Abstract
The structure of wood is so compact that enzymes are too large to penetrate into the structure and thereby attack the wood components for modifications that can be valuable for various purposes. Here we present a pretreatment method based on traditional kraft pulping, which opens the wood structure, so that enzymes are able to attack the wood components. To study this kind of chemical pretreatment, spruce wood samples were treated at similar conditions used in kraft cooking at varying intensities (H-factors). To verify if the structure was “opened” for enzymes, the pretreated wood samples were incubated with a cellulolytic culture filtrate, and the released reducing sugar concentration after the enzymatic hydrolysis was measured. The results indicated that un-pretreated wood fibers could not be attacked by the enzymes, but already relatively mild pretreatment was sufficient for letting the culture filtrate attack wood polysaccharides, and more intensive treatments opened the structure further. The mildest treatments did not cause any significant yield losses of lignin (Klason lignin). Some galactogluco-mannans were however lost during the pretreatments. The mechanisms behind the effect and the technical significance of the method are discussed.
Download PDF
Full Article
Mild alkaline treatment activates spruce wood for enzymatic processing: A possible stage in bio-refinery processes
Yan Wang,a Mikael E. Lindström,a and Gunnar Henriksson a,*
The structure of wood is so compact that enzymes are too large to penetrate into the structure and thereby attack the wood components for modifications that can be valuable for various purposes. Here we present a pretreatment method based on traditional kraft pulping, which opens the wood structure, so that enzymes are able to attack the wood components. To study this kind of chemical pretreatment, spruce wood samples were treated at similar conditions used in kraft cooking at varying intensities (H-factors). To verify if the structure was “opened” for enzymes, the pretreated wood samples were incubated with a cellulolytic culture filtrate, and the released reducing sugar concentration after the enzymatic hydrolysis was measured. The results indicated that un-pretreated wood fibers could not be attacked by the enzymes, but already relatively mild pretreatment was sufficient for letting the culture filtrate attack wood polysaccharides, and more intensive treatments opened the structure further. The mildest treatments did not cause any significant yield losses of lignin (Klason lignin). Some galactogluco-mannans were however lost during the pretreatments. The mechanisms behind the effect and the technical significance of the method are discussed.
Keywords: Wood structure; Kraft pulping; Enzymatic treatment; Lignin-polysaccharide networks; Bio-refinery
Contact information: a: Wallenberg Wood Science Centre (WWSC), Department of Fibre and Polymer, Royal Institute of Technology, KTH, Stockholm, 10044, Sweden
* Corresponding author: Phone: +46 8 790 6163; fax: +46 8 790 6166; E-mail: ghenrik@kth.se
INTRODUCTION
Today a large part of consumable materials, organic chemicals, and liquid fuels are made from petroleum (Conaway 1999). This is problematic from sustainability point of view, since petroleum is a non-renewable resource, which in time will need to be replaced. It is problematic also from an environmental point of view, since burning of fossil fuels and the waste that contains plastic and other materials made of petroleum raw materials will generate pollutants with negative effects. The most important of these is CO2, which is suggested to contribute to climate changes (Rodhe 1990). It is therefore of fundamental importance to find renewable raw material that can replace the petroleum. The plant cell wall represents here an interesting alternative, since it is a very abundant material, and contains polymeric and monomeric material with large potential for the manufacture of material, chemicals, and fuels (Lucia 2008); cellulose is a crystalline polysaccharide with very high degree of polymerization, that is useable directly for various types of materials from paper and boards to films with very high young modulus, and after derivatization or regeneration for a large range of material applications (Heinze and Petzold 2008); hemicelluloses are amorphous heteropolysaccharides with relatively moderate degree of polymerization, which have potential use, for instance, various types of films and barriers, and as raw material for chemicals (Spiridon and Popa 2008). The third main component in wood, lignin, is not a polysaccharide but a complex and partly random polymer of phenyl propanoid units connected together with ethers and carbon-carbon bonds. Lignin-based products have variety physicochemical properties and large number of applications (Doherty et al. 2011). In modified form (lignosulphonate) lignin is used as a dispersing agent in applications such as concrete and oil drilling (Grieson et al. 2005). Lignin can also be used as a binder (Westin et al. 2003), and as a raw material for vanillin (Tarabanko et al. 1995). Cell walls from woody plants, i.e., trees, do not directly compete with food production, which can be the case when ethanol is for fuel production comes from sugar canes and corn, leading to concerns about food availability and pricing (Rudaheranwa 2009). Trees have also the advantage that they are harvested all around the year, which minimizes the need for long-time storage.
One problem with wood is that it is difficult to directly extract different wood components in high yield. This might be due to the fact that lignin covalently crosslinks the different wood polymers (Lawoko et al. 2005), thereby creating an obstacle for extraction. One possible way to overcome such obstacles is to treat the wood with specific enzymes that can degrade the network by specific catalysis. Enzymes with suitable specificity for cellulose-, hemicellulose-, and lignin structures are produced by different types of molds and wood-degrading microorganisms (Rabinovich et al. 2002; Shallom and Shoham 2003; Ten Have and Teuningson 2001). Many enzymes of this type are today commercially produced in large quantities and applied in, for instance, the textile and food industries (Kirk et al. 2002). In fact, cellulose-degrading enzymes, the cellulases, may be the most used enzymes, based on volume. Principally, two kinds of commercial enzyme products exist – culture filtrates, that consist of a more or less complex mixture of enzymes that may have diverse specificity, and monocomponent products, that are produced by genetic modified microbial strains and contain one more or less totally pure component (Kirk et al. 2002). Due to their higher specificity, monocomponent products have an increased use. There is, however, a problem in enzymatic treatment of wood; the structure of the lignified tissue is so compact that large molecules such as enzymes cannot penetrate into the lignified cell wall (Blanchette et al. 1997). Therefore, direct enzyme treatment of wood is expected to have very limited effects, and some kind of pretreatment of wood is therefore necessary. One interesting technique involving such a pretreatment is related to kraft pulping, which is a well-established method for chemical pulping and is easy to use in large scale application. Kraft pulping is able to degrade lignin efficiently, but also the wood polysaccharides, especially the hemicelluloses, which are damaged in the process (Brännvall 2009). So, the alkaline pretreatment in this paper is similar to traditional kraft pulping but with milder conditions.
The purpose of this work is to determine whether this kind of pretreatment method, especially the mild alkaline treatment, is able to open up the compact wood structure sufficiently for enzymes to attack the wood (Pedersen et al 2010), and also to investigate the wood structure opening efficiencies with different pretreatment severities. Here, a commercial cellulolytic culture filtrate (Novozym 342) was selected since it is able to degrade most cell wall polysaccharides. Because this additive releases reducing sugars when it is active, the effects were easily detected by monitoring the level of sugars (Liu et al. 2009; Žnidaršič-Plazl et al. 2009).
EXPERIMENTAL
Materials
The wood chips were from Norway spruce (Picea abies), and supplied by Hallsta Pappersruk. The chips were air-dried and hand-sorted to remove chips with bark and knots, and also to remove chips with thicknesses more than 8 mm and less than 2 mm. The enzyme used was Novozym 342 (Novozymes, Denmark), which is a cellulolytic culture filtrate containing mainly endoglucanase, cellobiohydrolase, β-glucosidase), and also xylanase and other hemicellulases (Liu et al. 2009; Žnidaršič-Plazl et al. 2009). All other chemicals were of analytical grade.
Methods
Wood pretreatment
Wood chips were pretreated in a manner similar to traditional kraft pulping in a laboratory circulation digester. Samples of 1 kg (o.d. weight) wood chips were treated according to two different conditions, extended impregnation and kraft cooking, where liquor to wood ratios, temperature, incubation times, and chemical concentrations are summarized in Table 1. The circulation of the cooking liquor was started after steaming for 5 min. The temperature was increased at a rate of 1ºC/min. from 100 ºC until the desired cooking temperature was reached, after which it was held constant for the specified cooking times. H-factors were calculated according to the Arrhenius equation by combining time and temperature in a single expression. After this alkaline treatment, the wood chips were washed with deionized water with a 1.8 dm3/min flow for more than 12 hours to get rid of the alkaline residue. Disintegration was carried out on the pretreated wood chips with 50,000 revolutions in a disintegrator designed according to ISO 5263-1:2004. The portion of the samples in the disintegrator was approximately 30 g dry materials to 2 dm3 deionised water. The subsequent defibration was done at 2 bars water pressure in a Nordiska Armatur Fabriken (NAF) water-jet defibrator with 1.5 mm perforations.
Enzymatic treatment
Wet samples of 5 mg (o.d. weight) were suspended in 20 mM sodium phosphate buffer, pH 7 with 5 µL Novozym 342 (corresponding to the recommended dosage is 0.5 to 1.0 kg of Novozym 342 per ton of pulp, as stated in the enzyme information sheet, Novo Nordisk, 1996) at 40 °C for varying times in a final volume of 1 mL. The incubation was performed in a Thermo mixer comfort (from Eppendorf AB Stockholm Sweden) for 600 rpm shaking. The reaction was terminated by increasing the temperature to higher than 90 °C.
Analysis
Released reducing sugars were determined using the dinitrosalicylic acid (DNS) method (Miller 1959). The DNS reagent has a color reaction with reducing sugar that can be used for concentration determination. One mL of the DNS reagent solution (1% dinitrosalicylic acid, 1% NaOH, 10% NaK tartrate, 0.05% Na2SO3, and 0.2% phenol, all dissolved in water) was mixed with 1 mL of samples. The solution was centrifuged for three minutes at the highest speed of a table centrifuge machine, 14,500 rpm, and then 1 mL of supernatant was transferred to a new tube and boiled for 5 minutes. In parallel with these samples, glucose standards with known concentrations from 0.5 mM up to 4 mM were treated in an identical way. Samples were cooled down with ice water, and their absorbance values at 575 nm were recorded by a Cary 100 UV/VIS spectrophotometer (Varian, Palo Alto, CA, USA). Concentrations of released reducing sugars were calculated by comparison with the glucose standards.
The amounts of Klason lignin were determined according to TAPPI T222 om-83. Simultaneously the sugar contents in the acidic hydrolysate from the Klason lignin determination were analyzed by high performance anion exchange chromatography (HPAEC-PAD, Dionex ICS-3000).
Specific parameters description
H-factor (Vroom 1957), provides a method of expressing the effect of cooking time and temperature as a single variable on the extent of lignin removal during kraft pulping. Vroom assumed an activation energy of 134 kJ/mol, and the definition is:
(1)
Sulfidity, is a measure of the relationship between hydrogensulfide ion concen-tration and hydroxide ion concentration in a kraft cooking liquor and is often expressed as a percentage:
(2)
RESULTS AND DISCUSSION
Norway spruce (Picea abies) chips were treated with white liquor (solution of Na2S and NaOH) at different intensities. The mild alkaline treatment “extended impregnation” was done at 110 ºC for 40 minutes and 2h, and the more intensive treatment “pulping” was done at 150 ºC for 30 min. to 4 h (Table 1). The delignification degree was expressed as Klason lignin. For the extended impregnation, 110 °C 40 min. and 110 °C 2 h, the defibration steps were more difficult than in the case of the 150 °C pretreated samples. After the defibration the amounts of the rejects were much larger than the defibrillated materials. Therefore, when the enzymatic hydrolysis was done, both of the rejects and the defibrillated materials were tested.
Table 1. Conditions for Pretreatment of Wood
Since more sugars would be released if the o.d. weight of samples were larger, their absorbance values at 575 nm would not be stable in the UV/VIS spectrophotometer and dilution was needed, which would influence the results. So, 5 mg o.d. weight was chosen to investigate the ability of enzymes to attack the wood. The pretreated materials were first incubated for 2 h with a commercial cellulolytic culture filtrate (Novozym 342), which is able to degrade most cell wall polysaccharides. Released reducing sugar was measured to determine whether the enzymes could attack the polysaccharides or not. The reducing sugar analysis results are shown in Fig. 1.
Fig. 1. Released reducing sugar content after 2 h enzymatic hydrolysis.
* w: orignal wood, 11040’: the released matter from 110°C 40 min treated sample, 1102h’: released from 110 °C 2h treated sample. The descriptions for other names are given in Table 2.
It is clear from the figures that the enzymes were not able to create significant amounts of reducing sugar in the case of the untreated wood. However, after the pretreatment, even after just the extended impregnation, the enzymes could produce reducing sugars. This indicates that already a relative mild treatment opens up the tight structure of wood so that enzymes can penetrate and attack the wood components. An interesting parallel to the findings in this study is that relatively short pretreatment of wood chips by white rot fungi in a similar way allows protein to penetrate the cell wall, also here with insignificant yield losses (Blanchette et al. 1997). Based on these results it appears likely that steric factors limit the ability of enzymes to attack the native wood. The native wood structure is so compact that the enzymes cannot come in contact with the polysaccharides of wood (Blanchette et al. 1997). Already after just the extended impregnation, the structure seems to have been opened, so that enzymes were able to get in contact with the substrate. Interestingly, the opening effect was accomplished without any significant delignification (Table 1). In other words, the chemical pretreatment can lead to a swelling of wood without dissolution of lignin to any large degree. One explanation for this is that non-phenolic β-O-4 ethers in lignin are cleaved under alkaline conditions (Gierer 1980), and covalent bonds between lignin and polysaccharides may also be broken. Such reactions may partly degrade the lignin-carbohydrate networks in wood, and thereby allow enzymes to penetrate into the structure and get access to the polysaccharides. It is also likely that some hemicelluloses, mainly galactoglucomannan which has already been suffered primary peeling reaction at lower temperature (ca. 100 ºC), was degraded, and also there was significant dissolution of acetyl-galactogluco-mannans during the pretreatment. Such effects might also contribute to the opening of the wood structure for enzymatic attack (see sugar data in Table 2). After an extended impregnation process, the losses of Klason lignin were insignificant, but the contents of galactose and mannose were sharply decreased, which means that the dissolution and the peeling reaction of galactoglucomannan had occurred. The result of the peeling reaction is a depolymerization of the glucomannan. Thus, the compact lignin polysaccharide networks might be destroyed by this reaction. Therefore, the ability of lignin in wood to prevent enzymatic attack is most likely not a classical inhibiting effect (i.e. the inhibiting substances binding to and inactivating the enzyme), but rather related to the compact nature of the LCC (lignin-carbohydrate complex) networks.
Table 2. Data of Pretreated Wood
* The sugar content is the relative amount which makes the total sugar amounts as 100%.
To confirm this result, longer incubation times, 5 h, 24 h, and 48 h, were chosen to do the hydrolysis, and the obtained reducing sugar contents are compared in Fig. 2. With the extension of the incubation time, the reducing sugar contents were also increased, which means that more materials were hydrolyzed. At longer enzyme incubation times the pretreated materials produced much larger amounts of released sugars. More intensive pretreatment led to an improved ability for the enzymes to degrade the polysaccharides. This effect was stronger for the longer incubation times with enzymes. This may be because the accessible amounts of carbohydrates were larger for the more pretreated material. It is more crucial for the longer enzyme incubations, since in the beginning of the incubations the enzymes might be “saturated” with substrate; by contrast in the course of longer incubations the degradation rates decrease when the easily degradable carbohydrates, such as hemicelluloses and un-ordered cellulose structures, have already been consumed, and the amounts of accessible carbohydrates are most likely much larger in the more intensively treated materials. It is confirmed that the structure of the pretreated wood chips was opened to the enzyme, not only on the surface.
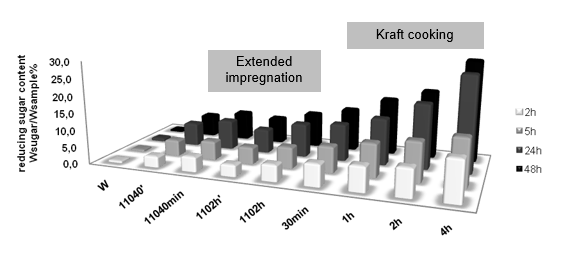
Fig. 2. Released reducing sugar content after 2h 5h, 24h, 48h enzymatic hydrolysis.
* w: orignal wood, 11040’: released from 110 °C 40 min treated sample, 1102h’: released from 110 °C 2h treated sample. The descriptions for other names are given in Table 2.
The dissolution of lignin does not proceed uniformly during kraft cooking. The middle lamella lignin requires a higher temperature in order to become dissolved because of the structural differences and/or to a lower accessibility of lignin (Saka et al. 1982). So the LCC networks of the pretreated fibres with extended impregnation (110 °C) were opened to a lesser degree than under the kraft cooking conditions (150 °C), no matter whether the time was longer or not. Here a parameter, H-factor, was introduced to connect the pretreated intensity with the enzyme effect. H-factor is the combination of temperature and time into a single variable that can represent the extent of this kind of pretreatment (shown in Table 2). The relationship between the pretreatment intensity (H-factor) and the efficiency of the enzymatic treatment intensity is shown in Fig. 3. It is clear from the figure that the structure opening efficiency was increased with the increasing of the pretreatment intensity. And also this kind of pretreatment was very efficient; even in cases where the pretreatment was mild, a clear effect of enzyme was observed.
Fig. 3. The relationship between the pretreatment intensities and the wood structure opening efficiencies
CONCLUSIONS
Intact spruce wood fibers could not be attacked to any significant degree by enzymes, which is in line with earlier studies by Blanchette et al. (1997). However, it was found that a relatively mild chemical treatment is sufficient to allow the enzymes to attack wood polysaccharides. Under the mild treatments the delignification was insignificant implying that the overall yield will remain high, especially lignin content. A more intensive treatment, where delignification was greater, gave more released reducing sugar when incubated with the culture filtrate. This is most likely due to exposure of more polysaccharides to the degrading enzymes, but such results do not necessary mean that the more intensive treatment is more beneficial for use of enzymes for degrading lignin polysaccharide networks in bio-refinery concepts.
Technical significance
This work indicates that a short pretreatment with white liquor is enough for opening up the wood structure for enzymatic attack. White liquor can easily be produced at a large scale for a low price in processes integrated in chemical recovery system of kraft mills. Therefore the possibilities to develop processes based on enzymatic treatment of wood biomass appear to be promising. A problem with this technique is, however, the relatively large losses of the hemicelluloses, mainly galactoglucomannan.
ACKNOWLEDGMENTS
This work was supported by Wallenberg Wood Science Centre (WWSC).
REFERENCES CITED
Blanchette, R. A., Kreuger, E. W., Haight, J. E., Akhtar, M., and Akin, D. E. (1997). “Cell wall alterations in loblolly pine wood decayed by the white rot fungus Ceriporiopsis subvermospora,” J. Biotechnol. V53(2-3), 203-213.
Brännvall, E. (2009). “Pulping technology,” In: Pulp and Paper Chemistry and Technology V2, Ek, M., Gellerstedt, G., and Henriksson, G. (eds.), De Gruyter, Berlin Germany ISBN 978-3-11-021341-6, pp. 121-147.
Conaway, C. F. (1999). The Petroleum Industry: A Nontechnical Guide, Penwell Publishing, Waltham Abbey, UK, ISBN 087814-763-2.
Doherty, O .S. M., Mousavioun, P., and Fellows, M. C. (2011). “Value-adding to cellulosic ethanol: Lignin polymers,” Industrial Crops and Products 33, 259-276.
Gierer, J. (1989). “Chemical aspects of kraft pulping,” Wood Sci. Technol. 14, 241-266.
Grierson, L. H., Knight, J. C., and Maharaj, R. (2005). “The role of calcium ions and lignosulphonate plasticiser in the hydration of cement,” Cem. Conc. Res. 35(4), 631-636.
Liu, H., Fu, S., Zhu, J. Y., Li, H., and Zhan, H. (2009). “Visualization of enzymatic hydrolysis of cellulose using AFM phase imaging,” Enzyme and Microbial Technology 45, 274-281.
Heinze, T., and Petzold, K. (2008). “Cellulose chemistry: Novel products and sythesis paths,” In: Monomers, Polymers and Composites from Renewable Resources, Belgacem, M. N., and Gandini, A. (eds.), Elsevier, Amsterdam, 343-368.
Kirk, O., Borchert, T. V., and Fuglsang, C. C. (2002). “Industrial enzyme applications,” Curr. Op. Biotechnol. 13, 345-351.
Lawoko, M., Henriksson, G., and Gellerstedt, G. (2006). “Characterisation of lignin carbohydrate complexes (LCCs) of spruce wood (Picea abies L.) isolated with two methods,” Holzforchung V60, 156-161.
Lucia, L. A. (2008). “Lignocellulosic biomass: Replace petroleum,” BioResources 3(4), 981- 982.
Miller, G. L. (1959). “Use of dinotrosalicylic acid reagents for determination of reducing sugars,” Anal. Chem. 31, 426-428.
Žnidaršič-Plazl, P., Rutar, V., and Ravnjak, D. (2009). “The effect of enzymatic treatments of pulps on fiber and paper properties,” Chem. Biochem. Eng. Q. 23(4), 497-506.
Pedersen, M., and Meyer, A. S. (2010). “Lignocellulose pretreatment severity- relating pH to biomatrix opening,” New Biotechnology V27 (6), 739-750.
Rabinovich, M. L., Melnick, M. S., and Bolobova, A. V. (2002). “The structure and mechanism of action of cellulolytic enzymes,” Biochem. Moscow V67 (8), 850-871.
Rodhe, H. (1990). “A comparison of the contribution of various gases to the greenhouse effect,” Science 248(4960), 1217-1219.
Rudaheranwa, N. (2009). “Biofuel subsidies and food prices in the context of WTO agreements,” Commonwealth Trade Hot Topics 63, 1- 5 ISSN 2071-9914.
Saka, S., Thomas, R. J., Gratzl, J. S., and Abson, D. (1982). “Topochemistry of delignification in Douglas-fir wood with soda, soda-anthraquinone and kraft pulping as determined by SEM-EDXA,” Wood Sci. Technol. 16, 139-153.
Shallom, D., and Shoham, Y. (2003). “Microbial hemicellulases,” Curr. Opinion Microbiol. 6, 219-228.
Spiridon, I., and Popa, V. I. (2008). “Hemicelluloses: Major sources, properties and applications,” In: Monomers, Polymers and Composites from Renewable Resources, Belgacem, M. N., and Gandini, A. (eds.), Elsevier, Amsterdam, 289-304.
Tarabanko, V. E., Fomova, N. A., Kunetsov, B. N., Ivanchenko, N. M., and Kudryashev, A.V. (1995). “On the mechanism of vannilin formation in the catalytic oxidation of lignin with oxygen,” React. Kinet. Catal. Lett. 55(1), 161-170.
Ten Have, R., and Teunissen, P. J. M. (2001). “Oxidative mechanisms involved in lignin degradation by white rot fungi,” Chem. Rev. 101, 3397-3413.
Vroom, K. E. (1957). “The H factor: A means of expressing cooking times and temperatures as a single variable,” Pulp Pap. Mag. Can. 58(3), 228-231.
Westin, M., Simonson, R., and Östman, B. (2001). “Kraft lignin wood fiberboards – The effect of kraft lignin addition to wood chips or board pulp prior to fiberboard production,” Holz als Roh- und Werkstoff 58, 393-400.
Article submitted: April 1, 2011; Peer review completed: April 28, 2011; Revised version received and accepted: May 5, 2011; Published: May 7, 2011.
