Abstract
The presence of dissolved organic matter, scientifically known as humic substances, gives an undesirable color and taste to water. In addition, they are the precursors of carcinogenic disinfection by-products upon disinfection treatment. Adsorption provides a potential means of removal of humic substances, and lignocellulosic biomass serves as a promising candidate. In this paper, we report the application of modified coconut copra residues for adsorption of humic substances from peat swamp runoff. The FTIR spectra suggest that coconut copra residues are genuinely rich with carboxyl groups with long alkyl chains; this renders the material a natural biosorbent, attaining an average of 50% removal under the conditions of testing. Upon treatment, dissolution of lignin and hemicellulose with the enhancement of effective carboxyl groups occurs, improving the adsorption efficiency to 96%; the treated water is visibly clear. The relative band abundance and band shifts further confirm the involvement of the surface functional groups in the adsorption process. The modified coconut copra residue is an attractive biosorbent option for removal of humic substances. The operating conditions are mild, involving non-toxic chemicals, and no pH adjustment is necessary to allow adsorption.
Download PDF
Full Article
Modified Coconut Copra Residues as a Low Cost Biosorbent for Adsorption of Humic Substances from Peat Swamp Runoff
Siong Fong Sim,* Terri Zhuan Ean Lee, Nurul Aida Lu Mohd Irwan Lu, and Mohamed Murtedza
The presence of dissolved organic matter, scientifically known as humic substances, gives an undesirable color and taste to water. In addition, they are the precursors of carcinogenic disinfection by-products upon disinfection treatment. Adsorption provides a potential means of removal of humic substances, and lignocellulosic biomass serves as a promising candidate. In this paper, we report the application of modified coconut copra residues for adsorption of humic substances from peat swamp runoff. The FTIR spectra suggest that coconut copra residues are genuinely rich with carboxyl groups with long alkyl chains; this renders the material a natural biosorbent, attaining an average of 50% removal under the conditions of testing. Upon treatment, dissolution of lignin and hemicellulose with the enhancement of effective carboxyl groups occurs, improving the adsorption efficiency to 96%; the treated water is visibly clear. The relative band abundance and band shifts further confirm the involvement of the surface functional groups in the adsorption process. The modified coconut copra residue is an attractive biosorbent option for removal of humic substances. The operating conditions are mild, involving non-toxic chemicals, and no pH adjustment is necessary to allow adsorption.
Keywords: Coconut copra residues; Biosorbent; Adsorption; Humic substances; Peat swamp runoff
Contact information: Department of Chemistry, Universiti Malaysia Sarawak, 94300, Kota Samarahan, Malaysia; *Corresponding author: sfsim@frst.unimas.my
INTRODUCTION
The presence of dissolved organic matter in water typically causes undesirable color and taste; it is also a precursor of disinfection by-products. The organic materials, scientifically termed humic substances, are found abundantly in peat soil and are readily leached into rivers, contributing to difficulties in the treatability of the water. In Sarawak, there are numerous rivers tainted by peat swamp runoff, generally known as tropical black water, due to the vast peatland area. The rivers are an important source of water supply, and a previous study demonstrated that water obtained from peat swamp leachate contains a high level of trihalomethanes, sometimes exceeding the allowable level in drinking water (Sim and Mohamed 2005). Hence, it is crucial to minimize the humic content prior to disinfection.
Adsorption is a feasible alternative for the removal of humic substances from aqueous solutions. Various porous adsorbents have been studied, including activated carbon and resins. Nevertheless, they are revealed to be less effective, as the pores are not available for adsorption of humic substances because of their macromolecular structure (Kilduff et al. 1996). Fundamentally, humic molecules contain both hydrophobic and hydrophilic moieties with diverse functional groups, i.e., carboxylic, phenolic, carbonyl, and hydroxyl groups; they are often negatively charged in the aquatic environment (Cornel et al. 1986). Their heterogeneous nature indicates that adsorbents with appropriate surface functional groups would enable adsorption of humic substances via electrostatic linkages, cation bridging, hydrophobic interactions, and hydrogen bonding (Bai and Zhang 2001, 2003; Zhang and Bai 2002a,b).
Several types of biomass, such as modified polyacrylonitrile fibers (Deng and Bai 2003), modified rice husk (Imyim and Prapalimrungsi 2010), and fungi (Zhou and Banks 1991; Deng et al. 2006), have been reported to remove humic acids. They are potential biosorbents due to the presence of numerous functional groups serving as binding sites for adsorption. A wide range of natural biomass has been studied as biosorbents (Lau et al. 2003; Ashraf et al. 2011a,b, 2012; Hossain et al. 2012; Achak et al. 2009; Chuah et al. 2005; Saha et al. 2013; Contreras et al. 2012; Kiran et al. 2013; Singha and Das 2012). Bhatnagar et al. (2010) provides a comprehensive review of the use of coconut-based biomass as biosorbents, as which they are extensively applied for heavy metals and dyes. However, to the best of our knowledge, no research has been carried out to investigate the adsorption ability of coconut copra residues for the removal of humic substances in natural water.
In this paper, modified coconut copra residue was employed as a low-cost biosorbent for adsorption of ‘real world’ humic substances from peat swamp runoff. According to Deng and Bai (2003), natural humic substances are different from the standard humic acid due to interactions with other ions in water. Our earlier study on the chemical characteristics of indigenous humic substances also suggests that tropical humic matters may be smaller in molecular size and richer in acidic functional groups due to the consistently wet and warm weather conditions that have accelerated the oxidative degradation (Sim and Mohamed 2007). In this study, the spectroscopic approach, typically Ultraviolet-Visible and Fourier Transform Infrared are employed to demonstrate the removal of humic substances from natural water by chemically modified coconut copra. The model of standard humic acid was used to access the prediction performance based on UV-Vis approach and to evaluate the adsorption behavior of coconut copra residue.
MATERIALS AND METHODS
Sample Preparation
The coconut copra residues were obtained from the local market. They were washed thoroughly to remove the coconut milk and dried in an oven at 105 C. The biomass was then refluxed in distilled water for 1 h at 100 C to remove the fatty acid residues and for hydrolysis of hemicellulose and lignin. The refluxed biomass was then washed and dried and subsequently agitated in 0.5 M citric acid for 1 h at room temperature. The treated biomass was once again washed to pH 3 and dried.
Adsorption of Humic Substances from Peat Swamp Runoff
Peat swamp runoff collected from Asa Jaya vicinity (surrounded by peat soil) was treated with raw and modified coconut copra residues in batches. The water was filtered through a 0.45-m membrane prior to treatment, and no pH adjustment was done. The pH of the peat swamp runoff is characteristically acidic and is typically between 4 and 5. Approximately 1 g of biomass with pH 3 was agitated in 20 mL of peat swamp runoff for 15 min, filtered, and subjected to UV irradiation.
Note that the contact time, pH, and dosage were optimized at room temperature (25 to 28 C) according to a one-factor-at-a-time approach. The results are not included for brevity; generally, a lower pH appears to improve the adsorption process. The observation, according to Vermeer et al. (1998), is associated with the configuration of humic substances. At low pH, humic substances are more spherical; this has allowed various surface interactions to take place of which electrostatic interaction dominates as humic substance in natural water is often negatively charged whilst the functional groups of biomass are protonated (Deng and Bai 2003).
The UV-Vis spectra of humic substances are characteristically featureless as a result of overlapping absorbance caused by its complicated structures, where the absorbance decreases almost exponentially toward longer wavelength. Apparently, the absorbance of peat swamp runoff is markedly reduced throughout the UV-Vis region after treatment (spectra not shown). This approach is a rapid technique for characterisation and quantification of humic substances; numerous wavelengths have been used for example, 250, 254, 272, 280, 285, 330, 365, 400, 465, and 665 nm (Hautala et al. 2000; Chen et al. 2002; Świetlik and Sirkorska 2005; Zbytniewski and Buszewski 2005; Gan et al. 2003; Ghabbour and Davies, 2009).
The absorbance at 465 nm was measured to indicate the removal efficiency. According to Hautala et al. (2000), the absorbance at 465 nm is the most recommended for measurement of the color in water caused by a given fraction of humic matter. Nonetheless, it is important to note that part of the humic molecules cannot absorb radiation in the visible region. To confirm the appropriateness of 465 nm for quantification of humic acid, a series of standard humic acid solution at various concentrations were scanned from 200 to 800 nm in six replicates. The samples were divided into 100 training and test sets where the training samples were used to predict the concentrations of the test samples according to simple linear regression. At wavelength < 220 nm, the models were constantly characterised by high root mean squares errors of prediction (RMSEP), suggesting the absorbance in this region is unsuitable for the quantification of humic acid. However the prediction error was seen to be reduced from 221 to 530 nm, beyond which the error began to increase. The observation indicates that the wavelength at 465 nm is acceptable to be employed for quantification. Note that in the study of prediction, standard humic acid was employed as a model to confirm the suitability of the wavelength.
Hypothetically, a linear relationship is expected between the absorbance and humic content; if adsorption has taken place, a reduction in absorbance is therefore anticipated. The absorbance measurements acquired after treatment were compared with the untreated peat swamp runoff to determine the removal efficiency. To determine the adsorption capacity of coconut copra residues, 1 g of biomass was continuously treated with an increasing volume of peat swamp runoff, starting at 20 mL with increments of 10 mL, for 15 min at each increment.
(1)
Isotherm Studies
A total of 1 g of untreated and treated coconut copra was agitated in 20, 40, 60, 80, 100, and 120 mg/L of standard humic acid (Sigma-Aldrich) for 15 min at room temperature. Note that the standard humic acid was dissolved in 0.1 M KOH, re-precipitated with concentrated HNO3 and oven-dried at 35 to 40 C. The removal percentage was similar suggested based on the absorbance at 465 nm. The adsorption equilibrium was characterized using Langmuir and Freundlich isotherm models. Langmuir isotherm model deals with monolayer adsorption while Freundlich isotherm model deals with heterogeneous adsorption.
The Langmuir equation can be expressed as,
(2)
where qmax is the maximum adsorption capacity (mgg-1), b is the adsorption energy, and q is the amount of humic acid adsorbed at equilibrium,
(3)
The Freundlich equation can be expressed as,
(4)
where K is the adsorption capacity and n is the adsorption intensity.
Fourier Transform Infrared Spectroscopy (FTIR)
The biomass, including raw, refluxed, citric acid-treated, and humic-adsorbed coconut copra residues, was analyzed with a ThermoScientific FTIR spectrometer (Thermo Nicolet Analytical Instruments, Madison, WI) using the KBr disc method with 2 mg of sample in 100 mg of KBr. The samples were scanned in six replicates in the scanning range of 4000 to 400 cm-1 at a resolution of 4 cm-1 to illustrate the consistency of the bands detected.
The spectra were analyzed with the peak detection and matching algorithm reported by Sim and Ting (2012) to produce a peak table with rows and columns corresponding to samples and wavenumbers, respectively. The bands detected in individual samples were then represented by the peak area in the peaks table. In short, the algorithm allows absorption bands to be identified automatically from each sample, and the bands are subsequently matched (bands corresponding to the same functional groups are aligned in the peak table) according to a set of user-defined parameters. Two main parameters, namely, the peak threshold and the peak matching window, were set at 3 and 0, respectively, to permit small but definable bands to be detected. Additionally, the bands were aligned on perfect matching so that shifts could be detected. The peak threshold indicates the peak noise factor, while the peak matching window defines the tolerance between the wavenumber of the target band and the matching bands.
Scanning Electron Microscope (SEM)
The morphological characteristics were observed using a scanning electron microscope (Model JEOL JSM-6390LA, Japan) with an accelerating voltage of 5 kV at ×500 magnification. The samples were coated with a thin film of conducting material prior to examination.
RESULTS AND DISCUSSION
According to Ofomaja and Ho (2006), coconut copra residues are an agricultural by-product characterized by the presence of lignocellulosic compounds with various functional groups, as confirmed by FTIR with absorption bands at 3600 to 3000 cm-1(-OH), 3000 to 2250 cm-1 (R-NH3+), 1300 to 1000 cm-1 (-C-O), 1800 to 1650 (C=O), and 1225 to 1150 cm-1 (C-C). In this study, it was found that coconut copra residues are rather different from other agricultural biomasses previously examined, i.e., coconut husk, sago hampas, oil palm empty fruit bunch, saw dust, groundnut shell, sugarcane bagasse, rice husk, and banana trunk.
Fig. 1. FTIR spectra of various agricultural biomasses
As illustrated in Fig. 1, the FTIR spectrum of coconut copra residues is distinguishable from other biomasses, as the absorption bands at 2922, 2858, 2811, 1780, 1745, 1700, and 1158 cm-1 are more prominent. The disparities suggest that coconut copra residues are genuinely richer in carboxyl groups, and the more intense peaks at 2922 cm-1 and 2850 cm-1 further indicate the presence of long alkyl chains. The relative abundance could not be compared with the findings of Ho and Ofomaja (2007), as a spectrum was not shown in their paper. Despite the wide ranging studies on coconut-based adsorbents (Bhatnagar et al. 2010), there have been only a few reports concerning coconut copra (Ho and Ofomaja 2006; Ofomaja and Ho 2007); hence, limited data concerning its chemical characteristics are available.
Raw and modified coconut copra residues (refluxed and citric acid-treated) were introduced to peat swamp runoff. Apparently, coconut copra residue is a natural biosorbent in which an absorbance of close to 50% is recorded without any treatment. Our experience with other biomasses, including rice husk, oil palm empty fruit bunch, sago hampas, saw dust, coconut husk, banana trunk and etc. show little adsorption if untreated. Some materials used as sorbents contribute to more color, possibly due to the leaching of tannins. Further modification with citric acid has been shown to improve the adsorption ability of most biomass types. Table 1 summarizes the average removal percentage of various untreated and treated biomass-based biosorbents.
Table 1. Average Removal Percentage of Various Biomass-based Sorbents
On the other hand, peat swamp runoff treated with modified coconut copra exhibited a remarkable improvement, with an average removal of 96%, resulting in visibly clear water. Figure 2 shows the removal percentage of humic substances from water treated with raw coconut copra residues as well as refluxed and citric acid-treated biomass.
The inset of Fig. 2 illustrates the visual observation of treatment with citric acid-modified biomass. The adsorption performance of refluxed alone residue exhibited slight improvement with an average removal of 57%, suggesting that the process rendered the biomass more ready for adsorption. However, the surface functional groups may be yet to be optimally modified for efficient interactions with humic substances. An attempt was made to simplify the two-stage treatment to a one-pot process, where the biomass was refluxed in citric acid directly, but the removal efficiency was compromised, yielding an average removal of 86%.
Fig. 2. The removal efficiency of raw, refluxed, and citric acid-treated coconut copra residues
The isotherm studies were carried out to describe the amount of adsorbate adsorbed onto the surface of the adsorbent. In this paper, the standard humic acid of Sigma Aldrich was used; however it is important to note that the models may not describe the behavior of indigenous humic substances satisfactorily due to the differences in chemical characteristics. The isotherm coefficients are used to compare the adsorption ability of untreated and treated coconut copra for humic acid. Table 2 summarises the Langmuir and Freundlich parameters for adsorption of standard humic acid using untreated and treated coconut copra. The Langmuir isotherm constant of the raw coconut copra was characterised by a negative value, suggesting inadequacy of the model in explaining the sorption process as the adsorption behavior may not comply with the model’s assumption. Generally, the adsorption capacity is interpretable with qmax, K, and n; these values are evidenced to increase considerably with the treated coconut copra confirming its improved adsorption ability after treatment.
Table 2. Langmuir and Freundlich Parameters for Adsorption of Standard Humic Acid using Untreated and Treated Coconut Copra
The FTIR profile can suggest the changes encountered over the two-stage treatments and the interactions involved between humic substances and the modified coconut copra residues. Figure 3 illustrates the FTIR profiles of raw, refluxed, citric acid-treated, and humic-adsorbed coconut copra. Apparently, the spectra profiles of biomass generated in various phases are very similar, so differences are not easily discernable. The resultant peak table of the peak detection algorithm is employed to facilitate comparisons of the band abundance and shifting, allowing interpretation of the underlying alterations.
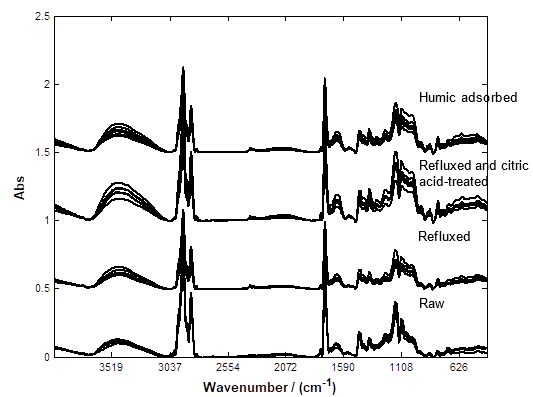
Fig. 3. FTIR spectra of raw and treated coconut copra residues
Citric acid treatment, according to Thanh and Nhung (2009), could lead to an acetylation reaction between cellulose and citric acid, resulting in the formation of ester linkages. In this study, enhanced C=O groups were observed in the FTIR spectra; specifically, there were several absorption bands in the regions of 1750 to 1735 cm-1 (conjugated C=O stretching), 1730 to 1700 cm-1 (free C=O stretching), and 1260 to 1230 cm-1 (C-C(O)-C stretching), corroborating the findings of Thanh and Nhung (2009). Figure 4 shows the alterations in abundance of several potential distinguishing bands with different treatments.
The band at 1700 cm-1, indicative of carboxylic acids, was observed to diminish after reflux, possibly due to the removal of fatty acid residues. Subsequent citric acid treatment demonstrated replenishment of the functional groups, corroborating the acetylation reaction postulated by Thanh and Nhung (2009). The bands at 1260 to 1230 cm-1 and 1750 to 1735 cm-1, designated as C-O stretching and conjugated C=O, respectively, also increase with organic acid treatment, further confirming the incorporation of carbonyl groups. After loading with humic substances, this band was interestingly reduced in intensity, suggesting interactions between humic substances and the biomass surface. On the other hand, the band ascribed to C=C aromatic rings of lignin at 1505 cm-1 is distinctively identified in raw coconut copra. However, after reflux, the band was completely missing. This implies the effective dissolution of the lignin, exposing the cellulose hydroxyl group that allows effective chemical modification to take place and eventually improve the adsorption performance.
Fig. 4. The relative abundance and the corresponding spectral region of the potential distinguishable bands
The band attributable to glycosidic linkages at 1159 cm-1demonstrates the reduced abundance and degradation of hemicellulose. Delignification and degradation of hemicellulose are common observations in biomass pretreatment (Sim et al.2012b). The absorption bands detected over the entire IR region were examined; some band shifts were obvious, where a band consistently found at an identical frequency for a specific group of samples was uniformly shifted after treatment. For example, the absorption band attributable to glycosidic linkages was detected at 1160 cm-1 in all replicates of untreated coconut copra residues. After adsorption with humic substances, the band was found at 1158 cm-1, indicating the occurrence of interactions.
The abundance of absorption bands over the entire IR region is shown in the Supplementary Information. Noticeably, most absorption bands experienced a reduction in intensity after reflux, but subsequent citric acid treatment improved the functional groups’ properties; upon loading with humic substances, the bands were once again reduced concurrently with band shifting. This suggests the involvement of various functional groups in the adsorption process, consistent with the findings of Etim et al. (2012). The complexity of the humic molecule allowed adsorption to occur via multiple interactions that have been confirmed in other related studies. The polar counterpart, according to Lin and Xing (2008), could interact with hydrophilic functionalities through hydrogen bonding, while aromatic moieties could interact with electron-rich sites via – interactions, where substitution of the rings with electron-donating or -withdrawing groups further encourages interactions promoting adsorption (Zhu et al. 2004; Chen et al. 2007). The alkyl and aromatic moieties could also contribute to hydrophobic and/or Van der Waals bonding (Pan and Xing 2008; Wang et al. 2011). The various interaction possibilities provide an explanation for the changes observed involving almost all absorption bands. Morphologically, no severe destruction was seen, apart from the surface of the treated biomass, which appears to be slightly rougher; this may be associated with the removal of lignin (SEM micrographs not shown).
Fig 5. The relative abundance of absorption bands detected over the IR region of various treated and untreated coconut copra residues
CONCLUSIONS
- Coconut copra residue is a natural biosorbent that removed an average of 47% of humic substances from peat swamp runoff under the conditions of testing.
- The FTIR spectrum indicates that raw coconut copra is rich with carboxyl groups that are intrinsically important for adsorption of humic substances when adsorption is carried out at a suitably low pH.
- The reflux process removes lignin and hemicellulose, increasing the availability of hydroxyl groups that render them more readily available for citric acid modification and adsorption.
- The modified coconut copra residue is a green and attractive biosorbent option for removal of humic substances.
- The operating conditions are mild and involve non-toxic chemicals. In addition, no pH adjustment is necessary to facilitate adsorption.
ACKNOWLEDGEMENTS
The authors are grateful for the support of the Ministry of Science and Technology (MOSTI), Grant. No. 06-01-09-SF0086.
REFERENCES CITED
Achak, M., Hafidi, A., Quazzani, N., Sayadi, S., and Mandi, L. (2009). “Low cost biosorbent “banana peel” for removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies,” J. Hazard. Mater. 166(1), 117-125.
Ashraf, M. A., Maah, M. J., and Yusoff, I. (2011a). “Study of mango biomass (Mangifera indica L.) as a cationic biosorbent,” Int. J. Environ. Sci. Tech. 7(3), 581-590.
Ashraf, M. A., Wajid, A., Mahmood, K., Maah, M. J., and Yusoff, I. (2011b). “Low cost biosorbent banana peel (Musa sapientum) for the removal of heavy metals,” Sci. Res. Essays6(19), 4055-4064.
Ashraf, M. A., Maah, M. J., and Yusoff, I. (2012). “Batchwise biosorption of Sn2+ ions by using chemically treated banana peel,” Res. J. Biotechnol. 7(3), 855-864.
Bai, R. B., and Zhang, X. (2001). “Polypyrrole-coated granules for humic acid removal,” J. Colloid Interface Sci. 243(1), 52-60.
Bhatnagar, A., Vilar, V. J. P., Botelho, C. M. S., and Boaventura, R. A. R. (2010). “Coconut-based biosorbents for water treatment – A review of the recent literature,” Adv. Colloid Interface Sci.160(1-2), 1-15.
Chen, J., Gu, B.H., LeBoeuf, E.J., Pan, H.J. and Dai, S. (2002). “Spectroscopic characterisation of the structural and functional properties of natural organic matter fractions,” Chemosphere48(1), 59-68.
Chen, W., Duan, L., and Zhu, Q. D. (2007). “Adsorption of polar and non-polar organic chemicals to carbon nanotubes,” Environ. Sci. Technol. 41(24), 8295-8300.
Chuah, T. G., Jumasiah, A., Azni, I., Katayon, S., and Choong, S. Y. T. (2005). “Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview,” Desalination175(3), 305-316.
Contreras, E., Sepulveda, L., and Palma, C. (2012). “Valorization of agroindustrial wastes as biosorbent for the removal of textile dyes from aqueous solutions,” Int. J. Chem. Eng. 2012, 679352.
Cornel, P. K., Summers, R. S., and Roberts, P. V. (1986). “Diffusion of humic acid in dilute aqueous solutions,” J. Colloid Interface Sci. 110(1), 149-164.
Deng, S., and Bai, R. B. (2003). “Aminated polyacrylonitrile fibers for humic acid adsorption: Behaviours and mechanisms,” Environ. Sci. Technol. 37, 5799-5808.
Deng, S. B., Yu, G., and Ting, Y. P. (2006). “Removal of humic acid using PEI-modified fungal biomass,” Sep. Sci. Technol. 41, 2989-3002.
Etim, U. J., Umoren, S. A., and Eduok, U. M. (2012). “Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution,” J. Saudi Chem. Soc. In Press.
Gan, E., Kotob, S.I. and Walia, D.S. (2007). “Evaluation of a spectrophotometric method for practical and cost effective quantification of fulvic acid,” Annals of Environmental Sciences 1, 11-15.
Ghabbour, E.A. and Davies, G. (2009). “Spectrophotometric analysis of fulvic acid – A second look,” Annals of Environmental Sciences 3, 131- 138.
Hautala, K., Peuravuori, J., and Pihlaja, K. (2000). “Measurement of aquatic humus content by spectroscopic analyses,” Water Res. 34(1), 246-258.
Ho, Y. S., and Ofomaja, A. E. (2006). “Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent,” Biochem. Eng. J. 30(2), 117-123.
Hossain, M. A., Ngo, H. H., Guo, W. S., and Nguyen, T. V. (2012). “Biosorption of Cu(II) from water by banana peel biosorbent: Experiments and models of adsorption and desorption,” J. Water Sustainability 2, 87-104.
Imyim, A., and Prapalimrungsi, E. (2010). “Humic acids removal from water by aminopropyl functionalized rice husk ash,” J. Hazard. Mater. 184(1-3), 775-781.
Kilduff, J. E., Karanfil, T., and Weber, W. J. (1996). “Adsorption of natural organic polyelectrolytes by activated carbon: A size-exclusion chromatography study,” Environ. Sci. Tech. 30(4), 1336-1343.
Kiran, B. M., Srikantaswamy, S., Manoj, P. H. V., and Tasneem, T. (2013). “A study on utilization of groundnut shell as biosorbents for heavy metals removal,” J. Environ. Sci. 2(1), 173-186.
Lau, T. C., Ang, P. O., and Wong, P. K. (2003). “Development of seaweed biomass as a biosorbent for metal ions,” Water Sci. Technol. 47(10), 49-54.
Lin, D. H., and Xing, B. S. (2008). “Tannic acid adsorption and its role for stabilizing carbon nanotube suspensions,” Environ. Sci. Technol. 42(16), 5917-5923.
Ofomaja, A. E., and Ho, Y. S. (2007). “Effect of pH on cadmium biosorption by coconut copra meal,” J. Hazard. Mater. 139(2), 356-362.
Pan, B., and Xing, B. S. (2008). “Adsorption mechanisms of organic chemicals on carbon nanotubes,” Environ. Sci. Technol.42(24), 9005-9013.
Saha, R., Saha, I., Nandi, R., Ghosh, A., Basu, A., Ghosh, S. K., and Saha, B. (2013). “Application of Chattim tree (devil tree, Alstonia scholaris) saw dust as a biosorbent for removal of hexavalent chromium from contaminated water,” Canadian J. Chem. Eng. 91(5), 814-821.
Sim, S. F., and Mohamed, M. (2005). “Occurrence of trihalomethanes in drinking water tainted by peat swamp runoff in Sarawak,” J. Sci. Technol. Tropics 1, 132-135.
Sim, S. F., and Mohamed, M. (2007). “Chemical characterisation of humic substances occurring in the peats of Sarawak, Malaysia,” Org. Geochem. 38(6), 967-976.
Sim, S. F., and Ting, W. (2012a). “An automated approach for analysis of fourier transform infrared (FTIR) spectra of edible oils,” Talanta 88(15), 537-543.
Sim, S. F., Mohamed, M., Mohd Irwan Lu, N. A. L., Sarman, N. S. P., and Samsudin, S. N. S. (2012b). “Computer-assisted analysis of Fourier transform infrared (FTIR) spectra for characterisation of various treated and untreated agriculture biomass,” BioResources 7(4), 5346-5380.
Singha, B., and Das, S. K. (2012). “Removal of Pb(II) ions from aqueous solution and industrial effluent using natural biosorbents,” Environ. Sci. Pollut. Res. Int. 19(6), 2212-2226.
Świetlik, J. and Sirkorska, E. (2005). “Characterisation of natural organic matter fractions by high pressure size exclusion chromatography, specific UV absorbance and total luminescence spectroscopy,” Pol. J. Environ. Stud. 15(1), 145-153.
Thanh, N. D., and Nhung, H. L. (2009) “Cellulose modified with citric acid and its absorption of Pb2+ and Cd2+ ion,” in: Proceedings of the 13th International Electronic Conference on Synthetic Organic Chemistry, Sciforum Electronic Conferences Series f003, pp. 1-13.
Vermeer, A.W.P., van Riemsdijk, W.H., Koopal, L.K. (1998). “Adsorption of humic acid to mineral particles. 1. Specific and electrostatic interaction,” Langmuir, 14, 4210-4216.
Wang, X., Shu, L., Wang, Y., Xu, B., Bai, Y., Tao, S., and Xing, B. (2011). “Sorption of peat humic acids to multi-walled carbon nanotubes,” Environ. Sci. Technol. 45(21), 9276-9283.
Zbytniewski, R. and Buszewski, B. (2005). “Characterisation of natural organic matter (NOM) derived from sewage sludge compost. Part 1: chemical and spectroscopic properties,” Bioresource Technol. 96(4), 471-478.
Zhang, X., and Bai, R. B. (2002a). “Adsorption behaviour of humic acids onto polypyrrole-coated nylon 66 granules,” J. Mater. Chem. 12(1), 2733-39.
Zhang, X., and Bai, R. B. (2002b). “Deposition/adsorption of colloids to surface-modified granules: Effect of surface interaction,” Langmuir 18(9), 3459-3465.
Zhang, X., and Bai, R. B. (2003). “Mechanisms and kinetics of humic acid adsorption onto chitosan coated granules,” J. Colloid Interface Sci. 264(1), 30-38.
Zhou, J. L., and Banks, C. J. (1991). “The adsorption of humic acid fractions by fungal biomass,” Environ. Technol. 12(6), 519-529.
Zhu, D. Q., Hyun, S. H., Pignatello, J. J., and Lee, L. S. (2004). “Evidence for pi-pi electron donor acceptor interactions between pi-donor aromatic compounds and pi-acceptor sites in soil organic matter through pH effects on sorption,” Environ. Sci. Technol. 38(16), 4361-4368.
Article submitted: July 18, 2013; Peer review completed: October 9, 2013; Revised version received: December 3, 2013; Accepted: December 6, 2013; Published: December 20, 2013.
