Abstract
Diverse cell wall compositions were subjected to pretreatment and saccharification to produce bioethanol from 20 Erianthus arundinaceus accessions. Using four typical pairs of biomass samples, various physical and chemical pretreatments were employed to extract cell wall polymers. Mild chemical pretreatment (2% NaOH and 50 °C) yielded complete biomass saccharification, whereas the liquid hot water pretreatment achieved the highest bioethanol yield with a full sugar-ethanol conversion rate. Notably, the extraction of the lignin p-coumaryl alcohol (H) monomer greatly enhanced biomass saccharification, which may be attributed either to the improved accessibility of cellulose to enzymes after effective removal of lignin or to the maintained native cellulose microfibrils from the relatively less co-extraction of hemicellulose. Hence, the results suggested that the H-monomer-rich lignin may slightly associate with cell wall networks for greatly enhanced lignocellulose enzymatic hydrolysis after mild pretreatments. The present findings provide a strategy for both cost-effective biomass process technology and precise lignocellulose modification for bioenergy.
Download PDF
Full Article
Mild Physical and Chemical Pretreatments to Enhance Biomass Enzymatic Saccharification and Bioethanol Production from Erianthus arundinaceus
Ao Li,a,b Qiaomei Yang,a,b Yu Li,a,c Shiguang Zhou,a,b Jiangfeng Huang,a,b Meng Hu,a,b Yuanyuan Tu,a,b Bo Hao,a,d Liangcai Peng,a,b and Tao Xia a,d,*
Diverse cell wall compositions were subjected to pretreatment and saccharification to produce bioethanol from 20 Erianthus arundinaceus accessions. Using four typical pairs of biomass samples, various physical and chemical pretreatments were employed to extract cell wall polymers. Mild chemical pretreatment (2% NaOH and 50 °C) yielded complete biomass saccharification, whereas the liquid hot water pretreatment achieved the highest bioethanol yield with a full sugar-ethanol conversion rate. Notably, the extraction of the lignin p-coumaryl alcohol (H) monomer greatly enhanced biomass saccharification, which may be attributed either to the improved accessibility of cellulose to enzymes after effective removal of lignin or to the maintained native cellulose microfibrils from the relatively less co-extraction of hemicellulose. Hence, the results suggested that the H-monomer-rich lignin may slightly associate with cell wall networks for greatly enhanced lignocellulose enzymatic hydrolysis after mild pretreatments. The present findings provide a strategy for both cost-effective biomass process technology and precise lignocellulose modification for bioenergy.
Keywords: Cell wall polymers; Lignin; p-Coumarin; Alcohol monomer; Physical and chemical pretreatments; Biomass saccharification; Polymer features
Contact information: a: Biomass and Bioenergy Research Centre, Huazhong Agricultural University, Wuhan 430070, China; b: College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; c: Guangdong Provincial Bioengineering Institute and Guangzhou Sugarcane Industry Research Institute, Guangzhou 510316, China; d: College of Life Science and Technology, Huazhong Agricultural University, Wuhan 430070, China;
*Corresponding author: xiatao@mail.hzau.edu.cn
INTRODUCTION
Because of the foreseeable depletion of fossil fuels, biomass feedstocks, which can be converted into fuels via bioconversion, are considered to be a partial alternative for renewable fuels (Alvira et al. 2010; Limayem and Ricke 2012; Chen et al. 2015; Zhu et al. 2015b; Rebaque et al. 2017). Erianthus arundinaceus is a typical highly photosynthetic-efficient C4 plant that belongs to Andropogoneae, which is a tribe of the grass family, Gramineae (Amalraj and Balasundaram 2006). Despite E. arundinaceus being a wild relative of the genus Saccharum, it has attracted the interest of sugarcane breeders worldwide for its valuable agronomic traits used in sugarcane genetic improvement, such as high biomass production, ratoonability, vigor, and resistance to biotic and abiotic stresses (D’Hont et al. 1995; Rott et al. 1997; Piperidis et al. 2000; Ram et al. 2001; Cai et al. 2005; Amalraj and Balasundaram 2006; Li et al. 2018). In general, E. arundinaceus can produce biomass yields that range from 4000 Mg/km2yr to 6000 Mg/km2yr (Mislevy et al. 1997; Hattori and Morita 2010), which is higher than that of other bioenergy plants, e.g., 1200 Mg/km2yr to 4000 Mg/km2yr for Miscanthus and 700 Mg/km2yr to 3500 Mg/km2yr for switchgrass (Hattori and Morita 2010). Therefore, E. arundinaceus is regarded as a potentially desirous biofuel and industrial feedstock. However, little has been reported about the processing of this biomass into biofuels, as well as the biofuel productivity of E. arundinaceus.
Generally, the lignocellulose-based process to obtain valuable liquid components, including fuels, involves three major steps: physical and chemical pretreatments for cell wall polymer disassociation, enzymatic degradation for soluble sugar release, and yeast fermentation for ethanol production (Caspeta et al. 2014; Zhu et al. 2015a). However, biomass conversion is currently a costly process because of lignocellulosic recalcitrance (Wang et al. 2016). Therefore, it is essential to select the desired E. arundinaceus accession used for breeding and to identify the key factors of plant cell walls that determine biomass enzymatic saccharification and bioethanol production after various physical and chemical pretreatments.
Plant cell walls are mainly composed of cellulose, hemicellulose, and lignin. Cellulose is a linear polymer composed of β-1,4-glucans (Wang et al. 2016) and its crystallinity index (CrI) and degree of polymerization (DP) are the major properties that negatively affect biomass enzymatic digestion in plants (Zhang et al. 2013; Huang et al. 2015; Wang et al. 2016). Xylans are major components of hemicelluloses in grass plants (Scheller and Ulvskov 2010). Although the arabinose substitution degree of xylans negatively affects the cellulose crystallinity for high biomass enzymatic digestibility in Miscanthus (Xu et al. 2012; Li et al. 2013a), the impacts of hemicellulose on the biomass digestibility vary depending upon various chemical pretreatments of wheat, corn, and sweet sorghum (Wu et al. 2013; Jia et al. 2014; Li et al. 2014a). Lignin is a stable and complex polymer that is biosynthesized from three major phenolic units, which are p-coumaryl (H), coniferyl (G), and sinapyl (S) alcohols (Ralph et al. 2004; Sun et al. 2013). Recently, it has been found that lignin plays dual roles in biomass enzymatic digestion, which is probably because the three monolignol concentrations are distinct in different plant species (Boudet et al. 2003; Ziebell et al. 2010; Xu et al. 2012; Wu et al. 2013; Azelee et al. 2014; Jia et al. 2014; Li et al. 2014a,b,c, 2015; Si et al. 2015; Jin et al. 2016). However, little is known about the effects of plant cell wall polymers on biomass digestibility in E. arundinaceus.
As the initial step for biomass saccharification, various pretreatments are extensively performed on biomass samples using chemicals (acids and alkali) and liquid hot water (Yu et al. 2013; Li et al. 2013b; Jiang et al. 2015; Chen et al. 2016; Jin et al. 2016). In principle, alkali pretreatment under a mild temperature mainly extracts lignin, whereas acid pretreatment leads to the release of hemicelluloses. In comparison, pretreatment with liquid hot water is a relatively inexpensive process with less secondary contamination (Robinson et al. 2015). Despite E. arundinaceus being considered a desired bioenergy crop, few reports have studied its optimum pretreatment for high biomass saccharification and bioethanol production. In this study, a total of 20 representative E. arundinaceus accessions that displayed a diverse cell wall composition and biomass digestibility were initially determined. Using four typical pairs of E. arundinaceus samples, this study further examined the cell wall polymer features and compared the biomass saccharification and bioethanol productivity with different pretreatments. Finally, this study interpreted the predominant role of lignin H-monomer in biomass enzymatic saccharification by efficient cell wall polymer extraction with mild physical and chemical pretreatments.
EXPERIMENTAL
Materials
A total of 20 E. arundinaceus accessions were collected from Guangdong, China. The stem and leaf (sheath included) tissues of mature straw were separately harvested, dried at 50 °C, and ground through a 40-mesh screen. The well-mixed powders were stored in a sealed dry container until use.
Methods
Cell wall polymer extraction and determination
The plant cell wall fractionation method was used to extract cellulose and hemicelluloses, as was previously described by Peng et al. (2000) and Jin et al. (2016). The cellulose was measured by a hexoses assay, and the hemicelluloses were calculated by determining the total hexoses and pentoses in the hemicellulose fraction. All of the experiments were performed in biological triplicate. The hexoses and pentoses in the biomass residues were determined with an ultraviolet-visible spectrometer (V-1100D, Shanghai MAPADA Instruments Co., Ltd., Shanghai, China) using glucose and xylose as the standard curves, which was previously described by Peng et al. (2000) and Jin et al. (2016). Because a high pentoses content interferes with absorbance reading at 620 nm for the hexoses assay, the pentose reading at 660 nm was deducted for the final hexoses calculation, which was verified by gas chromatography/mass spectroscopy analysis. All of the samples were detected in biological triplicate.
Total lignin and monolignol detection
The total lignin content was measured by the two-step acid hydrolysis method, according to Sluiter et al. (2008) with the minor modifications described by Wu et al. (2013). All of the samples were analyzed in biological triplicate. Three monolignols were detected by high performance liquid chromatography according to the method described by Li et al. (2014b).
Cellulose crystallinity detection
The cellulose crystallinity was determined by detecting the biomass CrI using the X-ray diffraction method, as described by Zhang et al. (2013) and Li et al. (2015). The standard error of the CrI method was within 0.05 to 0.15 (n = 5).
Biomass pretreatments
For the NaOH pretreatment, the well-mixed biomass samples were treated with 6 mL of NaOH at various concentrations (1%, 2%, 4%, and 8%) for 2 h at 50 °C. The pellets were washed with 10 mL of distilled water approximately 5 to 6 times until a pH of 7.0 was reached. A biomass sample was added to only 6 mL of distilled water under shaking for 2 h at 50 °C as an experimental control.
For the H2SO4 pretreatment, the well-mixed biomass samples were treated with 6 mL of H2SO4 at various concentrations (1%, 2%, 4%, and 8%). The sealed sample tubes were heated at 121 °C for 20 min in an autoclave (103 kPa), and then shaken at 150 rpm and 50 °C for 2 h. A biomass sample was added to only 6 mL of distilled water under shaking for 2 h at 50 °C as an experimental control.
For the liquid hot water pretreatment, biomass samples well-mixed with 2.4 mL of distilled water were added into well sealed stainless-steel bombs and heated at 200 °C under shaking at 150 rpm for 8 min, 16 min, 32 min, and 64 min. The sealed bombs were cooled down immediately and centrifuged at 3000g for 5 min.
The supernatants of each pretreatment were combined for determination of the pentoses and hexoses and the remaining pellets were used for enzymatic hydrolysis as described below. All of the samples were analyzed in biological triplicate.
Detection of the enzymatic hydrolysis
The remaining residues from the chemical (acid and alkali) and liquid hot water pretreatments were washed approximately 5 to 6 times with 10 mL of distilled water until the supernatants reached a pH of 7.0 and then once more with 10 mL of mixed cellulase reaction buffer (200 mol/ mL acetic acid–sodium acetate and pH = 4.8). The washed residues were incubated with 6 mL (2.0 g/L) of mixed-cellulases containing β-glucanase (≥ 3.73 × 104 U), cellulase (≥ 373 U), and xylanase (≥ 6 × 104 U) purchased from Imperial Jade Bio-technology Co., Ltd (Ningxia, China) and shaken at 150 rpm and 50 °C for 48 h. All of the supernatants collected after enzymatic hydrolysis were used for determination of the total pentoses and hexoses. Samples added to only 6 mL of reaction buffer were used as the experimental control. All of the experiments were conducted in biological triplicate.
Yeast fermentation and ethanol measurement
The yeast fermentation procedure used was described by Jin et al. (2016). Saccharomyces cerevisiae (Angel yeast Co., Ltd., Binzhou, China) was used in the fermentation process at 37 °C for 48 h. Ethanol was measured using the K2Cr2O7 method, which was previously described by Li et al. (2014a). All of the experiments were performed in biological triplicate.
Statistical calculation of the correlation coefficients
Correlation coefficients were calculated based on the spearman rank correlation analysis for all of the measured parameters for the eight samples of E. arundinaceus with three different pretreatments (Xu et al. 2012; Li et al. 2013a). All of the analyses used the average values calculated from the original determination values.
RESULTS AND DISCUSSION
Diversity of the Cell Wall Composition and Biomass Saccharification in the E. arundinaceus Accessions
In this study, the cell wall compositions (cellulose, hemicelluloses, and lignin) were determined in the stem and leaf tissues for a total of 20 representative E. arundinaceus accession samples (Fig. 1). In the stem tissues, the E. arundinaceus samples exhibited various cellulose, hemicellulose, and lignin levels from 31.9% to 42.5%, 21.4% to 27.0%, and 18.0% to 23.1%, respectively (Fig. 1a; Table S1). In comparison, the E. arundinaceus samples had relatively low variations in the cell wall compositions in the leaf tissues, including cellulose, hemicellulose, and lignin contents that ranged from 28.3% to 36.4%, 23.2% to 26.6%, and 19.2% to 22.3%, respectively. Additionally, the E. arundinaceus samples showed a relatively high average cellulose level (36%) in the stem tissue and high hemicellulose content (25%) in the leaf tissue with similar lignin levels (21%) in both the stem and leaf tissues.
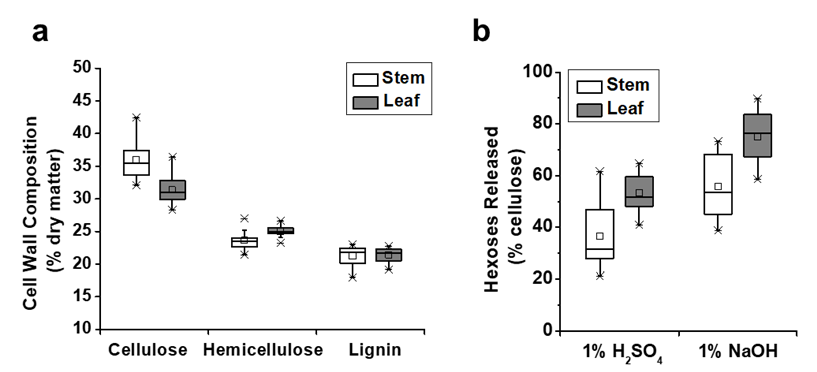
Fig. 1. Diverse cell wall compositions and biomass saccharification in the stem and leaf tissues of 20 E. arundinaceus samples (Table S1): (a) variations in the cellulose, hemicellulose, and lignin contents (n = 20); and (b) variations in the hexoses yields released during enzymatic hydrolysis after the 1% NaOH and 1% H2SO4 pretreatments (n = 20)
The biomass enzymatic digestibility (saccharification) is determined by calculating the hexoses yields (% cellulose) released during lignocellulose enzymatic hydrolysis after various pretreatments or total sugars yields (hexoses and pentoses; % dry matter) released during both enzymatic hydrolysis and pretreatment (Wu et al. 2013). In the present study, the hexoses yields were determined in the stem and leaf tissues of 20 E. arundinaceus samples after pretreatments with 1% H2SO4 and 1% NaOH. The E. arundinaceus samples exhibited large variations in the hexoses yields (% cellulose) that ranged from 21% to 73% in the stems and 41% to 90% in the leaf tissues (Fig. 1b; Table S1). The results also indicated that the leaf tissues released higher average hexoses yields (53% and 75%) than the stem tissues did (37% and 56%) after the 1% H2SO4 and 1% NaOH pretreatments. Additionally, the pretreatment with 1% NaOH led to higher hexoses yields in both the stem and leaf tissues compared with the 1% H2SO4 pretreatment. Hence, the results indicated that diverse cell wall compositions of the 20 E. arundinaceus samples could lead to greatly different biomass enzymatic saccharification processes, which was consistent with previous reports for Miscanthus, corn, and sweet sorghum (Xu et al. 2012; Jia et al. 2014; Li et al. 2014a). The results also suggested that lignocellulose features may have largely affected biomass saccharification for the E. arundinaceus samples.
High Biomass Digestibility after Mild Physical and Chemical Pretreatments
Among the 20 E. arundinaceus accessions examined above, four typical pairs of E. arundinaceus samples were selected based on the distinct cell wall compositions of each of the pairs (Table S2), including stem (Pairs I and II) and leaf tissues (Pairs III and IV) (Fig. 2; Table S3), In Pairs III and IV, the EaL40 and EaL33 leaf samples exhibited almost complete biomass enzymatic digestion with hexoses yields close to 100% (% cellulose) after the 2% NaOH pretreatment, whereas their paired samples (EaL29 and EaL30) had the highest hexoses yields after the 4% NaOH pretreatment (Table S3; Fig. 2a). However, the EaS19 and EaS40 stem samples in Pairs I and II showed almost complete biomass
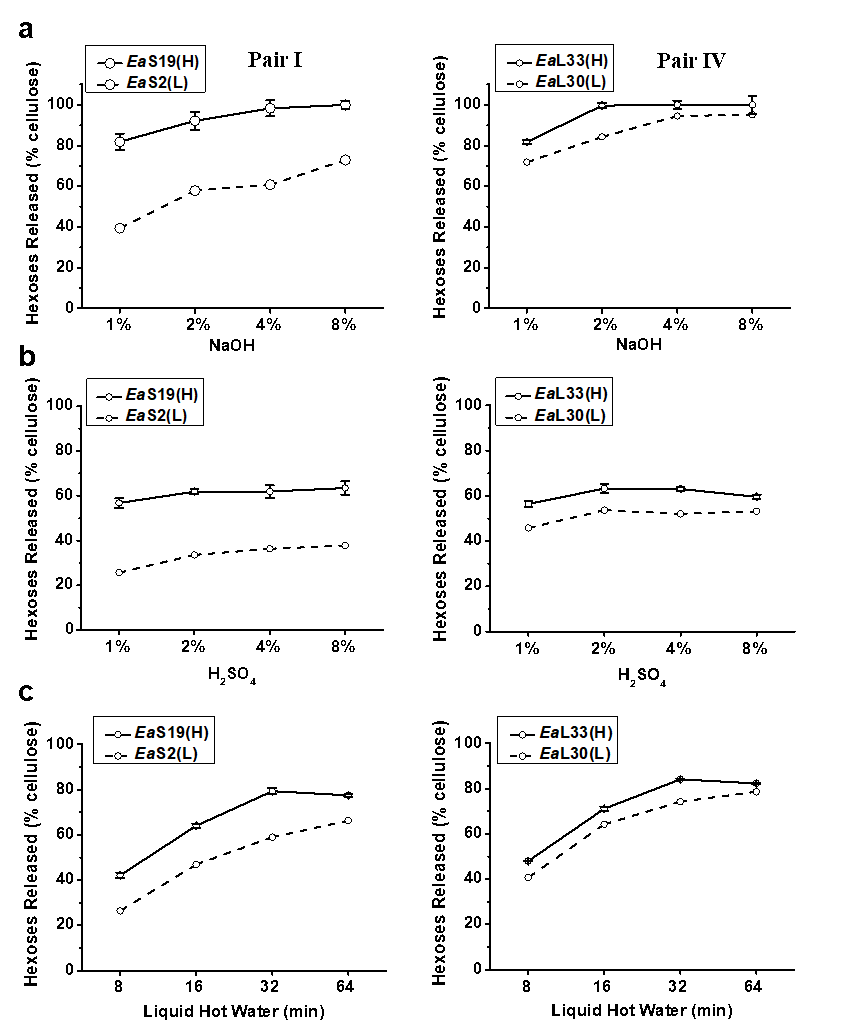
Fig. 2. Biomass saccharification after three pretreatments for two typical pairs of E. arundinaceus samples (Table S3); hexoses yields released during enzymatic hydrolysis after (a) pretreatment with NaOH at four concentrations; (b) pretreatment with H2SO4 at four concentrations; and (c) pretreatment with liquid hot water at 200 °C four times; all of the data is the mean ± the standard deviation (SD) (n = 3)
digestion after the 4% and 8% NaOH pretreatments, but their paired EaS2 and EaS3 samples had the highest hexoses yields at 73% and 88% after the 8% NaOH pretreatment, respectively, which confirmed that leaf tissues were more effective for biomass saccharification. In comparison, the biomass samples had hexoses yields of up to 63% after the pretreatments with four concentrations of H2SO4 (Table S3; Fig. 2b). However, the liquid hot water pretreatment (200 °C and 32 min) could have led to the highest hexoses yields, which were up to 80% in the EaS19 and EaL33 samples (Table S3; Fig. 2c). A prolonged time (64 min) for the liquid hot water pretreatments did not enhance the hexoses yields for the most of the examined samples. Hence, two samples of each pair underwent different biomass enzymatic saccharification processes, probably because of their distinct cell wall compositions and polymer features. Additionally, the pretreatments with NaOH and liquid hot water were more effective for biomass enzymatic digestion of the E. arundinaceus samples than the H2SO4 pretreatment (Fig. 2).
Bioethanol Productivity with the Liquid Hot Water Pretreatment
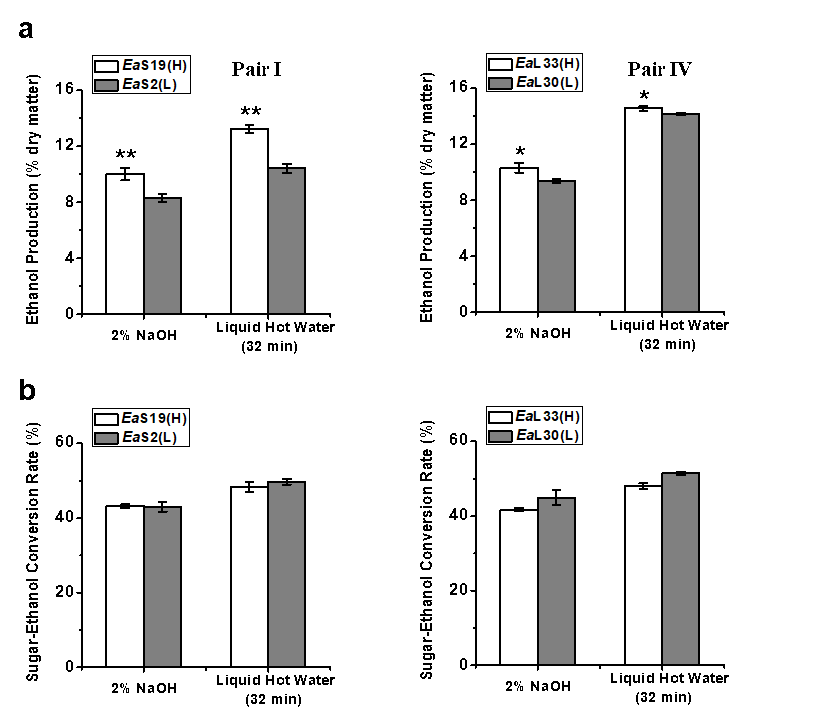
Fig. 3. Bioethanol productivity released during yeast fermentation using sugars obtained from the pretreatments and enzymatic hydrolysis for two pairs of E. arundinaceus samples: (a) ethanol yields (% dry matter) and (b) sugar-ethanol conversion rate (%); all of the data is the mean ± the SD (n = 3); * and ** significant differences between two samples of each pair for p < 0.05 and 0.01 Based on the relatively high biomass saccharification after the pretreatments with 2% NaOH and liquid hot water for 32 min, yeast fermentation experiments for bioethanol production were performed using Pairs I (stem) and IV (leaf) of the E. arundinaceus samples (Fig. 3; Table S4). In general, the two pairs had a relatively higher ethanol production after the liquid hot water pretreatment than after the 2% NaOH pretreatment (Fig. 3a), and had higher sugar-ethanol conversion rates after the liquid hot water pretreatment (Fig. 3b). The sugar-ethanol conversion rates reached 48% to 51% after the liquid hot water pretreatment, but only reached 42% to 45% after the 2% NaOH pretreatment (Table S4). Furthermore, although the two samples of Pair IV showed different hexoses yields after the liquid hot water pretreatment, they both had higher ethanol productions of up to 14% to 15% (% dry matter). Hence, the results indicated that the liquid hot water pretreatment (200 °C and 32 min) could lead to a highly efficient sugar-ethanol conversion of up to 51%, which is close to the theoretical conversion rate. The results also suggested that the liquid hot water pretreatment may produce fewer compounds that inhibit yeast fermentation for bioethanol production compared with the 2% NaOH pretreatment.
Distinct Impacts of the Lignin and Hemicelluloses Extractions on the Biomass Saccharification
It has been determined that cell wall polymer extraction with various physical and chemical pretreatments could distinctively affect the enzymatic digestibility of grass plants (Si et al. 2015; Jin et al. 2016). In this study, the extracted lignin and hemicellulose levels from three optimal pretreatments of four pairs of E. arundinaceus samples were determined (Table S5). In comparison, the 2% NaOH pretreatment extracted 34% to 43% hemicelluloses in the four-pair samples, whereas both the 2% H2SO4 and liquid hot water (200 °C and 32 min) pretreatments led to 80% to 88% hemicelluloses removal, which was consistent with previous reports on distinctive hemicellulose extraction with alkali and acid pretreatments (Hendriks and Zeeman 2009; Si et al. 2015). Despite the fact that the 2% H2SO4 pretreatment could extract 36% to 44% lignin, the 2% NaOH pretreatment had a slightly higher lignin extraction of 49% to 57%, which was consistent with previous reports that alkali pretreatment is favorable for high lignin extraction from grasses (Hendriks and Zeeman 2009; Si et al. 2015). Notably, it was found that the liquid hot water pretreatment also led to a relatively high and large range of lignin extraction of 43% to 57%. The data suggested that the three pretreatments are distinctive for hemicelluloses and lignin extraction from the E. arundinaceus samples.
Furthermore, a correlation analysis was performed between the cell wall polymer extraction and biomass saccharification (Fig. 4). Significantly, the extracted lignin amounts positively correlated with the hexoses yields released from enzymatic hydrolysis after the three pretreatments at a p less than 0.01 (n = 24), whereas the remaining lignin levels showed a negative correlation at a p less than 0.01 (Figs. 4a and 4b). In comparison, the remaining hemicellulose levels in the pretreated biomass residues positively correlated with the hexoses yields, which was consistent with previous reports of the positive impact of hemicelluloses on biomass saccharification with Miscanthus and rice (Xu et al. 2012; Li et al. 2015). However, despite the fact that the hemicellulose level showed a positive correlation with the hexoses yield, the R2 value remained low, and the extracted hemicellulose amounts did not show any significant correlation (Figs. 4c and 4d). Hence, the results indicated that only lignin extraction had a positive effect on biomass saccharification with E. arundinaceus samples after various pretreatments.
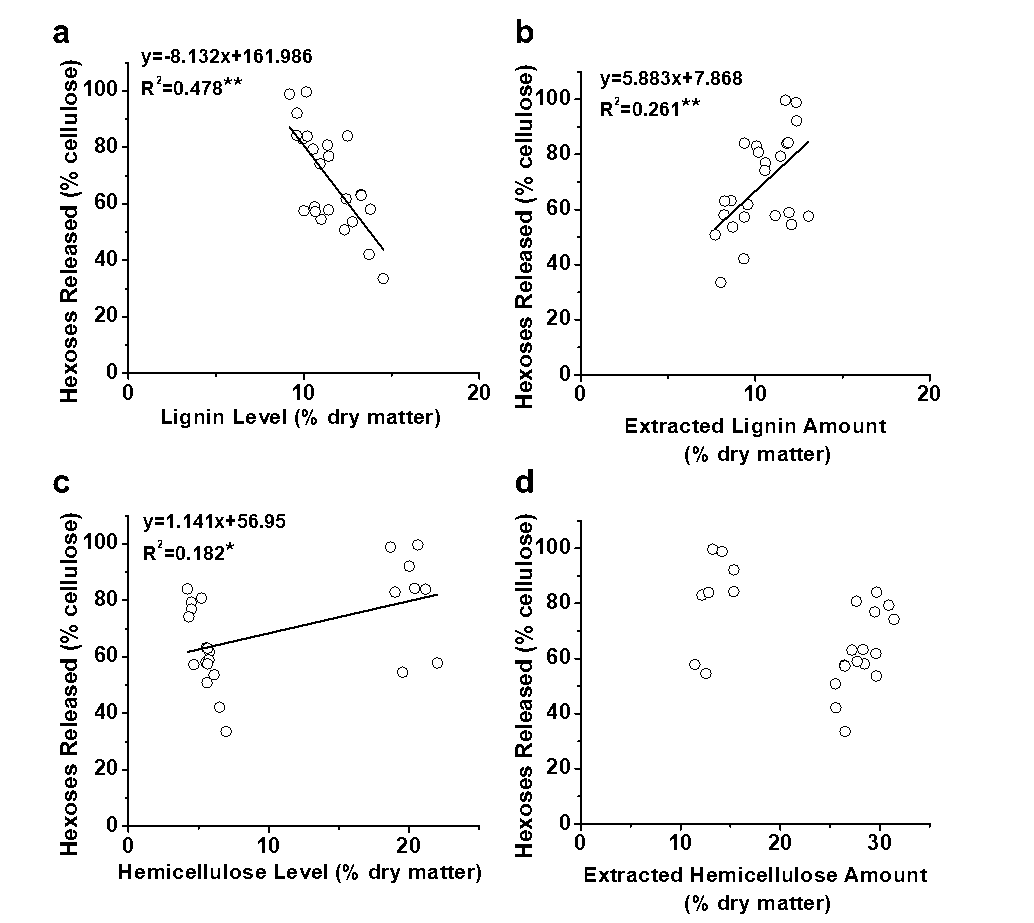
Fig. 4. Correlation analysis between the hexoses yields and lignin extractions from the three pretreatments (a) with lignin levels (% dry matter) of the pretreated biomass residues; (b) with the extracted lignin amounts (% dry matter) from three pretreatments; (c) with hemicellulose levels (% dry matter) of the pretreated biomass residues; and (d) with the extracted hemicellulose amounts (% dry matter) from three pretreatments; * and ** significant correlations for p < 0.05 and 0.01 (n = 24)
Predominant Impact of H-monomer Extraction on the Biomass Digestibility
Because of the positive effect of lignin extraction on the biomass digestibility, a correlation analysis was performed between the hexoses yields and monomer (H, G, and S) levels extracted after the three pretreatments of the four pairs of E. arundinaceus samples. Only the extracted amounts of H-monomer exhibited a positive correlation with the hexoses yields released during enzymatic hydrolysis, with a p less than 0.01 (n = 24) and high R2 value of 0.692, whereas the extracted G- and S-monomers did not show any significant correlation (Fig. 5a). Furthermore, it was found that the extracted rates of the H-monomer, rather than the G- and S-monomers, also positively correlated with the hexoses yields, with a p less than 0.01 and high R2 value of 0.657 (Fig. 5b). Thus, the data indicated that the H-monomer extraction predominately determined the biomass enzymatic digestibility with the E. arundinaceus samples after various pretreatments.
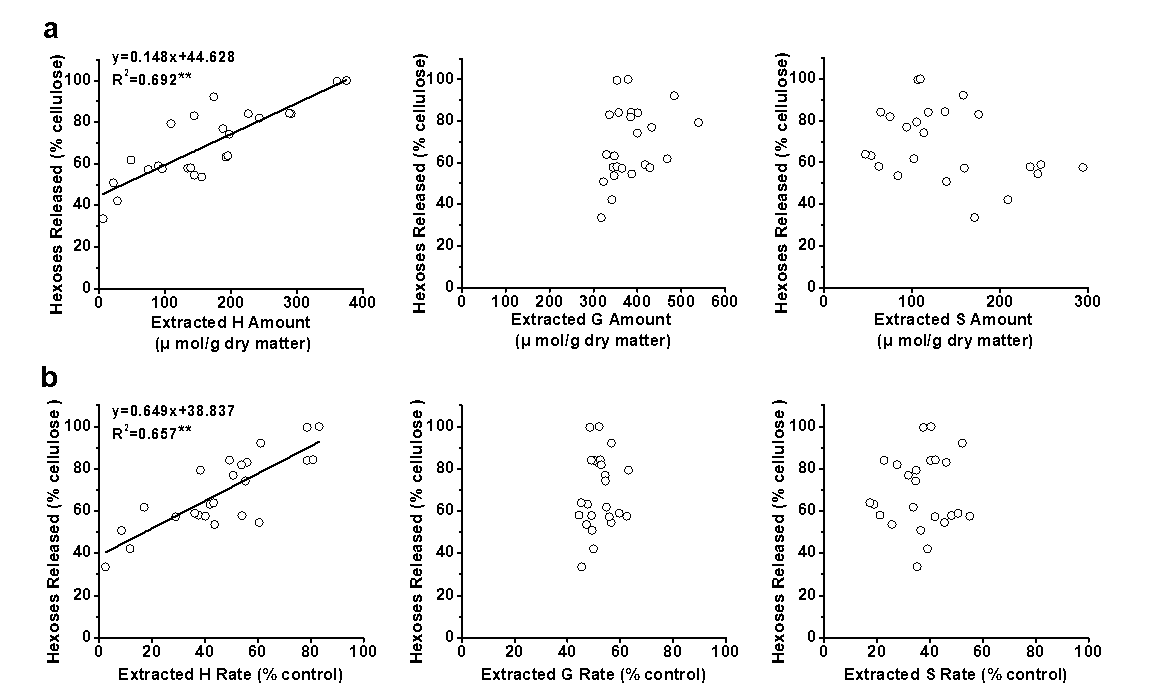
Fig. 5. Correlation analysis between the hexoses yields and three monolignol extractions from three pretreatments (a) with the extracted amounts of the three monolignols and (b) with the extracted rates of the three monolignols; ** significant correlation for p < 0.01 (n = 24)
Mechanism of the Efficient H-monomer Extraction for High Biomass Enzymatic Digestibility
Because plant cell walls are of dynamic networks, correlation analysis has been performed for proposing the mechanism models that highlight wall polymer extraction impacts on biomass enzymatic saccharification (Zhang et al. 2013; Wang et al. 2016; Hu et al. 2018). To understand why the H-monomer extraction could lead to high biomass saccharification, the cellulose CrI values of the biomass residues obtained after the three pretreatments of the four pairs of E. arundinaceus samples were determined (Table S6). Significantly, the CrI values of the pretreated biomass residues exhibited a negative correlation with the hexoses yields from enzymatic hydrolysis at a p less than 0.05 (n = 24), but the R2 value remained relatively low, probably due to abnormal sample distribution (Fig. 6a). Because the cellulose crystallinity is affected by the cell wall polymers through their associations via hydrogen bonds, a correlation analysis of the cellulose CrI values and cell wall polymer levels in the biomass residues obtained from the three pretreatments was performed. The hemicellulose levels of the pretreated biomass residues negatively correlated with the cellulose CrI at a p less than 0.05 (Fig. 6b), which explained why hemicellulose positively affected the hexoses yields (Fig. 4c). Its association with cellulose via hydrogen bonds likely reduced the cellulose crystallinity. In contrast, the extracted hemicellulose amounts showed a positive correlation with the increased cellulose CrI values, which confirmed that hemicellulose had a negative impact on the cellulose crystallinity of the E. arundinaceus samples (Fig. 6b). Furthermore, despite the fact that
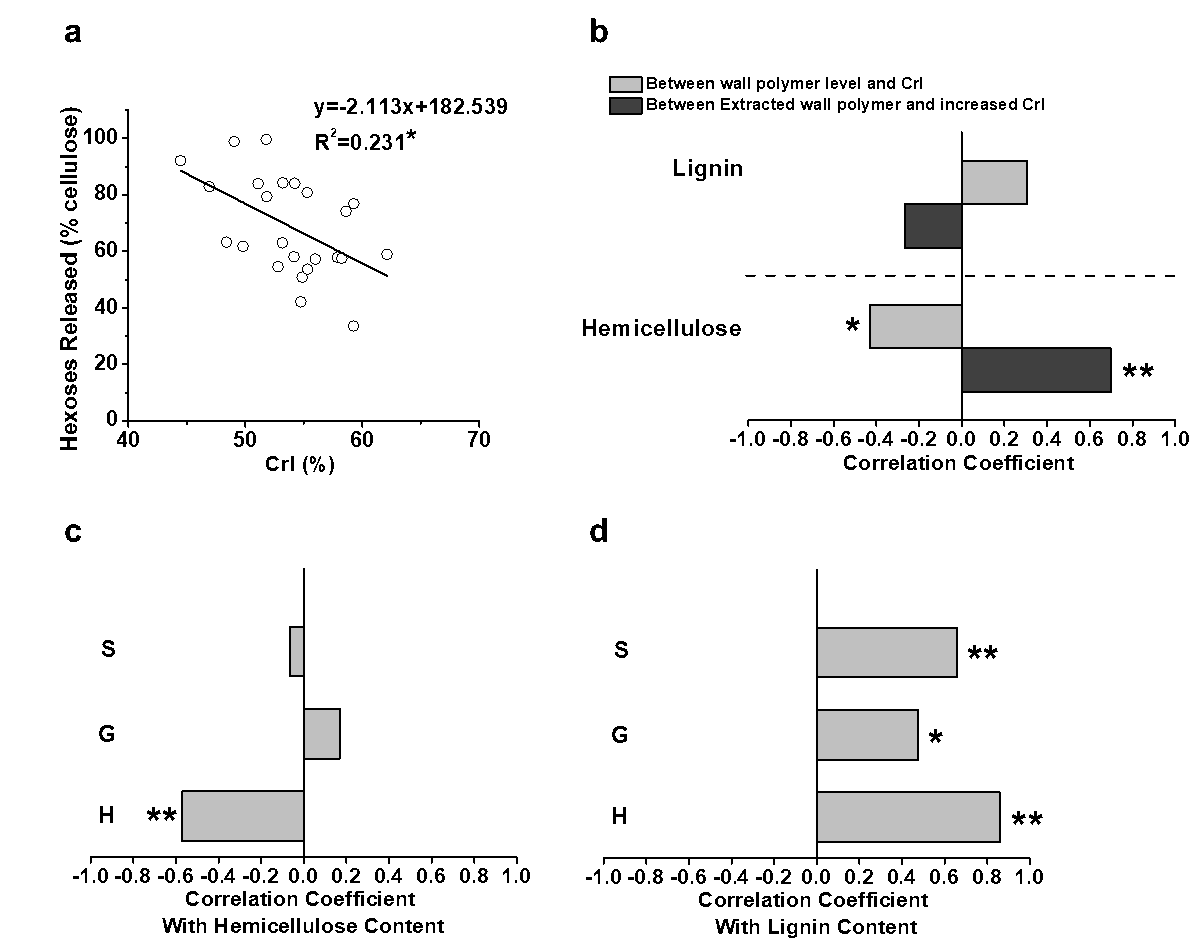
Fig. 6. Correlation analysis among the hexoses yields, cellulose CrI, and cell wall polymers under three pretreatments: (a) correlation between the hexoses yields and cellulose CrI; (b) correlations between the CrI/increased CrI and cell wall polymers levels/extracted cell wall polymer amounts in the pretreated biomass residues; (c) correlation between the three monolignol levels and hemicellulose content in the pretreated biomass residues; and (d) correlation between the three monolignol levels and lignin content in the pretreated biomass residues; * and ** significant correlations for p < 0.05 and 0.01 (n = 24)
lignin did not show any significant correlation with the cellulose crystallinity for the pretreated biomass residues (Fig. 6b), and only the H-monomer levels negatively correlated with the hemicellulose contents in the pretreated biomass residues (Fig. 6c). Additionally, it was found that the H-monomer level had the highest correlative coefficient value (0.86) with the lignin content (p < 0.01) compared with the G- and S-monomers from the pretreated biomass residues (Fig. 6d). Therefore, it was suggested that the E. arundinaceus samples containing a high H-monomer content may cause efficient lignin removal and relatively less hemicellulose co-extraction during the various pretreatments. The efficient H-monomer extraction should have led to more space for cellulases accession and loading for lignocellulose enzymatic hydrolysis (Li et al. 2014b). In contrast, the relatively high hemicellulose content may have maintained a native and low-crystalline cellulose microfibrils for the efficient enzymatic digestion of the E. arundinaceus samples from the pretreated biomass residues.
CONCLUSIONS
A total of 20 E. arundinaceus accessions showed diverse cell wall compositions and various biomass saccharification processes.
Mild alkali pretreatment (2% NaOH at 50 °C) led to almost complete biomass enzymatic saccharification of the leaf samples.
The liquid hot water pretreatment (200 °C and 32 min) resulted in the highest bioethanol yields because of the high sugar-ethanol conversion rates in both the stem and leaf tissues.
The H-monomer extraction predominately affected biomass enzymatic saccharification after the mild liquid hot water and chemical pretreatments, which provided a potential strategy for biomass process technology and bioenergy crop biotechnology.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Key Research and Development Program (2016YFD0800804), the Fundamental Research Funds for the Central Universities of China (2662015PY018), and the 111 Project of the Ministry of Education of China (B08032).
REFERENCES CITED
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresource Technol. 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093
Amalraj, V. A., and Balasundaram, N. (2006). “On the taxonomy of the members of ‘Saccharum complex’,” Genet. Resour. Crop Ev. 53(1), 35-41. DOI: 10.1007/s10722-004-0581-1
Azelee, N. I. W., Jahim, J. M., Rabu, A., Abdul Murad, A. M., Abu Bakar, F. D., and Illias, I. M. (2014). “Efficient removal of lignin with the maintenance of hemicellulose from kenaf by two-stage pretreatment process,” Carbohyd. Polym. 99, 447-453. DOI: 10.1016/j.carbpol.2013.08.043
Boudet, A. M., Kajita, S., Grima-Pettenati, J., and Goffner, D. (2003). “Lignins and lignocellulosics: A better control of synthesis for new and improved uses,” Trends Plant Sci. 8(12), 576-581. DOI: 10.1016/j.tplants.2003.10.001
Cai, Q., Aitken, K. S., Fan, Y. H., Piperidis, G., Jackson, P., and McIntyre, C. L. (2005). “A preliminary assessment of the genetic relationship between Erianthus rockii and the ‘Saccharum complex’ using microsatellite (SSR) and AFLP markers,” Plant Sci. 169(5), 976-984. DOI: 10.1016/j.plantsci.2005.07.002
Caspeta, L., Caro-Bermúdez, M. A., Ponce-Noyola, T., and Martinez, A. (2014). “Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol,” Appl. Energ. 113, 277-286. DOI: 10.1016/j.apenergy.2013.07.036
Chen, H., Jia, Z., Hu, T., Zhao, X., and Liu, D. (2015). “A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: Substrate digestibility, fermentability and structural features,” Appl. Energ. 150, 224-232. DOI: 10.1016/j.apenergy.2015.04.030
Chen, L., Li, J., Lu, M., Guo, X., Zhang, H., and Han, L. (2016). “Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover,” Carbohyd. Polym. 141, 1-9. DOI: 10.1016/j.carbpol.2015.12.079
D’Hont, A., Rao, P. S., Feldmann, P., Grivet, L., Islam-Faridi, N., Taylor, P., and Glaszmann, J. C. (1995). “Identification and characterisation of sugarcane intergeneric hybrids, Saccharum officinarum x Erianthus arundinaceus, with molecular markers and DNA in situ hybridization,” Theor. Appl. Genet. 91(2), 320-326. DOI: 10.1007/BF00220894
Hattori, T., and Morita, S. (2010). “Energy crops for sustainable bioethanol production; which, where and how?,” Plant Prod. Sci. 13(3), 221-234. DOI: 10.1626/pps.13.221
Hendriks, A. T. W. M., and Zeeman, G. (2009). “Pretreatments to enhance the digestibility of lignocellulosic biomass,” Bioresource Technol. 100(1), 10-18. DOI: 10.1016/j.biortech.2008.05.027
Huang, Y., Wei, X., Zhou, S., Liu, M., Tu, Y., Li, A., Chen, P., Wang, Y., Zhang, X., Tai, H., et al. (2015). “Steam explosion distinctively enhances biomass enzymatic saccharification of cotton stalks by largely reducing cellulose polymerization degree in G. barbadense and G. hirsutum,” Bioresource Technol. 181, 224-230. DOI: 10.1016/j.biortech.2015.01.020
Hu, M., Yu, H., Li, A., Cai, Q. M., Liu, P., Tu, Y., Wang, Y. T., Hu, R. F., Peng, L. C., and Xia, T. (2018). “Distinct polymer extraction and cellulose DP reduction for complete cellulose hydrolysis under mild chemical pretreatments in sugarcane,” Carbohydr. Polym. 202, 434-443. DOI:10.1016/j.carbpol.2018.08.039
Jia, J., Yu, B., Wu, L., Wang, H., Wu, Z., Li, M., Huang, P., Feng, S., Chen, P., Zheng, Y., et al. (2014). “Biomass enzymatic saccharification is determined by the non-KOH-extractable wall polymer features that predominately affect cellulose crystallinity in corn,” PLoS One 9(9). DOI: 10.1371/journal.pone.0108449
Jiang, W., Chang, S., Li, H., Oleskowicz-Popiel, P., and Xu, J. (2015). “Liquid hot water pretreatment on different parts of cotton stalk to facilitate ethanol production,” Bioresource Technol. 176, 175-180. DOI: 10.1016/j.biortech.2014.11.023
Jin, W., Chen, L., Hu, M., Sun, D., Li, A., Li, Y., Hu, Z., Zhou, S., Tu, Y., Xia, T., et al. (2016). “Tween-80 is effective for enhancing steam-exploded biomass enzymatic saccharification and ethanol production by specifically lessening cellulase absorption with lignin in common reed,” Appl. Energ. 175, 82-90. DOI: 10.1016/j.apenergy.2016.04.104
Li, F., Ren, S., Zhang, W., Xu, Z., Xie, G., Chen, Y., Tu, Y., Li, Q., Zhou, S., Li, Y., et al. (2013a). “Arabinose substitution degree in xylan positively affects lignocellulose enzymatic digestibility after various NaOH/H2SO4 pretreatments in Miscanthus,” Bioresource Technol. 130(1), 629-637. DOI: 10.1016/j.biortech.2012.12.107
Li, F., Zhang, M., Guo, K., Hu, Z., Zhang, R., Feng, Y., Yi, X., Zou, W., Wang, L., Wu, C., et al. (2015). “High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants,” Plant Biotechnol. J. 13(4), 514-525. DOI: 10.1111/pbi.12276
Li, H.-Q., Li, C.-L., Sang, T., and Xu, J. (2013b). “Pretreatment on Miscanthus lutarioriparious by liquid hot water for efficient ethanol production,” Biotechnol. Biofuels 6(1), 76. DOI: 10.1186/1754-6834-6-76
Li, M., Feng, S., Wu, L., Li, Y., Fan, C., Zhang, R., Zou, W., Tu, Y., Jing, H.-C., Li, S., et al. (2014a). “Sugar-rich sweet sorghum is distinctively affected by wall polymer features for biomass digestibility and ethanol fermentation in bagasse,” Bioresource Technol. 167, 14-23. DOI: 10.1016/j.biortech.2014.04.086
Li, M., Si, S., Hao, B., Zha, Y., Wan, C., Hong, S., Kang, Y., Jia, J., Zhang, J., Li, M., et al. (2014b). “Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus,” Bioresource Technol. 169, 447-454. DOI: 10.1016/j.biortech.2014.07.017
Li, Y., Zhuo, J., Liu, P., Chen, P., Hu, H., Wang, Y., Zhou, S., Tu, Y., Peng, L., and Wang, Y. (2018). “Distinct wall polymer deconstruction for high biomass digestibility under chemical pretreatment in Miscanthus and rice,” Carbohyd. Polym. 192, 273-281. DOI: 10.1016/j.carbpol.2018.03.013
Li, Z., Zhao, C., Zha, Y., Wan, C., Si, S., Liu, F., Zhang, R., Li, F., Yu, B., Yi, Z., et al. (2014c). “The minor wall-networks between monolignols and interlinked-phenolics predominantly affect biomass enzymatic digestibility in Miscanthus,” PLoS One 9(8). DOI: 10.1371/journal.pone.0105115
Limayem, A., and Ricke, S. C. (2012). “Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects,” Prog. Energ. Combust. 38(4), 449-467. DOI: 10.1016/j.pecs.2012.03.002
Mislevy, P., Martin, F. G., Adjei, M. B., and Miller, J. D. (1997). “Harvest management effects on quantity and quality of Erianthus plant morphological components,” Biomass Bioenerg. 13(1-2), 51-58. DOI: 10.1016/S0961-9534(97)00023-8
Peng, L., Hocart, C. H., Redmond, J. W., and Williamson, R. E. (2000). “Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production,” Planta 211(3), 406-414. DOI: 10.1007/s004250000301
Piperidis, G., Christopher, M. J., Carroll, B. J., Berding, N., and D’Hont, A. (2000). “Molecular contribution to selection of intergeneric hybrids between sugarcane and the wild species Erianthus arundinaceus,” Genome 43(6), 1033-1037. DOI: 10.1139/gen-43-6-1033
Ralph, J., Lundquist, K., Brunow, G., Lu, F., Kim, H., Schatz, P. F., Marita, J. M., Hatfield, R. D., Ralph, S. A., Christensen, J. H., et al. (2004). “Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids,” Phytochem. Rev. 3(1-2), 29-60. DOI: 10.1023/B:PHYT.0000047809.65444.a4
Ram, B., Sreenivasan, T. V., Sahi, B. K., and Singh, N. (2001). “Introgression of low temperature tolerance and red rot resistance from Erianthus in sugarcane,” Euphytica 122(1), 145-153. DOI: 10.1023/A:1012626805467
Rebaque, D., Martínez-Rubio, R., Fornalé, S., García-Angulo, P., Alonso-Simón, A., Álvarez, J. M., Caparros-Ruiz, D., Acebes, J. L., and Encina, A. (2017). “Characterization of structural cell wall polysaccharides in cattail (Typha latifolia): Evaluation as potential biofuel feedstock,” Carbohyd. Polym. 175, 679-688. DOI: 10.1016/j.carbpol.2017.08.021
Robinson, T., Mood, M., Balaji, C., Soumya, S., Tamal, B., Vaibhav, V. G. (2015). “Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: a comparative study,” Biomass Bioenergy, 81, 9-18. DOI: 10.1016/j.biombioe.2015.05.006
Rott, P., Mohamed, I. S., Klett, P., Soupa, D., de Saint-Albin, A., Feldmann, P., and Letourmy, P. (1997). “Resistance to leaf scald disease is associated with limited colonization of sugarcane and wild relatives by Xanthomonas albilineans,” Phytopathology 87(12), 1202-1213. DOI: 10.1094/PHYTO.1997.87.12.1202
Scheller, H. V., and Ulvskov, P. (2010). “Hemicelluloses,” Annu. Rev. Plant Biol. 61, 263-289. DOI: 10.1146/annurev-arplant-042809-112315
Si, S., Chen, Y., Fan, C., Hu, H., Li, Y., Huang, J., Liao, H., Hao, B., Li, Q., Peng, L., et al. (2015). “Lignin extraction distinctively enhances biomass enzymatic saccharification in hemicelluloses-rich Miscanthus species under various alkali and acid pretreatments,” Bioresource Technol. 183, 248-254. DOI: 10.1016/j.biortech.2015.02.031
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2008). Determination of Structural Carbohydrates and Lignin in Biomass (NREL/TP-510-42618), National Renewable Energy Laboratory, Golden, CO.
Sun, H., Li, Y., Feng, S., Zou, W., Guo, K., Fan, C., Si, S., and Peng, L. (2013). “Analysis of five rice 4-coumarate:coenzyme A ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice,” Biochem. Bioph. Res. Co. 430(3), 1151-1156. DOI: 10.1016/j.bbrc.2012.12.019
Wang, Y., Fan, C., Hu, H., Li, Y., Sun, D., Wang, Y., and Peng, L. (2016). “Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops,” Biotechnol. Adv. 34(5), 997-1017. DOI: 10.1016/j.biotechadv.2016.06.001
Wu, Z., Zhang, M., Wang, L., Tu, Y., Jing, Z., Xie, G., Zou, W., Li, F., Guo, K., Li, Q., et al. (2013). “Biomass digestibility is predominantly affected by three factors of wall polymer features distinctive in wheat accessions and rice mutants,” Biotechnol. Biofuels 6(1), 183-196. DOI: 10.1186/1754-6834-6-183
Xu, N., Zhang, W., Ren, S., Liu, F., Zhao, C., Liao, H., Xu, Z., Huang, J., Li, Q., Tu, Y., et al. (2012). “Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus,” Biotechnol. Biofuels 5(1), 58. DOI: 10.1186/1754-6834-5-58
Yu, Q., Zhuang, X., Lv, S., He, M., Zhang, Y., Yuan, Z., Qi, W., Wang, Q., Wang, W., and Tan, X. (2013). “Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes,” Bioresource Technol. 129, 592-598. DOI: 10.1016/j.biortech.2012.11.099
Zhang, W., Yi, Z., Huang, J., Li, F., Hao, B., Li, M., Hong, S., Lv, Y., Sun, W., Ragauskas, A., et al. (2013). “Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus,” Bioresource Technol. 130, 30-37. DOI: 10.1016/j.biortech.2012.12.029
Zhu, M.-Q., Wen, J.-L., Wang, Z.-W., Su, Y.-Q., Wei, Q., and Sun, R.-C. (2015a). “Structural changes in lignin during integrated process of steam explosion followed by alkaline hydrogen peroxide of Eucommia ulmoides Oliver and its effect on enzymatic hydrolysis,” Appl. Energ. 158, 233-242. DOI: 10.1016/j.apenergy.2015.08.085
Zhu, S., Huang, W., Huang, W., Wang, K., Chen, Q., and Wu, Y. (2015b). “Pretreatment of rice straw for ethanol production by a two-step process using dilute sulfuric acid and sulfomethylation reagent,” Appl. Energ. 154, 190-196. DOI: 10.1016/j.apenergy.2015.05.008
Ziebell, A., Gracom, K., Katahira, R., Chen, F., Pu, Y., Ragauskas, A., Dixon, R. A., and Davis, M. (2010). “Increase in 4-coumaryl alcohol units during lignification in alfalfa (Medicago sativa) alters the extractability and molecular weight of lignin,” J. Biol. Chem. 285(50), 38961-38968. DOI: 10.1074/jbc.M110.137315
Article submitted: July 23, 2018; Peer review completed: September 9, 2018; Revised version received: November 15, 2018; Accepted: November 17, 2018; Published: December 3, 2018.
DOI: 10.15376/biores.14.1.650-668
APPENDIX
Supplementary
Table S1. Cell Wall Composition and Lignocellulose Enzymatic Digestibility of Erianthus arundinaceus Sample
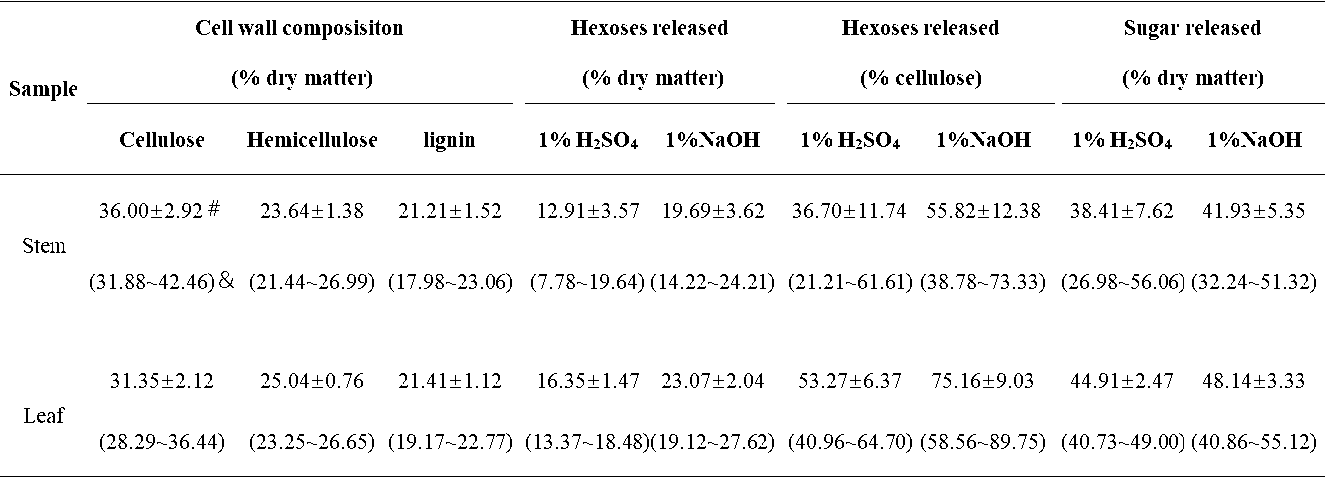
# Mean value. & Minimum and maximum values. Sugar released: sugar yield released from both pretreatment and enzymatic hydrolysis of samples
Table S2. Cell Wall Composition and Biomass Digestibility of Four Groups of Erianthus arundinaceus Samples
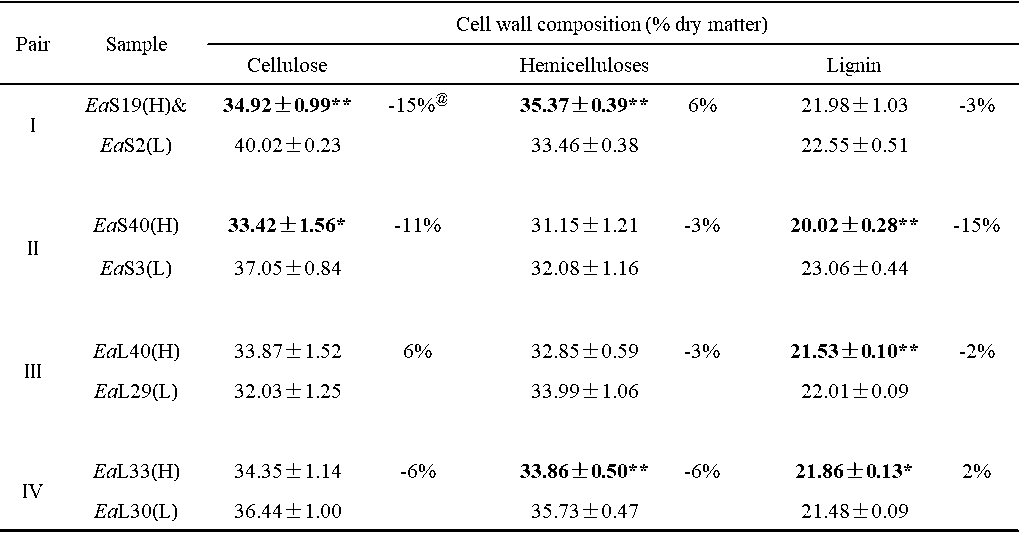
& (H) or (L) indicated the sample in the pair with high (H) or low (L) biomass digestibility. All data as means ± SD (n = 3). Ea: Erianthus arundinaceus ; S: stem; L: leaf. * and ** Indicated significant difference at pair by t-test at p <0.05 and 0.01 (n = 3); @ Percentage of the increased or decreased level at pair: subtraction of two samples by low value at pair.
Table S3. Hexose Yields (% cellulose) Released from Enzymatic Hydrolysis after Three Pretreatments in Four Pairs of Erianthus arundinaceus Samples
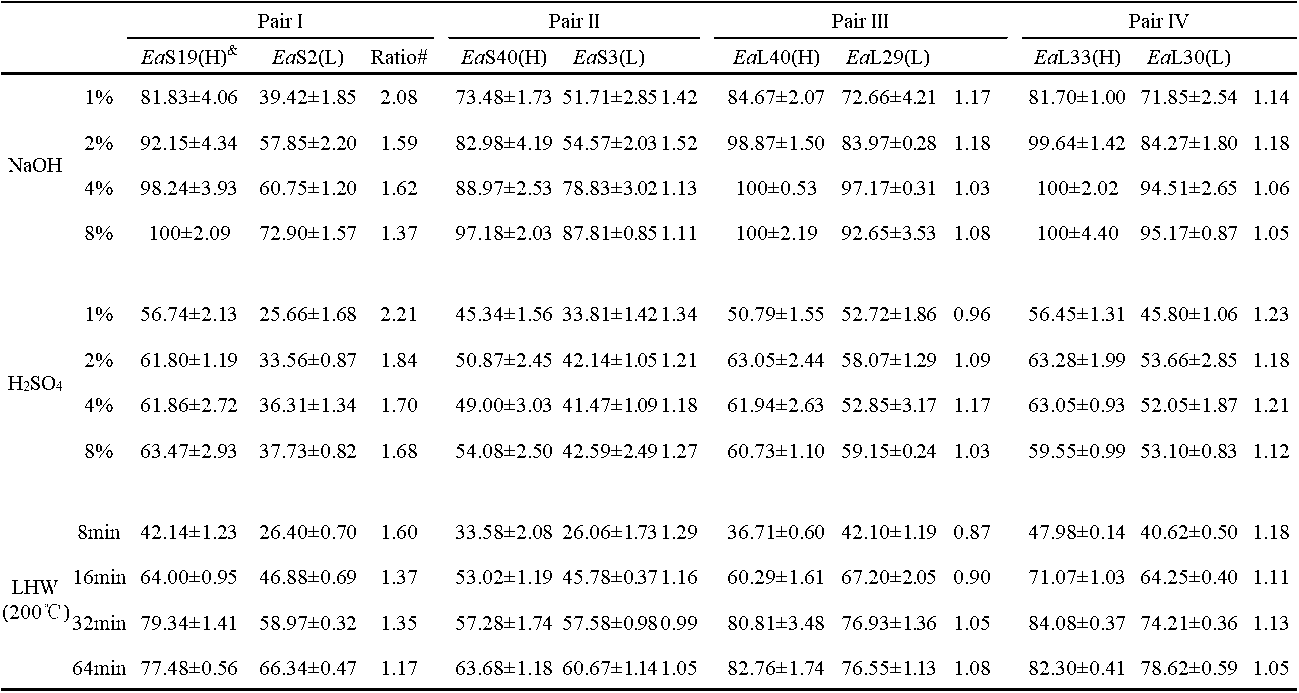
& (H) or (L) indicated the sample in the pair with high (H) or low (L) biomass digestibility. All data as means ± SD (n = 3). Ea: Erianthus arundinaceus ; S: stem; L: leaf. * and ** Indicated significant difference at pair by t-test at p <0.05 and 0.01 (n = 3); @ Percentage of the increased or decreased level at pair: subtraction of two samples by low value at pair.
Table S4. Bioethanol Productivity Released from Yeast Fermentation Using Sugars Obtained from Pretreatments and Enzymatic Hydrolysis in Two Pairs of Erianthus arundinaceus Samples
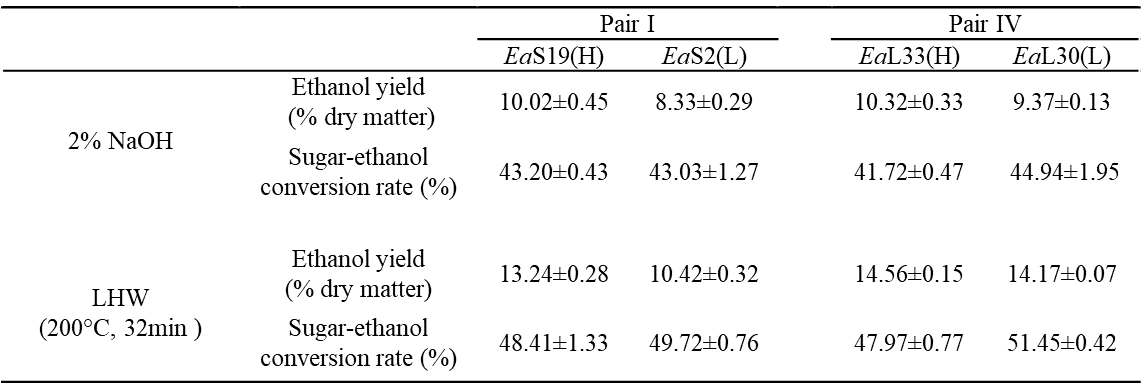
All data as means ± SD (n = 3). &, (H) or (L) Indicated the sample in the pair with high (H) or low (L) biomass digestibility.
Table S5. Hemicelluloses and Lignin Extraction Rates from the Three Pretreatments in Four Pairs of Erianthus arundinaceus Samples
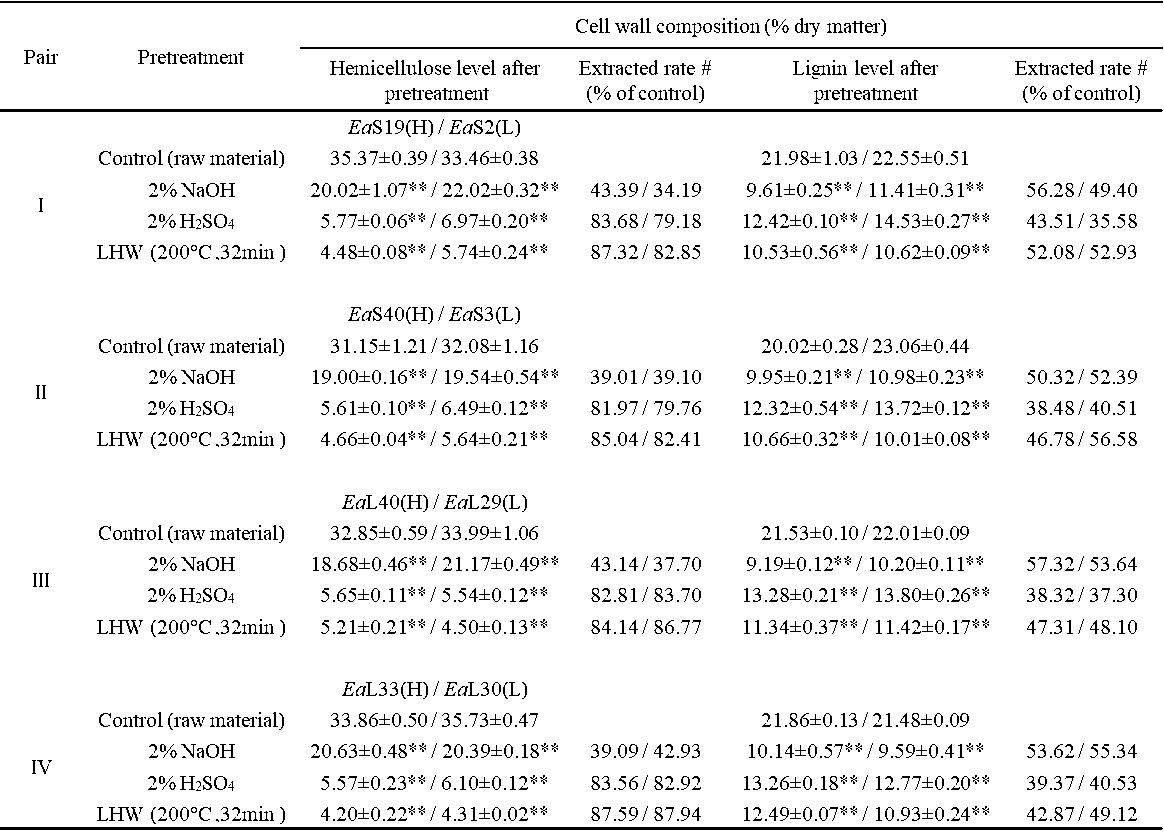
All data as means ± SD (n = 3). # Indicated the plant cell wall polymer extraction rates: subtraction between hemicelluloses/lignin level of biomass residue with the control value (raw material) divided by control value, ** Indicated significant difference between the raw material and pretreated residue by t-test at p <0.01 (n = 3).
Table S6. Cellulose CrI of the Biomass Residues Obtained from the Three Pretreatments in Four Pairs of Erianthus arundinaceus Samples
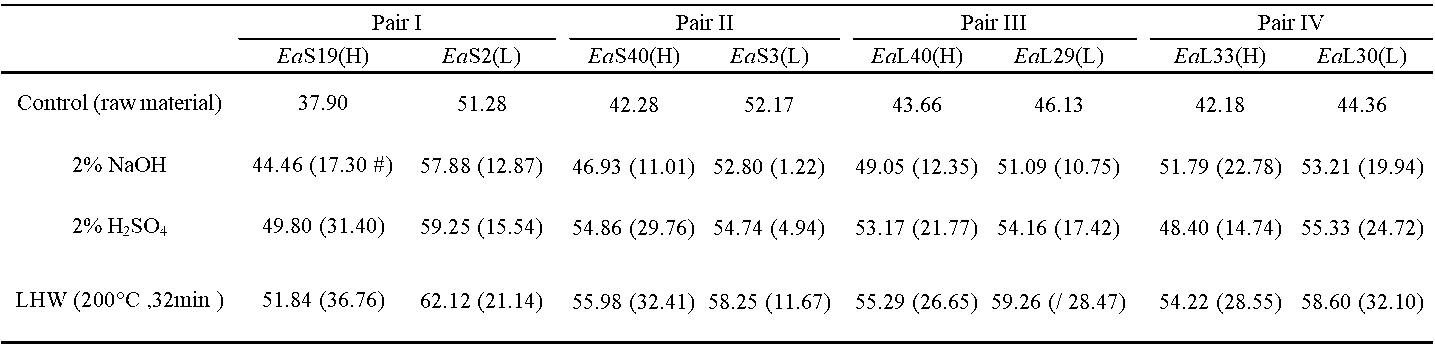
Percentage of increased level between the raw materials and pretreated residues by subtraction of two values divided by value of the raw materials (brackets).
