10.1002/bit.24595
Li, Y., Fei, X., and Deng, X. (2012). “Novel molecular insights into nitrogen starvation-induced triacylglycerols accumulation revealed by differential gene expression analysis in green algae Micractinium pusillum,” Biomass and Bioenergy 42, 199-211. DOI:10.1016/j.biombioe.2012.03.010
Li, Y., Han, D., Hu, G., Sommerfeld, M., and Hu, Q. (2010). “Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii,” Biotechnology and Bioengineering 107(2), 258-268. DOI:10.1002/bit.22807
Li, Y., Xu, H., Han, F., Mu, J., Chen, D., Feng, B., and Zeng, H. (2015b). “Regulation of lipid metabolism in the green microalga Chlorella protothecoides by heterotrophy–photoinduction cultivation regime,” Bioresource Technology 192, 781-791. DOI:10.1016/j.biortech.2014.07.028
Lin, I. P., Jiang, P.-L., Chen, C.-S., and Tzen, J. T. C. (2012). “A unique caleosin serving as the major integral protein in oil bodies isolated from Chlorella sp. cells cultured with limited nitrogen,” Plant Physiology and Biochemistry 61, 80-87. DOI:10.1016/j.plaphy.2012.09.008
Liu, J., Huang, J., Sun, Z., Zhong, Y., Jiang, Y., and Chen, F. (2011). “Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production,” Bioresource Technology 102(1), 106-110. DOI:10.1016/j.biortech.2010.06.017
Liu, J., Sun, Z., Gerken, H., Liu, Z., Jiang, Y., and Chen, F. (2014). “Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential,” Marine Drugs 12(6), 3487-3515. DOI:10.3390/md12063487
Liu, Z., Liu, C., Hou, Y., Chen, S., Xiao, D., Zhang, J., and Chen, F. (2013). “Isolation and characterization of a marine microalga for biofuel production with astaxanthin as a co-product,” Energies 6(6), 2759-2772. DOI:10.3390/en6062759
López Barreiro, D., Prins, W., Ronsse, F., and Brilman, W. (2013). “Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects,” Biomass and Bioenergy 53, 113-127. DOI:10.1016/j.biombioe.2012.12.029
Lu, C., Rao, K., Hall, D., and Vonshak, A. (2001). “Production of eicosapentaenoic acid (EPA) in Monodus subterraneus grown in a helical tubular photobioreactor as affected by cell density and light intensity,” Journal of Applied Phycology 13(6), 517-522. DOI:10.1023/A:1012515500651
Manikan, V., Nazir, M. Y. M., Kalil, M. S., Isa, M. H. M., Kader, A. J. A., Yusoff, W. M. W., and Hamid, A. A. (2015). “A new strain of docosahexaenoic acid producing microalga from Malaysian coastal waters,” Algal Research 9(0), 40-47. DOI:10.1016/j.algal.2015.02.023
Marchal, L., Mojaat-Guemir, M., Foucault, A., and Pruvost, J. (2013). “Centrifugal partition extraction of b-carotene from Dunaliella salina for efficient and biocompatible recovery of metabolites,” Bioresource Technology 134(0), 396-400. DOI:10.1016/j.biortech.2013.02.019
Matsui, M., Muizzuddin, N., Arad, S., and Marenus, K. (2003). “Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo,” Applied Biochemistry and Biotechnology 104(1), 13-22. DOI:10.1385/ABAB:104:1:13
Mendes, A., Reis, A., Vasconcelos, R., Guerra, P., and Lopes da Silva, T. (2009). “Crypthecodinium cohnii with emphasis on DHA production: A review,” Journal of Applied Phycology 21(2), 199-214. DOI:10.1007/s10811-008-9351-3
Mendes, R. L., Nobre, B. P., Cardoso, M. T., Pereira, A. P., and Palavra, A. F. (2003). “Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae,” Inorganica Chimica Acta 356, 328-334. DOI:10.1016/S0020-1693(03)00363-3
Mercer, P., and Armenta, R. E. (2011). “Developments in oil extraction from microalgae,” European Journal of Lipid Science and Technology 113(5), 539-547. DOI:10.1002/ejlt.201000455
Michalska, J., Zauber, H., Buchanan, B. B., Cejudo, F. J., and Geigenberger, P. (2009). “NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts,” Proceedings of the National Academy of Sciences 106(24), 9908-9913. DOI:10.1073/pnas.0903559106
Mitra, D., van Leeuwen, J., and Lamsal, B. (2012). “Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products,” Algal Research 1(1), 40-48. DOI:10.1016/j.algal.2012.03.002
Mitra, M., Patidar, S. K., George, B., Shah, F., and Mishra, S. (2015). “A euryhaline Nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production,” Algal Research 8, 161-167. DOI:10.1016/j.algal.2015.02.006
Mizuno, Y., Sato, A., Watanabe, K., Hirata, A., Takeshita, T., Ota, S., Sato, N., Zachleder, V., Tsuzuki, M., and Kawano, S. (2013). “Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species,” Bioresource Technology 129, 150-155. DOI:10.1016/j.biortech.2012.11.030
Mozaffarian, D., and Wu, J. H. Y. (2011). “Omega-3 fatty acids and cardiovascular disease. Effects on risk factors, molecular pathways, and clinical events,” Journal of the American College of Cardiology 58(20), 2047-2067. DOI:10.1016/j.jacc.2011.06.063
Msanne, J., Xu, D., Konda, A. R., Casas-Mollano, J. A., Awada, T., Cahoon, E. B., and Cerutti, H. (2012). “Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169,” Phytochemistry 75, 50-59. DOI:10.1016/j.phytochem.2011.12.007
Nakamura, Y., and Imamura, M. (1983). “Change in properties of starch when photosynthesized at different temperatures in Chlorella vulgaris,” Plant Science Letters 31(2-3), 123-131. DOI:10.1016/0304-4211(83)90048-2
Nakamura, Y., and Miyachi, S. (1982a). “Change in starch photosynthesized at different temperatures in Chlorella,” Plant Science Letters 27(1), 1-6. DOI:10.1016/0304-4211(82)90065-7
Nakamura, Y., and Miyachi, S. (1982b). “Effect of temperature on starch degradation in Chlorella vulgaris 11h cells,” Plant and Cell Physiology 23(2), 333-341.
Nascimento, I. A., Cabanelas, I. T. D., Santos, J. N. d., Nascimento, M. A., Sousa, L., and Sansone, G. (2015). “Biodiesel yields and fuel quality as criteria for algal-feedstock selection: Effects of CO2-supplementation and nutrient levels in cultures,” Algal Research 8, 53-60. DOI:10.1016/j.algal.2015.01.001
Neto, A. M. P., Sotana de Souza, R. A., Leon-Nino, A. D., da Costa, J. D. a. A., Tiburcio, R. S., Nunes, T. A., Sellare de Mello, T. C., Kanemoto, F. T., Saldanha-Corrêa, F. M. P., and Gianesella, S. M. F. (2013). “Improvement in microalgae lipid extraction using a sonication-assisted method,” Renewable Energy 55, 525-531. DOI:10.1016/j.renene.2013.01.019
Oliveira, M. A. C. L. d., Monteiro, M. P. C., Robbs, P. G., and Leite, S. G. F. (1999). “Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures,” Aquaculture International 7(4), 261-275. DOI:10.1023/a:1009233230706
Osei, G., Arthur, R., Afrane, G., and Agyemang, E. O. (2013). “Potential feedstocks for bioethanol production as a substitute for gasoline in Ghana,” Renewable Energy 55(0), 12-17. DOI:10.1016/j.renene.2012.12.012
Padmaja, K. V., Atheya, N., and Bhatnagar, A. K. (2009). “Upgrading of Candelilla biocrude to hydrocarbon fuels by fluid catalytic cracking,” Biomass and Bioenergy 33(12), 1664-1669. DOI:10.1016/j.biombioe.2009.08.011
Palmquist, D. L., and Jenkins, T. C. (2003). “Challenges with fats and fatty acid methods,” Journal of Animal Science 81(12), 3250-3254.
Pancha, I., Chokshi, K., Maurya, R., Trivedi, K., Patidar, S. K., Ghosh, A., and Mishra, S. (2015). “Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077,” Bioresource Technology 189, 341-348. DOI:10.1016/j.biortech.2015.04.017
Park, K., Whitney, C., McNichol, J., Dickinson, K., MacQuarrie, S., Skrupski, B., Zou, J., Wilson, K., O’Leary, S. B., and McGinn, P. (2012). “Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: Potential applications for wastewater remediation for biofuel production,” Journal of Applied Phycology 24(3), 339-348. DOI:10.1007/s10811-011-9772-2
Patil, G., Chethana, S., Madhusudhan, M. C., and Raghavarao, K. S. M. S. (2008). “Fractionation and purification of the phycobiliproteins from Spirulina platensis,” Bioresource Technology 99(15), 7393-7396. DOI:10.1016/j.biortech.2008.01.028
Perez-Garcia, O., De-Bashan, L. E., Hernandez, J.-P., and Bashan, Y. (2010). “Efficiency of growth and nutrient uptake from wasterwater by heterotrophic, autotrophic and mixotrophic cultivation of Chlorella vulgaris immobilized with azospirillum brasilense1,” Journal of Phycology 46(4), 800-812. DOI:10.1111/j.1529-8817.2010.00862.x
Pieber, S., Schober, S., and Mittelbach, M. (2012). “Pressurized fluid extraction of polyunsaturated fatty acids from the microalga Nannochloropsis oculata,” Biomass and Bioenergy 47, 474-482. DOI:http://dx.doi.org/10.1016/j.biombioe.2012.10.019
Popper, Z. A., Michel, G., Hervé, C., Domozych, D. S., Willats, W. G. T., Tuohy, M. G., Kloareg, B., and Stengel, D. B. (2011). “Evolution and diversity of plant cell walls: from algae to flowering plants,” Annual Review of Plant Biology 62(1), 567-590. DOI:10.1146/annurev-arplant-042110-103809
Pramanik, K. (2003). “Properties and use of Jatropha curcas oil and diesel fuel blends in compression ignition engine,” Renewable Energy 28(2), 239-248. DOI:10.1016/S0960-1481(02)00027-7
Pulz, O., and Gross, W. (2004). “Valuable products from biotechnology of microalgae,” Applied Microbiology and Biotechnology 65(6), 635-648. DOI:10.1007/s00253-004-1647-x
Radakovits, R., Eduafo, P. M., and Posewitz, M. C. (2011). “Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum,” Metabolic Engineering 13(1), 89-95. DOI:10.1016/j.ymben.2010.10.003
Radakovits, R., Jinkerson, R. E., Darzins, A., and Posewitz, M. C. (2010). “Genetic engineering of algae for enhanced biofuel production,” Eukaryot Cell 9(4), 486-501. DOI:10.1128/EC.00364-09
Ramos, M. J., Fernández, C. M., Casas, A., Rodríguez, L., and Pérez, Á. (2009). “Influence of fatty acid composition of raw materials on biodiesel properties,” Bioresource Technology 100(1), 261-268. DOI:10.1016/j.biortech.2008.06.039
Rao, A. R., Sarada, R., and Ravishankar, G. A. (2007). “Stabilization of astaxanthin in edible oils and its use as an antioxidant,” Journal of the Science of Food and Agriculture 87(6), 957-965. DOI:10.1002/jsfa.2766
Raposo, M., de Morais, R., and Bernardo de Morais, A. (2013). “Bioactivity and applications of sulphated polysaccharides from marine microalgae,” Marine Drugs 11(1), 233-252. DOI:10.3390/md11010233
Ratledge, C. (2004). “Fatty acid biosynthesis in microorganisms being used for single cell oil production,” Biochimie 86(11), 807-815. DOI:10.1016/j.biochi.2004.09.017
Ratledge, C. (2010). “Single cell oils for the 21st century,” in: Single Cell Oils. Microbial and Algal Oils, Cohen, Z. and Ratledge, C. (ed.), AOCS Press, Urbana, 3
Raven, J. A. (2010). “Inorganic carbon acquisition by eukaryotic algae: Four current questions,” Photosynthesis Research 106(1-2), 123-134. DOI:10.1007/s11120-010-9563-7
Ren, L.-J., Ji, X.-J., Huang, H., Qu, L., Feng, Y., Tong, Q.-Q., and Ouyang, P.-K. (2010). “Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp,” Applied Microbiology and Biotechnology 87(5), 1649-1656. DOI:10.1007/s00253-010-2639-7
Renaud, S. M., Parry, D. L., and Thinh, L.-V. (1994). “Microalgae for use in tropical aquaculture I: Gross chemical and fatty acid composition of twelve species of microalgae from the Northern Territory, Australia,” Journal of Applied Phycology 6(3), 337-345. DOI:10.1007/BF02181948
Renaud, S. M., Thinh, L.-V., Lambrinidis, G., and Parry, D. L. (2002). “Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures,” Aquaculture 211(1-4), 195-214. DOI:10.1016/s0044-8486(01)00875-4
Roesler, K., Shintani, D., Savage, L., Boddupalli, S., and Ohlrogge, J. (1997). “Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds,” Plant Physiology 113(1), 75-81. DOI:10.1104/pp.113.1.75
Rosa, S. M., Soria, M. A., Vélez, C. G., and Galvagno, M. A. (2010). “Improvement of a two-stage fermentation process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21 applying statistical experimental designs and data analysis,” Bioresource Technology 101(7), 2367-2374. DOI:10.1016/j.biortech.2009.11.056
Rosenberg, J. N., Kobayashi, N., Barnes, A., Noel, E. A., Betenbaugh, M. J., and Oyler, G. A. (2014). “Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana,” PloS one 9(4), e92460.
Rosenberg, J. N., Oh, V. H., Yu, G., Guzman, B. J., Oyler, G. A., and Betenbaugh, M. J. (2015). “Chapter 22 – Exploiting the molecular genetics of microalgae: From strain development pipelines to the uncharted waters of mass production,” in: Handbook of Marine Microalgae, Kim, S.-K. (ed.), Academic Press, Boston, 331-352. DOI:10.1016/B978-0-12-800776-1.00022-4
Rosenberg, J. N., Oyler, G. A., Wilkinson, L., and Betenbaugh, M. J. (2008). “A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution,” Current Opinion in Biotechnology 19(5), 430-436. DOI:10.1016/j.copbio.2008.07.008
Ross, A. B., Biller, P., Kubacki, M. L., Li, H., Lea-Langton, A., and Jones, J. M. (2010). “Hydrothermal processing of microalgae using alkali and organic acids,” Fuel 89(9), 2234-2243. DOI:10.1016/j.fuel.2010.01.025
Santana, A., Jesus, S., Larrayoz, M. A., and Filho, R. M. (2012). “Supercritical carbon dioxide extraction of algal lipids for the biodiesel production,” Procedia Engineering 42, 1755-1761. DOI:10.1016/j.proeng.2012.07.569
Scott, S. A., Davey, M. P., Dennis, J. S., Horst, I., Howe, C. J., Lea-Smith, D. J., and Smith, A. G. (2010). “Biodiesel from algae: Challenges and prospects,” Current Opinion in Biotechnology 21(3), 277-286. DOI:10.1016/j.copbio.2010.03.005
Singh, A., Sharma, P., Saran, A. K., Singh, N., and Bishnoi, N. R. (2013). “Comparative study on ethanol production from pretreated sugarcane bagasse using immobilized Saccharomyces cerevisiae on various matrices,” Renewable Energy 50, 488-493. DOI:10.1016/j.renene.2012.07.003
Singh, S., Kate, B. N., and Banerjee, U. C. (2005). “Bioactive compounds from cyanobacteria and microalgae: An overview,” Critical Reviews in Biotechnology 25(3), 73-95. DOI:10.1080/07388550500248498
Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A. (2006). “Commercial applications of microalgae,” Journal of Bioscience and Bioengineering 101(2), 87-96. DOI:10.1263/jbb.101.87
Staats, N., De Winder, B., Stal, L., and Mur, L. (1999). “Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum,” European Journal of Phycology 34(2), 161-169. DOI:10.1080/09670269910001736212
Stephenson, P. G., Moore, C. M., Terry, M. J., Zubkov, M. V., and Bibby, T. S. (2011). “Improving photosynthesis for algal biofuels: toward a green revolution,” Trends in Biotechnology 29(12), 615-623. DOI:10.1016/j.tibtech.2011.06.005
Sukenik, A. (1991). “Ecophysiological considerations in the optimization of eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae),” Bioresource Technology 35(3), 263-269. DOI:10.1016/0960-8524(91)90123-2
Sun, N., Wang, Y., Li, Y.-T., Huang, J.-C., and Chen, F. (2008). “Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta),” Process Biochemistry 43(11), 1288-1292. DOI:10.1016/j.procbio.2008.07.014
Sun, X., Cao, Y., Xu, H., Liu, Y., Sun, J., Qiao, D., and Cao, Y. (2014a). “Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process,” Bioresource Technology 155, 204-212. DOI:10.1016/j.biortech.2013.12.109
Sun, Y., Wang, H., Guo, G., Pu, Y., and Yan, B. (2014b). “The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana,” Carbohydrate Polymers 113(0), 22-31. DOI:10.1016/j.carbpol.2014.06.058
Tanadul, O. U., VanderGheynst, J. S., Beckles, D. M., Powell, A. L., and Labavitch, J. M. (2014). “The impact of elevated CO2 concentration on the quality of algal starch as a potential biofuel feedstock,” Biotechnology and Bioengineering 111(7), 1323-1331. DOI:10.1002/bit.25203
Tang, S., Qin, C., Wang, H., Li, S., and Tian, S. (2011). “Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae,” The Journal of Supercritical Fluids 57(1), 44-49. DOI:10.1016/j.supflu.2011.01.010
Taraldsvik, M., and Myklestad, S. (2000). “The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum,” European Journal of Phycology 35(2), 189-194. DOI:10.1080/09670260010001735781
Thyssen, C., Schlichting, R., and Giersch, C. (2001). “The CO2-concentrating mechanism in the physiological context: Lowering the CO2 supply diminishes culture growth and economises starch utilisation in Chlamydomonas reinhardtii,” Planta 213(4), 629-639. DOI:Doi 10.1007/S004250100534
Tredici, M. R., Carlozzi, P., Chini Zittelli, G., and Materassi, R. (1991). “A vertical alveolar panel (VAP) for outdoor mass cultivation of microalgae and cyanobacteria,” Bioresource Technology 38(2-3), 153-159. DOI:10.1016/0960-8524(91)90147-c
Tsigie, Y. A., Huynh, L. H., Ismadji, S., Engida, A. M., and Ju, Y.-H. (2012). “In situ biodiesel production from wet Chlorella vulgaris under subcritical condition,” Chemical Engineering Journal 213, 104-108. DOI:10.1016/j.cej.2012.09.112
van Eijck, J., Batidzirai, B., and Faaij, A. (2014). “Current and future economic performance of first and second generation biofuels in developing countries,” Applied Energy 135(0), 115-141. DOI:10.1016/j.apenergy.2014.08.015
Velasquez-Orta, S. B., Lee, J. G. M., and Harvey, A. (2012). “Alkaline in situ transesterification of Chlorella vulgaris,” Fuel 94(0), 544-550. DOI:10.1016/j.fuel.2011.11.045
Walker, G. M. (2011). “125th anniversary review: Fuel alcohol: Current production and future challenges,” Journal of the Institute of Brewing 117(1), 3-22. DOI:10.1002/j.2050-0416.2011.tb00438.x
Wan, M., Zhang, J., Hou, D., Fan, J., Li, Y., Huang, J., and Wang, J. (2014). “The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation,” Bioresource Technology 167(0), 276-283. DOI:10.1016/j.biortech.2014.06.030
Wang, H., Xiong, H., Hui, Z., and Zeng, X. (2012). “Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids,” Bioresource Technology 104, 215-220. DOI:10.1016/j.biortech.2011.11.020
Wang, Y., Guo, W., Yen, H.-W., Ho, S.-H., Lo, Y.-C., Cheng, C.-L., Ren, N., and Chang, J.-S. (2015). “Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production,” Bioresource Technology 198, 619-625. DOI:10.1016/j.biortech.2015.09.067
Wang, Y., Liu, Z., and Qin, S. (2013). “Effects of iron on fatty acid and astaxanthin accumulation in mixotrophic Chromochloris zofingiensis,” Biotechnology Letters 35(3), 351-357. DOI:10.1007/s10529-012-1096-z
Wang, Z. T., Ullrich, N., Joo, S., Waffenschmidt, S., and Goodenough, U. (2009). “Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii,” Eukaryotic cell 8(12), 1856-1868. DOI:10.1128/ec.00272-09
Wen, Z.-Y., and Chen, F. (2003). “Heterotrophic production of eicosapentaenoic acid by microalgae,” Biotechnology Advances 21(4), 273-294. DOI:10.1016/S0734-9750(03)00051-X
Wen, Z.-Y., Jiang, Y., and Chen, F. (2002). “High cell density culture of the diatom Nitzschia laevis for eicosapentaenoic acid production: Fed-batch development,” Process Biochemistry 37(12), 1447-1453. DOI:10.1016/S0032-9592(02)00034-1
Wen, Z. Y., and Chen, F. (2001). “A perfusion–cell bleeding culture strategy for enhancing the productivity of eicosapentaenoic acid by Nitzschia laevis,” Applied Microbiology and Biotechnology 57(3), 316-322. DOI:10.1007/s002530100786
Wu, H., and Miao, X. (2014). “Biodiesel quality and biochemical changes of microalgae Chlorella pyrenoidosa and Scenedesmus obliquus in response to nitrate levels,” Bioresource Technology 170, 421-427. DOI:10.1016/j.biortech.2014.08.017
Xia, J.-R., and Gao, K.-S. (2005). “Impacts of elevated CO2 concentration on biochemical composition, carbonic anhydrase, and nitrate reductase activity of freshwater green algae,” Journal of Integrative Plant Biology 47(6), 668-675. DOI:10.1111/j.1744-7909.2005.00114.x
Yao, C., Ai, J., Cao, X., Xue, S., and Zhang, W. (2012). “Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation,” Bioresource Technology 118, 438-444. DOI:10.1016/j.biortech.2012.05.030
Yao, C.-H., Ai, J.-N., Cao, X.-P., and Xue, S. (2013a). “Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions,” Applied Microbiology and Biotechnology 97(13), 6099-6110. DOI:10.1007/s00253-013-4983-x
Yao, C.-H., Ai, J.-N., Cao, X.-P., and Xue, S. (2013b). “Salinity manipulation as an effective method for enhanced starch production in the marine microalga Tetraselmis subcordiformis,” Bioresource Technology 146, 663-671. DOI:10.1016/j.biortech.2013.07.134
Yeh, K.-L., and Chang, J.-S. (2012). “Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31,” Bioresource Technology 105, 120-127. DOI:10.1016/j.biortech.2011.11.103
Yen, H.-W., Hu, I. C., Chen, C.-Y., Ho, S.-H., Lee, D.-J., and Chang, J.-S. (2013). “Microalgae-based biorefinery – From biofuels to natural products,” Bioresource Technology 135, 166-174. DOI:10.1016/j.biortech.2012.10.099
Yim, J. H., Kim, S. J., Ahn, S. H., and Lee, H. K. (2007). “Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03,” Bioresource Technology 98(2), 361-367. DOI:10.1016/j.biortech.2005.12.021
Yin, L.-J., Jiang, S.-T., Pon, S.-H., and Lin, H.-H. (2010). “Hydrolysis of Chlorella by Cellulomonas sp. YJ5 cellulases and its biofunctional properties,” Journal of Food Science 75(9), H317-H323. DOI:10.1111/j.1750-3841.2010.01867.x
Yu, G., Zhang, Y., Schideman, L., Funk, T., and Wang, Z. (2011). “Hydrothermal liquefaction of low lipid content microalgae into bio-crude oil,” Trans. ASABE 54(1), 239-246. DOI:10.13031/2013.36241
Yuan, X., Shi, X., Zhang, D., Qiu, Y., Guo, R., and Wang, L. (2011). “Biogas production and microcystin biodegradation in anaerobic digestion of blue algae,” Energy & Environmental Science 4(4), 1511-1515. DOI:10.1039/C0EE00452A
Zachleder, V. (1994). “The effect of hydroxyurea and fluorodeoxyuridine on cell cycle events in the chlorococcal alga Scenedesmus quadricauda (Chlorophyta)1,” Journal of Phycology 30(2), 274-279. DOI:10.1111/j.0022-3646.1994.00274.x
Zachleder, V. (1995). “Regulation of growth processes during the cell cycle of thechlorococcal alga Scenedesmus quadricauda under a DNA replication block1,” Journal of Phycology 31(6), 941-947. DOI:10.1111/j.0022-3646.1995.00941.x
Zeeman, S. C., Kossmann, J., and Smith, A. M. (2010). “Starch: Its metabolism, evolution, and biotechnological modification in plants,” Annual Review of Plant Biology 61(1), 209-234. DOI:10.1146/annurev-arplant-042809-112301
Zhang, T.-Y., Wu, Y.-H., Zhu, S.-f., Li, F.-M., and Hu, H.-Y. (2013). “Isolation and heterotrophic cultivation of mixotrophic microalgae strains for domestic wastewater treatment and lipid production under dark condition,” Bioresource Technology 149, 586-589. DOI:10.1016/j.biortech.2013.09.106
Zhu, S., Huang, W., Xu, J., Wang, Z., Xu, J., and Yuan, Z. (2014). “Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis,” Bioresource Technology 152, 292-298. DOI:10.1016/j.biortech.2013.10.092
Zhu, X.-G., Long, S. P., and Ort, D. R. (2008). “What is the maximum efficiency with which photosynthesis can convert solar energy into biomass?,” Current Opinion in Biotechnology 19(2), 153-159. DOI:10.1016/j.copbio.2008.02.004
Zhukova, N. V., and Aizdaicher, N. A. (1995). “Fatty acid composition of 15 species of marine microalgae,” Phytochemistry 39(2), 351-356. DOI:10.1016/0031-9422(94)00913-E
Articles submitted: July 1, 2015; Resubmitted in expanded form: August 21, 2015; Peer review completed: October 3, 2015; Revised version received and accepted: October 24, 2015; Published: November 16, 2015.
DOI: 10.15376/biores.11.1.Tang
