Abstract
The microbiological diversity of cultivable bacteria was analyzed in an aerated facultative lagoon. The removal of specific compounds and measures of pollutant load was evaluated with isolated native bacteria, selected and identified in kraft cellulose effluent. The system was operated with an organic loading rate of 0.2 kgCODm-3d-1 for 60 days. Analyses of the fluorescence excitation-emission matrix, acute ecotoxicity, and microbiology were performed. Bioaugmentation tests were done to emphasize the removal of color, using promising species. The removals of biochemical oxygen demand, chemical oxygen demand, and total organic carbon in AFL were 94%, 51%, and 41%, respectively. Regarding color, removal was up to 4%, and the total phenolic compounds were not removed through biological treatment. The treatment also decreased turbidity by 94% and lignin derivatives by 12%. The bacteria identified through NCBI-BLAST and statistical similarity totaled 9 species in the cellulose effluent, three of which have the potential for color treatment: Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp. The Bacillus cereus combined with biomass removed color (69%), total phenolic compounds (37%), and compounds derived from lignin (53%). These species are promising for removing specific parameters combined with biomass from biological AFL treatment systems.
Download PDF
Full Article
Microbiological Diversity in an Aerated Lagoon Treating Kraft Effluent
Jackeline Valendolf Nunes,a,* Mac Wendell Barbosa da Silva,a Gustavo Henrique Couto,a Eduarda Roberta Bordin,a Wanessa Algarte Ramsdorf,a Izadora Cervelin Flôr,b Vânia Aparecida Vicente,b José Daniel de Almeida,c Fernanda Celinski,c and Claudia Regina Xavier a
The microbiological diversity of cultivable bacteria was analyzed in an aerated facultative lagoon. The removal of specific compounds and measures of pollutant load was evaluated with isolated native bacteria, selected and identified in kraft cellulose effluent. The system was operated with an organic loading rate of 0.2 kgCODm-3d-1 for 60 days. Analyses of the fluorescence excitation-emission matrix, acute ecotoxicity, and microbiology were performed. Bioaugmentation tests were done to emphasize the removal of color, using promising species. The removals of biochemical oxygen demand, chemical oxygen demand, and total organic carbon in AFL were 94%, 51%, and 41%, respectively. Regarding color, removal was up to 4%, and the total phenolic compounds were not removed through biological treatment. The treatment also decreased turbidity by 94% and lignin derivatives by 12%. The bacteria identified through NCBI-BLAST and statistical similarity totaled 9 species in the cellulose effluent, three of which have the potential for color treatment: Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp. The Bacillus cereus combined with biomass removed color (69%), total phenolic compounds (37%), and compounds derived from lignin (53%). These species are promising for removing specific parameters combined with biomass from biological AFL treatment systems.
Keywords: Aerated facultative lagoon; Bioaugmentation; Biological treatment; Kraft effluent; Pulp and paper mill; Recalcitrant compounds
Contact information: a: Academic Department of Chemistry and Biology, Federal Technological University of Paraná, Dep. Heitor Alencar Furtado Street, 5000 – Cidade Industrial de Curitiba, Curitiba – Paraná, Brazil; b: Department of Basic Pathology, Federal University of Paraná, Av. Cel. Francisco H. dos Santos, 100 – Jardim das Américas, Curitiba – Paraná, Brazil; c: Cocelpa CIA. of Cellulose and Paper of Paraná, Rodovia do Xisto, S/N – Km 14,5 – Jardim Alvorada, Araucária – Paraná, Brazil;
* Corresponding author: jacke.valendolf@hotmail.com
GRAPHICAL ABSTRACT
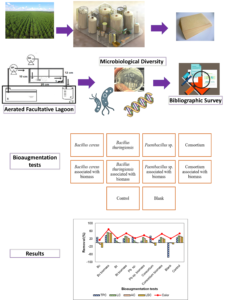
INTRODUCTION
The pulp and paper industry is important for the global and Brazilian economies (Ibá 2020). Pulp and paper production requires high water consumption, using 22 to 40 m3 per ton of pulp produced (Hubbe et al. 2016; Ibá 2020). The resulting effluents lead to environmental contamination due to the concentration of organic matter and compounds that are difficult to degrade (Kamali and Khodaparast 2015; Peitz and Xavier 2020). Cellulose effluent is treated in various ways, including biological systems, physical-chemical processes, adsorption, advanced oxidation, and membrane filtration (Majumdar et al. 2019). Except for the biological ones, these methods are expensive, which often makes their application unfeasible (Kamali and Khodaparast 2015; Kamali et al. 2019).
Activated sludge and aerated facultative lagoon (AFL) processes are the most used systems in the biological treatment of effluents from the pulp and paper industry (Kamali and Khodaparast 2015; Bailón-salas et al. 2017; Lewis et al. 2018). In this sense, AFLs are widely used in Brazil due to the country’s favorable climatic conditions and the large availability of area for the construction of these lagoons (Von Sperling 2016).
In addition, AFLs are simple to maintain, have low cost, and remove the biochemical oxygen demand (BOD5 between 80 and 95%) and the chemical oxygen demand (COD between 40 and 60%) in different types of effluents. Aerated facultative lagoons are stable in relation to shock loads, distributing the excess over their length, and have long hydraulic retention time (HRT) (2 to 10 days) (Swamy et al. 2011; Subashini 2015). In kraft effluents, lignin compounds and their derivatives persist in the cellulose effluent because of their recalcitrance. Therefore, efforts have been made to optimize this process for the removal of these specific compounds (Machado et al. 2018).
The bioaugmentation is based on the spontaneous and controlled action of microorganisms to increase their quantity and be able to degrade pollutants from soil, water bodies, and industrial effluents process (Ardeleanu 2011). This process is an alternative to improving the performance of AFL treatment in the removal of specific compounds, and it is a sustainable technology with a good benefit-cost ratio (Hossain and Ismail 2015). The challenge is to select the best microorganism to degrade the specific compounds in pulp industry effluents (Ghribi et al. 2016; Bailón-salas et al. 2017). Table 1 lists studies with specific bacteria used to remove recalcitrant compounds present in cellulose effluents, such as lignin compounds (LC) and their derivatives, aromatic compounds (AC), total phenolic compounds (TPC), and color, which show the efficiency in the use of bacteria for such treatment.
Among the microorganisms found in aerobic biological treatment systems are Bacillus sp., Brevibacillus parabrevis, Paenibacillus sp., and Serratia liquefaciens, which are efficient in the degradation of specific and hard-to-degrade compounds contained in cellulose effluents (Hooda et al. 2018; Singh et al. 2019; Sonkar et al. 2019).
The objective of this research was to evaluate the bacterial diversity in an AFL system with an organic loading rate (OLR) of 0.2 kgCODm-3d-1 and the removal of specific compounds through bioaugmentation with isolated native bacteria, selected and identified contained in kraft cellulose effluent.
EXPERIMENTAL
Effluent Sample Collection
The AFL influent was provided by an unbleached kraft pulp mill based in the metropolitan region of Curitiba, in the state of Paraná, Brazil. The samples were collected at the entrance of the treatment system, before primary settling basin, which is followed by biological treatment through aerated facultative and maturation lagoons. The samples were transported in 20 L containers and stored at 4 °C in the absence of light (Apha 2017). For treatment in the AFL, two sample collections were performed (Sample 1), and for bioaugmentation tests, a third sample (Sample 2) was collected.
Laboratory-scale aerated facultative lagoon
The AFL treatment was operated for 60 d with an OLR of 0.2 kgCODm-3d-1, and the addition of nutrients to the AFL influent remained in the proportion of 100:0.5:0.1 for COD:N:P, based on the ratio applied by the mill that provided the samples. The concentration of inoculated sludge was 70 mgL-1 (Von Sperling 2014). A Lutron Oxygen Meter DO-5519 (Taipei, Taiwan) was used in order to measure dissolved oxygen (DO), ICEL OR-2300 (Haifa, Israel) was used for redox potential (RP), and pH Meter CienlaB mPA-210 (Piracicaba, Brazil) for the pH. The system developed in laboratory conditions is represented in Fig. 1.
Fig. 1. Bench AFL scheme
Characterization of cellulose effluent samples
The sample provided by the industry and also the influent and effluent from AFL treatment were analysed. The samples were filtered with a nitrocellulose filter of 0.45 μm pore size and analyzed using the parameters of BOD5, COD, TOC, color (Vis440nm), turbidity, TPC, aromatic compounds (UV254nm), and compounds derived from lignin, that is, lignins (UV280nm) and lignosulfonic ones (UV346nm) (Çeçen 2003; Chamorro et al. 2009; Apha 2017). The color sample was analyzed at pH 9.0, and the sample of aromatic compounds and lignin derivatives was analyzed at pH 7.0 (Çeçen 2003), both using a Varian UV-VIS Cary-50 Spectrophotometer (Santa Clara, United States of America). All analyses were performed in triplicate.
In addition, the following analyses were also performed when the AFL reached steady-state: Fluorescence excitation-emission matrix (EEM), total and volatile suspended solids (TSS and VSS) on the biomass, and acute ecotoxicity of both the influent and effluent of the treatment with Daphnia magna (ABNT NBR 12713 2016).
Microbiological Diversity Analysis
Microbiological analyses were performed on biomass samples taken from the AFL at steady-state and guided by molecular identification based on 16S rRNA gene sequence analysis. First, bacteria were isolated from biomass using nutrient agar medium (Himedia, Mumbai, Índia) after incubation of plates at 30 °C for 48 h. Genomic DNA was extracted and purified from isolates in the kraft effluent (Vicente et al. 2008) and 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the universal primers 968F/1392R for the bacteria domain. Afterwards, PCR fragments were analyzed by electrophoresis through 1% agarose gels for 16S rRNA products followed by purification and sequencing on a Illumina MiSeq platform using the MiSeq Reagent Kit v3 600 (San Diego, USA).
Bacteria were identified by sequence alignment using the nucleotide Basic Local Alignment Search Tool (BLASTN) against the nucleotide collection database (NCBI database, https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Bioaugmentation Test
The bioaugmentation test was performed as presented by Saleem et al. (2014). Initially, Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp. were plated; subsequently, 2 mL of the solution of each isolated species were added to a 250 mL Erlenmeyer flask containing 20 mL of nutrient broth. These were stirred at 40 rpm in a shaker at 30 °C, and their growth was monitored every 1 h through the optical density analysis of the samples using a UV-Vis spectrophotometer at a wavelength of 600 nm (Bombardi et al. 2018). The results of these measurements were analyzed using the Chem Agilent software to determine the concentration of the colony-forming unit (CFU mL-1).
In the bioaugmentation process, the combinations shown in Fig. 2 were under aeration and agitation at 70 rpm in a shaker, at a temperature of 25 °C, for 2.1 d in order to have OLR conditions similar to those of the AFL with 0.2 kgCODm-3d-1. In experiments containing biomass, 70 mg VSS L-1 was used in each system. The influent of each system was 100 mL of the sample from the kraft pulp mill, with pH adjusted to 7.0 (0.02) and addition of nutrients for COD:N:P of 100:0.5:0.1. Figure 2 shows a flowchart with the layout of the tests for the bioaugmentation treatment.
Fig. 2. Layout of bioaugmentation tests
The isolated bacteria, isolated bacteria combined with biomass, consortium, and consortium combined with biomass were tested in triplicate. A blank was carried out containing only neutralized influent with the addition of nutrients, while in the control, in addition to the aforementioned content, 70 mgVSSL-1 of biomass were present.
The efficiency of the treatment was evaluated according to the removal of BOD5, COD, TPC, color, aromatic compounds, and compounds derived from lignin, that is, lignin and lignosulfonic compounds.
RESULTS AND DISCUSSION
Sample Characterization
Results for characterization of Samples 1 and 2 provided by the pulp mill are shown in Table 2. Sample 1 was used during the treatment with OLR of 0.2 kg COD m-3 d-1, and Sample 2, in the bioaugmentation test. The influent of Sample 1 presented a BOD5 / COD ratio of 0.28 and Sample 2 of 0.33. According to Jordão and Pessoa (2016), the values above 0.30 suggest good biodegradability, being favorable for biological treatment.
Fig. 3. Control parameters of AFL. OLR, organic loading rate; HRT, hydraulic retention time
AFL operating parameters
Figure 3 contains data on the AFL control parameters in relation to organic loading rate, pH, temperature, and hydraulic retention time. The average organic loading rate was 0.19 (0.02) kgCODm-3d-1, which is close to the predicted value that resulted in an average HRT of 2.1 d. The average temperature was 23.0 °C. The influent pH was set at 7.0 to enter the AFL treatment system; however, the average pH measured in the treatment effluent was 7.4. The RP measured in the AFL aerated zone was 42.0 mV, while in the sedimentation zone it was -23.5 mV, which is in line with the AFL system, in which an anoxic environment is observed in the sedimentation zone (Metcalf and Eddy 2016).
Evaluation of organic matter removal
Figure 4 shows the organic matter removal in terms of BOD5 and COD. The average values of BOD5 removal were greater than 90%, which is in line with previous reports, 50 to 95% of BOD5 removal in systems and conditions similar to those employed in this study (Machado et al. 2018; Peitz and Xavier 2020).
Fig. 4. Removal of organic matter in relation to BOD5 and COD
The COD removal ranged between 40 and 60% during the 60 d of operation. This result was similar to that obtained by Machado et al. (2018) using the organic load rate of 0.2 kgCODm-3d-1 in an aerated lagoon. Hubbe et al. (2016) and Kamali et al. (2019) reported the difficulty that biological systems present in treating cellulose effluents because the efficiency of COD removal is usually around 50%.
The analysis of TOC removal averaged 49% for the AFL with OLR of 0.2 kgCODm-3d-1. The results obtained in such OLR were similar to the result found by Lewis et al. (2018) in an aerated facultative lagoon.
In the state of Paraná, Brazil, the Conselho Estadual do Meio Ambiente [State Council for the Environment] (CEMA) establishes that for the pulp and paper industry, the limit of BOD5 in the effluent discharged into water bodies should be 50 mg L-1, and the COD should be 300 mg L-1 (CEMA Resolution 070/2009). Thus, the treatment performed was effective in adapting the effluent to the discharge criteria based on global organic matter.
Evaluation of the AFL specific compounds, color, and turbidity
Figure 5 presents the analysis of specific compounds, namely: total phenolic compounds, aromatic compounds, lignin compounds, lignosulfonic compounds, in addition to the AFL color and turbidity parameters.
Fig. 5. Evaluation of removal of specific compounds, color, and turbidity
Figure 5 shows that the TPC increased during the AFL treatment with an average of 26%. Some studies with kraft effluent showed an increase in total phenolic compounds in aerated biological systems (Chamorro et al. 2009; Duarte et al. 2018; Machado et al. 2018; Melchiors 2019; Peitz and Xavier 2020).
In relation to the other specific compounds of the kraft cellulose effluent, the removal of lignin compounds was around 13%. For aromatic compounds, the average removal was 16%, and the lignosulfonic compounds had an average removal of 8%.
The data verified that there was no color removal. The increase observed in the first 10 d of operation is related to the stabilization of the treatment system. The increase in color may be related to the process of biotransformation of chromophoric units and the condensation of color-forming compounds, without mineralization of the effluent (Lewis et al. 2018; Peitz and Xavier 2020). Low color removal has been observed during treatment by aerated lagoons (Kamali and Khodaparast 2015; Peitz and Xavier 2020).
Regarding the removal of turbidity, the system showed an average removal of 94%. In general, the AFL system showed good removal in this parameter, indicating potential for clarification of the effluent in the AFL sedimentation zone.
C:N:P ratio and the performance of aerated lagoons
In relation to the C:N:P ratio used, the AFL efficiency used in this study was compared with studies using different nutrient ratios. Table 3 shows the performance of aerated lagoons treating kraft cellulose effluent with different COD:N:P ratios.
In the studies by Machado et al. (2018) and Peitz and Xavier (2020), the nutrient ratio was 100:5:1; in the present study, it was 100:0.5:0.1, which is more similar to that of an AFL and to what is actually employed by the industry. The removal of biodegradable organic matter (BOD5) was greater in this study than in those with more use of nutrients. The lower color removal, however, may be associated with low redox potential in the AFL sedimentation zone. In general, it was observed that the demand for nutrients can be optimized, which can result in savings in the treatment process.
Analysis of fluorescence excitation-emission matrix (EEM)
Figure 6 shows the fluorescence excitation-emission matrices (EEM) of the treatment system. Fluorescence intensity (FI) is expressed in arbitrary units (a.u.).
Fig. 6. Fluorescence excitation-emission matrices of the treatment system by LAF a) EEM’s affluent, b) EEM’s effluent, c) 3D spectrum of fluorescence intensity (FI) of the fluorogenic compounds removed and increased during treatment in the AFL system
Figures 6a and 6b show different fluorescence peaks, represented by excitation (λEX) and emission (λEM) wavelengths. The observed peaks were named A, B, C, and D. Peaks A and B, located in the λEM < 380 nm region, were identified by Carstea et al. (2016) in several EEMs obtained from wastewater. These peaks are associated with by-products of organic matter biodegradation (Bridgeman et al. 2013). Peak C is related to the common chemical characteristics of cellulose industry effluents, such as compounds derived from lignin (Managó 2019). Peak D is related to fluorogenic compounds. Comparing Fig. 6a and 6b, it is possible to observe a small removal of the aforementioned compounds.
Figure 6c shows the 3D spectrum of the difference between the fluorescence intensity emitted by the AFL influent and effluent. The peaks of positive FI represent the fluorogenic compounds removed in the treatment, and negative FI indicates the intensity increased during the treatment (Melchiors 2019). The presentation of this spectrum corroborates the results of the present research, such as color production in the anoxic zone, TPC in the aerated zone, and the low removal of lignin derivatives. Other authors have also encountered the removal of fluorogenic compounds in cellulose effluents (Janhom et al. 2011; Murphy et al. 2011; Carstea et al. 2016; Melchiors 2019).
Biomass analysis
Regarding the biomass of the treatment system, it was observed that after 60 d of operation in the lagoon, the VSS reached 770 mg L-1 and the TSS was 1181 mg L-1. The average VSS/TSS ratio was 0.67, which indicates a stabilized biomass (Von Sperling 2014). The observed growth rate was 1100% and is comparable to that observed by Peitz 2018, in which the biomass grew from 70 mg L-1 of VSS to 1783 mg L-1 in 60 days of operation in an aerated lagoon, containing support medium with an OLR similar to the one in the present study.
Ecotoxicity analysis
The results of acute ecotoxicity were carried out with Daphnia magna with a 48 h exposure to the influent and the effluent treated by AFL. The toxicity factor obtained was 1 (TF = 1), which shows that the sample of effluent obtained from the pulp and paper industry did not present acute toxicity even at 100% concentration. These results are in line with those found by Machado et al. (2018) and Peitz and Xavier (2019) with Daphnia magna exposed to the same type of effluent. Thus, these data comply with the current state legislation by CEMA, resolution Nº 081/10 (CEMA Resolution 081/2010 (2010).).
Identification of Bacteria Contained in the Effluent
The comparison of the 16S rRNA gene sequences of the bacteria were carried out against the NCBI database to find regions of identity with statistical significance between deposited sequences. Table 4 shows the isolated bacteria identified (≥97% of query coverage) in the biomass of the AFL treating kraft pulp effluent. The total number of microorganisms collected from the aerated and sedimentation zones was 9, which were species of bacteria, as shown in Table 4.
The microorganisms identified in the AFL biomass have also been found in other studies with bacteria, such as the ones by Chandra et al. (2012), Raj et al. (2014), Saleem et al. (2014), Bailón-Salas et al. (2017), and Sonkar et al. (2019). Among such microorganisms, there was an emphasis on Bacillus cereus as promising for color removal in cellulose effluent, as suggested by Saleem et al. (2014).
Bioaugmentation Test
Subsequently, bioaugmentation tests were performed to emphasize the removal of color, using the following species: Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp. This parameter was considered to be a challenge for biological treatment due to the processes of depolymerization and molecular repolymerization in the different conditions employed. Following the increase in the concentration of bacteria, it was observed that the maximum growth occurred in 1.5 h for Bacillus cereus and Bacillus thuringienses, and in 6 h for Paenibacillus sp. under incubation conditions. The bacteria concentration was 6.2 × 108, 6.4 × 108, and 6.3 × 108 CFU mL-1 for the three species, respectively.
In the bioaugmentation, both organic matter and specific compounds were removed. In Fig. 7, these results are presented from the tests with Bacillus cereus, Bacillus cereus coupled with biomass, Bacillus thuringiensis, Bacillus thuringiensis coupled with biomass, Paenibacillus sp., Paenibacillus sp. coupled with biomass, mixed (Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp.), mixed coupled with biomass, blank and control.
Despite the performance of Paenibacillus sp. combined with biomass having been the best system for the removal of global organic matter, the treatment of specific compounds, especially color, was better in the system in which Bacillus cereus combined with biomass was used. Under these conditions, besides color (69%), TPC (37%), LC (53%), AC (50%), and lignosulfonic compounds (49%) were also removed. In the control test, the use of aeration alone caused an increase in the TPC in the medium, as observed in other studies (Chamorro et al. 2010; Melchiors 2019; Peitz and Xavier 2020).
Regarding the bacteria used in the present study, it is worth mentioning that Saleem et al. 2014 used Bacillus cereus alone for the treatment of cellulose effluent, removing BOD5, COD, and color by 66%, 61%, and 90%, respectively, with pH 6.5 in a batch reactor. Chandra et al. (2012) also employed Bacillus cereus in the treatment of cellulose effluent; however, they combined it with Serratia marcescens and Serratia liquifaciens, which had been identified in a cellulose effluent treatment system. They obtained the removal of 65% for color, 63% for TPC, 63% for COD, and 64% for BOD5 for a 7-day HRT. With these results, it is possible to affirm that the present bioaugmentation study using Bacillus cereus combined with biomass was close to that observed in the literature.
Fig. 7. Evaluation of removal of organic matter and specific compounds in bioaugmentation a) removal of organic matter in BOD5, and COD b) removal of specific compounds from kraft effluent, BC – Bacillus cereus Bt – Bacillus thuringiense Pb – Paenibacillus sp.
The bacterium Paenibacillus sp. was identified by Raj et al. (2014) in a batch reactor in a 6-day HRT in the treatment of cellulose effluent, in which removals of 68%, 54%, 86%, 83%, and 78% were obtained for color, lignin compounds, total phenol, BOD5, and COD, respectively. Chandra et al. (2008) identified the bacteria Bacillus sp. and Paenibacillus sp. in a cellulose effluent treatment system operating with 6-day HRT, pH of 7.6 and temperature of 30 °C, in which color was removed by 65% and 48% for the bacteria used separately in treatment by bioaugmentation.
Sonkar et al. (2019) identified the bacterium Bacillus thuringiensis in a cellulose effluent treatment system and found a 99% similarity to it in a batch reactor. The removal of BOD5, COD, TOC, and color was by 93%, 89%, 82%, and 73%, respectively, with a 3-day HRT. These species (Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp.), which were identified and used in the bioaugmentation tests, proved to be promising for removal of specific parameters combined with biomass from AFL biological treatment.
CONCLUSIONS
- In this study, the microbiological diversity of a kraft effluent treatment system by an aerated lagoon was analyzed, and 9 species of bacteria were identified, three of which have the potential for color treatment: Bacillus cereus, Bacillus thuringiensis, and Paenibacillus sp.
- There was efficiency by AFL in removing specific compounds from the kraft effluent with an OLR of 0.2 kgCODm-3d-1 being in the parameters of BOD5 (94%), COD (51%), TOC (49%). Regarding color, removal was up to 4%, and the total phenolic compounds were not removed through biological treatment. The treatment also decreased turbidity by 94% and lignin derivatives by 12%.
- In bioaugmentation tests, the treatment of specific compounds and especially the color was better in the system with Bacillus cereus associated with biomass, in which the removal were to color (69%), TPC (37%), LC ( 53%), AC (50%) and lignosulfonic compounds (49%).
- The bioaugmentation of the Bacillus cereus with the biomass of the treatment system is a sustainable and innovative alternative for the treatment of kraft effluent in OLR of 0.2 kg CODm-3d-1.
ACKNOWLEDGMENTS
The authors are grateful to the Cocelpa cellulose and paper supplying the effluent, to the Federal Technological University of Paraná, the Department of Chemistry and Biology, the Wastewater Treatment Laboratory, UTFPR Microbiology Laboratory, Ecotoxicology Laboratory, UFPR Microbiology and Parasitology Laboratory, and the Effluent Treatment Research Group (GTEF) for the support and opportunity to develop this work.
REFERENCES CITED
ABNT NBR 12713 (2016). “Ecotoxicologia aquática – Toxicidade aguda – Método de ensaio com Daphnia spp. (Cladocera, Crustacea) [Aquatic ecotoxicology – Acute toxicity – Test with Daphnia spp (Cladocera, Crustacea)],” Associação Brasileira de Normas Técnicas, Rio de Janeiro, Brazil.
APHA (2017). Standard Methods for the Examination of Water and Wastewater, 23rd Edition, E. W. Rice, R. B. Baird, A. D. Eaton (eds.), American Public Health Association, Washington, D.C.
Ardeleanu, E. R. (2011). “An approach to bioremediation,” Journal of Engineering Studies and Research 17(4), 7-12.
Bailón-Salas, A. M., Medrano-Roldán, H., Valle-Cervantes, S., Ordaz-Díaz, L. A., Urtiz-Estrada, N., and Rojas-Contreras, J. A. (2017). “Review of molecular techniques for the identification of bacterial communities in biological effluent treatment facilities at pulp and paper mills,” BioResources 12(2), 4384-4409. DOI: 10.15376/biores.12.2.Bailon_Salas
Bombardi, F. M. L., Ishii, F. K., Pelayo, J. S., Kolm, H. E., Müller, M., and Fabris, J. L. (2018). “Spectral monitoring of the growth dynamics of E. coli bacterial populations in water environment,” in: 26th International Conference on Optical Fiber Sensors, Lausanne, Switzerland, paper WF43. DOI: 10.1364/OFS.2018.WF43
Bridgeman, J., Baker, A., Carliell-Marquet, C., and Carstea, E. (2013). “Determination of changes in wastewater quality through a treatment works using fluorescence spectroscopy,” Environmental Technology 34(23), 3069-3077. DOI: 10.1080/09593330.2013.803131
Carstea, E. M., Bridgeman, J., Baker, A., and Reynolds, D. M. (2016). “Fluorescence spectroscopy for wastewater monitoring: A review,” Water Research 95, 205-219. DOI: 10.1016/j.watres.2016.03.021
Çeçen, F. (2003). “The use of UV-VIS measurements in the determination of biological treatability of pulp bleaching effluents,” in: Conf. Proc. – 7th International Water Association Symposium on Forest Industry Wastewaters, Seattle, WA, pp. 135-142.
CEMA Resolution 070/2009 (2009). “Dispõe sobre o licenciamento ambiental, estabelece condições e critérios e dá outras providências, para empreendimentos industriais [Provides for environmental licensing, establishes conditions and criteria and provides other measures for industrial enterprises],” Paraná – Governo do Estado, Curitiba, Paraná, Brazil.
CEMA Resolution 081/2010 (2010). “Dispõe sobre critérios e padrões de ecotoxicidade para o controle de efluentes líquidos lançados em águas superficiais no estado do Paraná [Provides for ecotoxicity criteria and standards for the control of liquid effluents discharged into surface waters in the state of Paraná],” Paraná – Governo do Estado, Curitiba, Paraná, Brazil.
Chamorro, S., Pozo, Z., Jarpa, M., Hernandes, V., Becerra, J., and Vidal, G. (2009). “Monitoring endocrine activity in kraft mill effluents treated by an aerobic moving bed bioreactor system,” Water Science and Technology 62(1), 154-161. DOI: 10.2166/wst.2010.297
Chandra, R., Singh, R., and Yadav, S. (2012). “Effect of bacterial inoculum ratio in mixed culture for decolorization and detoxification of pulp paper mill effluent,” Journal of Chemical Technology & Biotechnology 87(3), 436-444. DOI: 10.1002/jctb.2758
Chandra, R., Singh, S., Reddy, M. M. K., Patel, D. K., Purohit, H. J., and Kapley, A. (2008). “Isolation and characterization of bacterial strains Paenibacillus sp. and Bacillus sp. for kraft lignin decolorization from pulp and paper mill waste,” The Journal of General and Applied Microbiology 54(6), 399-407. DOI: 10.2323/jgam.54.399
Duarte, J. C., Peitz, P., and Xavier, C. R. (2018). “Avaliação do tratamento de efluente kraft com meio de suporte esponjoso em reator sequencial em batelada (RSB) [Evaluation of the treatment of kraft effluent with spongy support medium in sequential batch reactor],” in: Anais XIV Simpósio Ítalo-Brasileiro de Engenharia Sanitária Ambiental – SIBESA, Foz do Iguaçu, Brazil.
Ghribi, M., Meddeb-Mouelhi, F., and Beauregard, M. (2016). “Microbial diversity in various types of paper mill sludge: Identification of enzyme activities with potential industrial applications,” Springer Plus 5, 1492. DOI: 0.1186/s40064-016-3147-8
Hooda, R., Bhardwaj, N. K., and Singh, P. (2018). “Brevibacillus parabrevis MTCC 12105: A potential bacterium for pulp and paper effluent degradation,” World Journal of Microbiology and Biotechnology 34(2), 31-41. DOI: 10.1007/s11274-018-2414-y
Hossain, K., and Ismail, N. (2015). “Bioremediation and detoxification of pulp and paper mill effluent: A review,” Research Journal of Environmental Toxicology 9(3), 113-134. DOI: 10.3923/rjet.2015.113.134
Hubbe, M. A., Metts, J. R., Hermosilla, D., Blanco, M. A., Yerushalmi, L., Haghighat, F., Lindholm-Lehto, P., Khodaparast, K., Kamali M., and Elliott, A. (2016). “Wastewater treatment and reclamation: A review of pulp and paper industry practices and opportunities,” BioResources 11(3), 7953-8091. DOI: 10.15376/biores.11.3. 7953-8091
IBÁ – Indústria Brasileira de Árvores. (2020). “Relatório IBÁ 2020,” (https://iba.org/datafiles/publicacoes/relatorios/relatorio-iba-2020.pdf), Accessed 22 October 2020.
Janhom, T., Pavasant, P., and Wattanachira, S. (2011). “Profiling and monitoring of DOM in brewery wastewater and treated wastewater,” Environmental Monitoring and Assessment 176, 403-418. DOI: 0.1007/s10661-010-1592-3
Jordão, E. P. and Pessoa, C. A. (2016). Tratamentos de Esgotos Domésticos [Domestic Sewage Treatments], ABES, Rio de Janeiro, Brazil.
Kamali, M., Alavi-Borazjani, S. A., Khodaparast, Z., Khalaj, M., Jahanshahi, A., Costa, E., and Capela, I. (2019). “Additive and additive-free treatment technologies for pulp and paper mill effluents: advances, challenges and opportunities,” Water Resources and Industry 21, 100109. DOI: 10.1016/j.wri.2019.100109
Kamali, M., and Khodaparast, Z. (2015). “Review on recent developments on pulp and paper mill wastewater treatment,” Ecotoxicology Environmental Safety 114, 326-342. DOI: 10.1016/j.ecoenv.2014.05.005
Lewis, R., Cohen, J., Awad, J., Burger, H., Marzouk, J., Burch, G., Lewis, D. M., and Van Leeuwen, J. A. (2018). “Study of the impacts of process changes of a pulp and paper mill on aerated stabilization basin (ASB) performance,” Chemosphere 211, 767-774. DOI: 10.1016/j.chemosphere.2018.07.178
Machado, E. P., Xavier, C. R., and Couto, G. H. (2018). “Tratamento de efluente Kraft em lagoa aerada facultativa empregando enzimas lignolíticas [Kraft effluent treatment in an optional aerated lagoon using lignolytic enzymes],” Interciencia 43(8), 590-596.
Majumdar, S., Priyadarshinee, R., Kumar, A., Mandal, T., and Mandal, D. D. (2019). “Exploring Planococcus sp. TRC1, a bacterial isolate, for carotenoid pigment production and detoxification of paper mill effluent in immobilized fluidized bed reactor,” Journal of Cleaner Production 211, 1389-1402. DOI: 10.1016/j.jclepro.2018.11.157
Managó, B. (2019). Treatment of Pulp Wastewater by Membrane Bioreactor, Ph.D. Dissertation, Universidade Estadual do Centro-Oeste, Irati, Paraná, Brazil.
Melchiors, E. (2019). Avaliação do Desenvolvimento de Biofilme em Meio Suporte Esponjoso em Reator Biológico de Leito Móvel (MBBR) no Tratamento de Efluente de Indústria de Celulose [Evaluation of the development of biofilm in spongy support medium in biological reactor of mobile bed (MBBR) in the treatment of effluent from the cellulose industry], Master’s Thesis, Universidade Tecnológica Federal do Paraná – Curitiba, Paraná, Brazil.
Metcalf, L. and Eddy, H. P. (2016). Tratamento de Efluente e Recuperação de Recursos [Effluent Treatment and Resource Recovery], McGraw Hill Brasil, Porto Alegre, Brazil.
Murphy, K. R., Hambly, A., Singh, S., Henderson, R. K., Baker, A., Stuetz, R., and Khan, S. J. (2011). “Organic matter fluorescence in municipal water recycling schemes: toward a unified PARAFAC model,” Environmental Science Technology 45(7), 2909-2916. DOI: 10.1021/es103015e
Peitz, C., and Xavier. C. R. (2020). “Moving bed biofilm reactor for treatment of kraft pulp effluent with high organic load rate,” Revista Ambiente & Água 15(4), 1-10. DOI: 10.4136/ambi-agua.2512
Peitz, C. and Xavier. C. R. (2019). “Evaluation of aerated lagoon modified with spongy support medium treating kraft pulp mill efluent,” Revista Facultad de Ingeniería Universidad de Antioquia 92, 70-79. DOI: 10.17533/udea.redin.20190725
Peitz, C. (2018). Desempenho de Sistema Modificado de Lagoa Aerada com Meio de Suporte em Leito Móvel no Tratamento de Efluente de Celulose Kraft [Performance of Modified Aerated Lagoon System with Mobile Bed Support Medium in Kraft Cellulose Effluent Treatment], Master’s Thesis, Programa de Pós-Graduação em Ciência e Tecnologia Ambiental, Universidade Tecnológica Federal do Paraná, Curitiba, Paraná, Brazil.
Raj, A., Kumar, S., Haq, I., and Singh, S. K. (2014). “Bioremediation and toxicity reduction in pulp and paper mill effluent by newly isolated ligninolytic Paenibacillus sp.,” Ecological Engineering 71, 355-362. DOI: 10.1016/j.ecoleng.2014.07.002
Saleem, M, Ahmad, S., and Ahmad, M. (2014). “Potential of Bacillus cereus for bioremediation of pulp and paper industrial waste,” Annals of Microbiology 64(2), 823-829. DOI: 10.1007/s13213-013-0721-y
Singh, A. K., Yadav, P., Bharagava, R. N., Saratale, G. D., and Raj. A. (2019). “Biotransformation and cytotoxicity evaluation of kraft lignin degraded by ligninolytic Serratia liquefaciens,” Frontiers in Microbiology 10, 2364. DOI: 10.3389/fmicb.2019.02364
Sonkar, M., Kumar, M., Dutt, D., and Kumar, V. (2019). “Treatment of pulp and paper mill effluent by a novel bacterium Bacillus sp. IITRDVM-5 through a sequential batch process,” Biocatalysis and Agricultural Biotechnology 20, 101232.
Subashini, A. M. (2015). “Review on biological treatment processes of pulp and paper industry wastewater,” International Journal of Innovative Research in Science, Engineering and Technology 4(5), 3721-3725. DOI: 10.15680/IJIRSET.2015.0405195
Swamy, N. K., Singh, P., and Sarethy, I. P. (2011). “Aerobic and anaerobic treatment of paper industry wastewater,” Research in Environment Life and Science 4(4), 141-148.
Vicente, V. A., Attili-Angelis, D., Pie, M. R., Queiroz-Telles, F., Cruz, L. M., Najafzadeh, M. J., Hoog, G. S., Zhao J. S., and Pizziranikleiner, A. (2008). “Environmental isolation of black yeast-like fungi involved in human infection,” Studies in Mycology 61, 137-144.
Von Sperling, M. (2016). Urban Wastewater Treatment in Brazil (IDB-TN-970), Department of Sanitary and Environmental Engineering, Federal University of Minas Gerais, Brazil.
Von Sperling, M. (2014). “Introdução à qualidade das águas e ao tratamento de esgotos [Introduction to water quality and sewage treatment],” in: Princípios do tratamento biológico de águas residuárias [Principles of biological wastewater treatment], Belo Horizonte, Minas Gerais, Brazil.
Article submitted: March 20, 2021; Peer review completed: May 9, 2021; Revised version received and accepted: May 22, 2021; Published: June 1, 2021.
DOI: 10.15376/biores.16.3.5203-5219
