Abstract
The pyrolysis reaction of rice straw under microwave irradiation in bromide 1-ethyl-3-methyl imidazole ([Emim]Br) ionic liquid (IL) was investigated in this work. The effects of reaction temperature, mass ratio of IL to straw, reaction time, and microwave irradiation power on the yield of bio-oil were considered. An orthogonal experimental method was adopted to obtain the optimal technological conditions for pyrolysis: a reaction temperature of 160 °C, a mass ratio of IL to straw of 2:1, a reaction time of 15 min, and a microwave irradiation power of 700 W. The yield of bio-oil reached 28.2% at optimum conditions. The percent recovery of IL ranged from 84% to 87%. The recycled IL could be reused as straw pyrolysis solvent and did not affect the bio-oil yield because its structure was not damaged after being used in pyrolysis as a solvent.
Download PDF
Full Article
Microwave Irradiation Pyrolysis of Rice Straw in Ionic Liquid ([Emim]Br)
Shaoling Cheng,a,c Zhimin Zhang,b Dongming Zhang,a,c and Yu Deng b,c,*
The pyrolysis reaction of rice straw under microwave irradiation in bromide 1-ethyl-3-methyl imidazole ([Emim]Br) ionic liquid (IL) was investigated in this work. The effects of reaction temperature, mass ratio of IL to straw, reaction time, and microwave irradiation power on the yield of bio-oil were considered. An orthogonal experimental method was adopted to obtain the optimal technological conditions for pyrolysis: a reaction temperature of 160 °C, a mass ratio of IL to straw of 2:1, a reaction time of 15 min, and a microwave irradiation power of 700 W. The yield of bio-oil reached 28.2% at optimum conditions. The percent recovery of IL ranged from 84% to 87%. The recycled IL could be reused as straw pyrolysis solvent and did not affect the bio-oil yield because its structure was not damaged after being used in pyrolysis as a solvent.
Keywords: Rice straw; Ionic liquid; Pyrolysis; Bio-oil; Microwave irradiation
Contact information: a: College of Science, Tianjin University of Science & Technology, Tianjin 300457; b: College of Material Science and Chemical Engineering, Tianjin University of Science & Technology, Tianjin 300457; c: Tianjin Key Laboratory of Pulp & Paper, Tianjin 300457;
* Corresponding author: dengyu@tust.edu.cn
INTRODUCTION
As a renewable resource, biomass can be converted into bio-oil, which can be used as liquid fuel or can be the source of high value-added chemicals. How to convert biomass energy into useful resources has been the focus of many investigators all over the world. Biomass energy utilization techniques can be classified into three types (Bridgwater and Peacocke 2000; Olsson and Hahn-Hagerdal 1996; Hahn-Hagerdal et al. 2006): direct combustion, biochemical conversion, and thermochemical conversion. Direct combustion is the most common type used, but it is very low in thermal efficiency. Biochemical conversion is rather efficient at biomass energy conversion, but the slow transformation rate and waste liquid problems limit its application. Compared with the above two methods of biomass conversion, thermochemical conversion seems to be a promising route for producing bio-oils because of its fast pyrolysis and higher yields. However, pyrolysis technology usually requires a relatively high temperature (more than 500 °C) to maximum yields (Hyeon et al. 2010; Yang and Qiu 2008; Mullen et al. 2010; Wan et al. 2009; Yu et al. 2007; Wang et al. 2007). Thermal energy consumption is much higher for thermochemical conversion; therefore, it is necessary to explore a low energy consumption route for pyrolysis biomass to produce bio-oils.
Chemical reaction time can be shortened and reaction temperature can be reduced through microwave-assisted heating technology. There have been many reports about microwave-assisted pyrolysis of biomass in recent years (Miura et al. 2004; Jones et al. 2002). Various types of biomass have been used as feedstock for pyrolysis studies, such as wood (Miura et al. 2004), coffee hulls (Dominguez et al. 2007), waste tea (Yagmur et al. 2008), corn stover (Lei et al. 2009), and rice straw (Huang et al. 2010). Rice straw is one of the most abundant types of biomass, which has been pyrolyzed to produce hydrogen-rich fuel gas (Huang et al. 2010) and bio-oil (Du et al. 2010) under microwave irradiation. These pyrolysis reactions were all performed in heterogeneous reaction systems. In our work, rice straw pyrolysis reactions were conducted in a homogeneous system by using ionic liquid as solvent.
An ionic liquid is a non-volatile liquid solvent with a low melting point and has many practical applications, such as its use as a green solvent, catalyst, and microwave adsorbent. It has been reported that certain ionic liquids can dissolve cellulose well, so wood biomass is readily soluble in ionic liquid even under mild conditions (Swatloski et al. 2002; Kilpeläinen et al. 2007). Meanwhile, ionic liquids possess microwave adsorption characteristics because of their strongly polar structure. As a microwave adsorbent, ionic liquid has been used in many organic syntheses (Guo et al. 2007; Yu et al. 2011). It can be predicted that when biomass pyrolysis reactions are processed in ionic liquids, which act as a solvent and microwave adsorbent under microwave irradiation, ionic liquids will adsorb microwave energy effectively, leading to a rapid rise of reaction temperature and the promotion of biomass pyrolysis. The pyrolysis reactions of biomass in ionic liquids have the characteristics of low pyrolysis temperature, less energy consumption, little pollution of the environment, and the use of simple equipment. These characteristics will benefit the practical application for the biomass pyrolysis in ionic liquids. In this work, straw pyrolysis in ionic liquid under microwave irradiation was investigated. The influence of pyrolysis conditions on the yields of bio-oils was evaluated.
EXPERIMENTAL
Materials and Instruments
Dichloromethane was provided by Tianjin Jiangtian Chemical Technology Co., Ltd.. 1-ethyl-3-methylimidazole bromide ([Emim]Br) was synthesized according to known procedures (Burrell et al. 2007). All biomass of rice straw samples was provided by the Pulp Laboratory at Tianjin University of Science & Technology. The component analysis was conducted according to the method described by Shi and He (2006). The analysis results were as follows: water 8.61%, ash 12.78%, holocellulose 63.1%, pentosan 16.48%, benzene-alcohol extractions 7.45%, acid-soluble lignin 4.15%, and Klason lignin 11.42%.
A NJL07-3 laboratory microwave oven was purchased from Nanjing Jiequan Microwave Development Co., Ltd. (Nanjing, China). A WQF-510 Fourier transform Infrared Spectrometer was purchased from Beijing Second Optical Instrument Factory (Beijing, China).
Pyrolysis Method
Ten grams of dry straw and a certain amount of IL were mixed in a 250 mL round-bottom flask. Then, the flask was placed in the microwave oven and installed according to Fig. 1. The pyrolysis reaction proceeded at a certain microwave irradiation power and the bio-oil was collected in a receiving flask by condensing the gas from the reactor. In order to reduce the pollution of the environment, the non-condensable gases were washed with acid-solution and basic-solution before being exhausted out. Four single factors pyrolysis conditions were as follows: temperature, mass ratio of IL to straw, time, and power (see details given later). Based on the results of single factor experiments, three factors and three levels orthogonal experiments were designed in Table 1. The quality of bio-oil in the receiving flask was measured (m,g) after pyrolysis reaction ended. If the initial amount of dry straw was m0 (g), the yields of bio-oil were calculated as follows:
(1)
Fourier transform infrared (FTIR) analysis was performed at 4 cm-1 resolution and a scanning range of 500 cm-1 to 4200 cm-1.
Fig. 1. Schematic layout of the pyrolysis experimental setup. 1) Microwave oven; 2) Reaction flask; 3) Receiving flask; 4) Gas-washing bottle
Recovery of Ionic Liquid
After each pyrolysis reaction, ILs were recovered by washing with distilled water several times. The residues were filtered to remove the solid residue. The filtrate was then heated to remove water by rotary evaporation at 80 °C and dried in a vacuum to obtain the recovered ionic liquid.
RESULTS AND DISCUSSION
Effect of Single Factor Pyrolysis on the Yield of Bio-Oil
Effect of pyrolytic temperature on the yield of bio-oil
The effect of the reaction temperature on the yield of bio-oil was studied under the following conditions: reaction time 20 min, mass ratio of IL to straw 2:1, and microwave irradiation power 600 W. The yield of bio-oil was obtained under different reaction temperatures. The bio-oil yield first increased and then decreased with an increase in the pyrolytic temperature (Fig. 2). The maximum yield was reached at 220 °C. The pyrolysis reaction is an endothermic process, so the yield of bio-oil was increased by raising the reaction temperature. From Fig. 2, it can be seen that the yield of the bio-oil decreased when the pyrolytic temperature was above 220 °C. This was because some of the compounds of bio-oil may undergo secondary pyrolysis reactions when the temperature is too high (Pattanotai et al. 2013). However, a lot of aerial fog was given off when the pyrolysis temperature was higher than 150 °C. The aerial fog may carry the IL together into the bio-oil. Also, IL tend to decompose at high temperatures. So a pyrolytic temperature of 150 °C was judged to be the best choice.
Fig. 2. Effect of pyrolytic temperature on the yield of bio-oil
Effect of the mass ratio of IL to straw on the yield of bio-oil
The reaction conditions that were used to test the influence of the mass ratio of IL to straw on bio-oil yield were as follows: reaction time 20 min, reaction temperature 150 °C, and microwave irradiation power 600 W (Fig. 3). In Fig. 3, it is apparent that the yield of bio-oil first increased and then decreased with an increase in the amount of IL while the amount of straw was not changed. The crystalline structure of straw fibers was destroyed with an increase in the amount of ionic liquid due to the increased solubility of straw. With the crystalline structure of straw fibers destroyed intensively, the pyrolysis reaction rate of straw accelerated and the bio-oil yield increased. Bio-oil yield reached a maximum when the mass ratio of IL to straw was 1.5:1. When the amount of IL was increased further, the bio-oil yield decreased. As previously stated, straw fibers can be degraded in IL when the solution is heated. Straw fibers may be degraded intensively in heavy IL to give more non-condensable gases. The greater quantity of non-condensable gases there are, the lower the yield will be of bio-oil.
Fig. 3. Effect of mass ratio of IL to straw on bio-oil yield
Effect of pyrolytic time on the yield of bio-oil
In order to reveal the influence of the pyrolysis time on the bio-oil yield, the following reaction conditions were used: reaction temperature 150 °C, IL to straw mass ratio 2:1, and microwave irradiation power 600 W (Fig. 4). In the given reaction conditions, the pyrolysis reaction was completed in 10 min; IL may be decomposed with a further increase in heating time. Therefore, the optimum reaction time was 10 min.
Fig. 4. Effect of pyrolytic time on the yield of bio-oil
Effect of microwave irradiation power on the yield of bio-oil
The following conditions were used to test the effect of microwave irradiation power on the bio-oil yield: reaction temperature 150 °C, reaction time 10 min, and IL to straw mass ratio 2:1. The results of the bio-oil yield changing with different microwave irradiation power are shown in Fig. 5. The yield of bio-oil increased with an increase in microwave irradiation power and reached the maximum at 700 W, which is the maximum power of the microwave oven. As is known, microwave cavitation intensifies with an increase in irradiation power. Chemical bonds are readily broken by powerful cavitation.
Fig. 5. Effect of microwave irradiation power on the yield of bio-oil
The Orthogonal Experiments on Pyrolysis of Straw under Microwave Irradiation in IL
To obtain the optimal pyrolysis conditions, orthogonal experiments were carried out based on Table 1. According to the range R analysis, the order of the influencing factors on the yield of bio-oil was determined to be as follows: pyrolytic temperature > mass ratio of IL to straw > pyrolytic time. From Table 1, the optimal pyrolysis conditions were A3B3C3: mass ratio of IL to straw 2:1, pyrolytic time 15 min, pyrolytic temperature 160 °C, and microwave irradiation power 700 W. The yield of bio-oil reached 28.2% in optimum conditions. The maximum yield of bio-oil was lower than that reported in other studies, for which the bio-oil yield ranged from 35% to 53% (Li et al. 2012; Putun et al. 2004;Du et al. 2010). But the temperatures used in their experiments (usually more than 450 °C) were higher than that of ours (160 °C). So the energy consumption in our work is smaller than that of their works, although the yield is not high. Because of the low energy consumed, rice straw pyrolysis in IL under microwave irradiation technology has better application prospects.
Table 1. The Orthogonal Experiments
A: IL/straw (g/g), B: temperature (℃), C: time (min)
Analysis of IR Spectrum of Bio-oil
The IR spectrum of bio-oil produced under optimum conditions is shown in Fig. 6. From Fig. 6, strong adsorption peaks at 3600 cm-1 to 2700 cm-1 indicate that the bio-oil contained hydroxyl compounds, such as alcohol, phenol, carboxylic acid, etc. Adsorption peaks at 1640 cm-1 to 1710 cm-1 correspond to C=O stretching vibrations, which shows that there are aldehyde, ketone, carboxylic acid, and carboxylic acid derivatives. Peaks at 1600 cm-1 to 1450 cm-1 and 900 cm-1 to 600 cm-1 are the characteristic adsorption peaks of the aromatic compounds. The main compositions of rice straw are cellulose, hemicelluloses, and lignin. When pyrolysis reactions occur, cellulose and hemicelluloses are decomposed into alcohols, carboxylic acids, aldehydes, or ketones, while lignin is converted to phenols or other aromatic compounds (Demirbas 2000).
As shown in Fig. 6, the bio-oil products contained oxygen-containing functional group compounds such as alcohols, phenols, aldehydes, ketones, carboxylic acids, etc. If these compounds are purified further, many very valuable or high value-added chemicals can be obtained, such as furfural. But, if bio-oil is used as fuel, the combustion value is much lower because of its high oxygen content. Many investigators in the biomass pyrolysis field are researching ways to improve the quality of bio-oil.
Fig. 6. IR spectra of bio-oil
Recovery of Ionic Liquids and Reuse
Ionic liquids were recovered after each pyrolysis reaction and can be reused in other pyrolysis reactions. The IR spectra of recycled IL were principally consistent with the original (Fig. 7), so the structure of recovered IL was not damaged during the pyrolysis process.
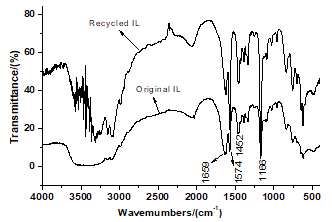
Fig. 7. IR spectra of original and recycled ILs
To examine the effect of recycled ionic liquid on the yield of bio-oil, straw pyrolysis reactions were repeated three times at the optimum conditions in recycled ionic liquid. The results of recovery percent and bio-oil yield are listed in Table 2. Table 2 shows that recovery percent of ionic liquid was about 84% to 87%. This may be caused by the carbonization or decomposition of ionic liquid or the adsorption by charcoal in the bottom of the reaction flask (Li et al. 2004). The yield of bio-oil did not change when the recycled ionic liquids were used.
Table 2. Recovery Percent of IL and the Effect of the Recycled IL on Bio-Oil Yield
CONCLUSIONS
The optimum conditions for microwave irradiation pyrolysis of straw in ionic liquid were as follows: pyrolytic temperature 160 °C, mass ratio of IL to straw 2:1, pyrolytic time 15 min, and microwave irradiation power 700 w. Under the above conditions, the yield of bio-oil was 28.2%. The recovery percent of ionic liquid was above 80%. The ionic liquid can be reused because the structure of the recycled ionic liquid is not damaged during processing. Bio-oil has a high oxygen content and needs to be refined before it is used for fuel.
ACKNOWLEDGMENTS
The authors are grateful for the financial support of the National Natural Science Foundation (21176195), Tianjin Key Laboratory of Pulp & Paper Fund (201104), and Tianjin University of Science & Technology Lab opening fund (1211A216).
REFERENCES CITED
Bridgwater, A. V., and Peacocke, G. V. C. (2000). “Fast pyrolysis processes for biomass,” Renewable Sustainable Energy Rev. 4(1), 1-73.
Burrell, A. K., Del Sesto, R. E., Baker, S. N., McCleskey, T. M., and Baker, G. A. (2007). “The large scale synthesis of pure imidazolium and pyrrolidinium ionic liquids,” Green Chem. 9 (5), 449–454.
Demirbas, A. (2000). “Mechanisms of liquefaction and pyrolysis reactions of biomass,” Energy Convers. Manage. 41, 633-646.
Dominguez, A., Menendez, J. A., Fernandez. Y., and Pis, J. J. (2007). “Evidence of self-gasification during the microwave-induced pyrolysis of coffee hulls,” Energy fuels 21, 373-378.
Du, J., Liu, P., Liu, Z. H., Sun, D. G., and Tao, C. Y. (2010). “Fast pyrolysis of biomass for bio-oil with ionic liquid and microwave irradiation,” J. Fuel Chem. & Technol . 38(5), 554-559.
Guo, S. R., Zhou, C. H., and Wang, Y. (2007). “Significantly enhanced reactivities of the nucleophilic substitution reactions induced by microwaves in ionic liquids,” Chem. Reagents29(10), 605-607.
Hahn-Hagerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Lidén, G., and Zacchi, G. (2006). “Bio-ethanol: The fuel of tomorrow from the residues of today,” Trends Biotechnol. 24(12), 549-556.
Huang, Y. F., Kuan, W. H., Lo, S. L., and Lin C. F. (2010). “Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis,” Bioresour. Technol. 101, 1968-1973.
Hyeon, S. H., Hyun, J. P., Park Y. K., Changkook, R., Dong, J. S., Young-Woong, S., Jin-Heong, Y., and Seung-Soo, K. (2010). “Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed,” Bioresource Technol. 101(1), S91-S96.
Jones, D. A., Lelyveld, T. P., Mavrofidis, S. D., Kingman, S. W., and Miles, N. J. (2002). “Microwave heating applications in environmental engineering–A review,” Resour. Conserv. Recy.34(2), 75-90.
Kilpeläinen, I., Xie, H. B., King, A., Granstrom, M., Heikkinen, S., and Argyropoulos, D. S. (2007). “Dissolution of wood in ionic liquids,” J. Agric. Food Chem. 55(22), 9142- 9148.
Lei, H. W., Ren, S.J., and Julson, J. (2009). “The effects of reaction temperature and time and particle size of corn stover on microwave pyrolysis,” Energy Fuels 23, 3254-3261.
Li, R., Zhong, Z. P., Jin, B. S., Jiang, X. X., Wang, C. H., and Zheng, A. J. (2012). “Influence of reaction conditions and red brick on fast pyrolysis of rice residue (husk and straw) in a spout-fluid bed,” Can. J. Chem. Eng. 90(5), 1202-1211.
Li, X. H., Zhang, L., Li, Q., Geng, W. G., Ye, Y. J., and Wang, L. F. (2004). “TGA-FTIR study of 1-n-butyl-3-methylimidazolium bromide ionic liquid,” Acta Phys. Chim. Sin. 20(12), 1465-1468.
Miura, M., Kaga, H., Sakurai, A., Kakuchi, T., and Takahashi, K. (2004). “Rapid pyrolysis of wood block by microwave heating,” J. Anal. Appl. Pyrolysis 71(1), 187-199.
Mullen, C. A., Boateng A. A., Goldberg, N. M., Liam, I. M., Laird, D. A., and Hicks, K. B. (2010). “Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis,” Biomass and Bioenergy 34(1), 67-74.
Olsson, L., and Hahn-Hagerdal, B. (1996). “Fermentation of lignocellulosic hydrolysates for ethanol production,” Enzyme Microbial Technol. 18(5), 312-331.
Pattanotai, T., Watanabe, H., and Okazaki, K. (2013). “Experimental investigation of intraparticle secondary reactions of tar during wood pyrolysis,” Fuel 104(2), 468-475.
Putun, A. E., Apaydin, E., and Putun, E. (2004). “Rice straw as a bio-oil source via pyrolysis and steam pyrolysis,” Energy (Oxford) 29(12), 2171-2180.
Swatloski, R. P., Spear, S. K., Holbrey, J. D., and Rogers, R. D. (2002). “Dissolution of cellulose with ionic liquids,” J. Am. Chem. Soc. 124(18), 4974-4975.Shi, S. L., and He, F. W. (2006). Analysis and Determination for Pulp and Paper, Beijing: China Light Industry Press, 22-50.
Wan, Y. Q., Chen, P., Zhang, B., Yang, C.Y., Liu, Y. H., Lin, X. Y., and Ruan, R. (2009). “Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity,” J. Anal. Appl. Pyrolysis 86(1), 161-167.
Wang, Q., Wang, S. R., Wang, L., Tan, H., Luo, C. Y., and Ling, K. F. (2007). “Experimental study of biomass flash pyrolysis for bio-oil production,” J. Eng. Thermophys. 28(1), 173-176.
Yagmur, E., Ozmak, M., and Aktas, Z. (2008). “A novel method for production of activated carbon from waste tea by chemical activation with microwave energy,” Fuel 87, 3278-3285.
Yang, S. W., and Qiu, K. Q. (2008). “Vacuum pyrolysis of Chinese fir sawdust for bio-oil production,” Chin. J. Process Eng. 8(6), 1143-1147.
Yu, F., Deng, S., Chen, P., Liu, Y., Wan, Y., Olson, A., Kittelson, D., and Ruan, R. (2007). “Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover,” Appl. Biochem. Biotechnol. 137-140(1-12), 957-970.
Yu, D. H., Wang, C. M., Yin, Y. Y., Zhang, A. J., Gao, G., and Fang X. X. (2011). “A synergistic effect of microwave irradiation and ionic liquids on enzyme-catalyzed biodiesel production,” Green Chem. 13(7), 1869-1875.
Article submitted: April 8, 2013; Peer review completed: May 8, 2013; Revised version received: May 30, 2013; Accepted: May 31, 2013; Published: June 6, 2013.
